Contrasting effect of perlecan on adipogenic and osteogenic differentiation of mesenchymal stem cells in vitro
Abstract
Perlecan, a basement membrane component, shows diverse functions in different organs and tissues. However, the role of perlecan in differentiation of mesenchymal stem cells (MSCs) has been barely investigated. In this study, we examined the effect of perlecan on adipogenic and osteogenic differentiation of MSCs in vitro by adding extrinsic perlecan to culture media or blocking the function of intrinsic perlecan expressed into culture media by differentiating MSCs. Extrinsic perlecan suppressed adipogenic differentiation; however, it promoted osteogenic differentiation. These functions were further confirmed by a study of blocking intrinsic perlecan. Perlecan treated with heparitinase-I also showed the suppressive effect on adipogenic differentiation. In contrast, the promotive effect on osteogenic differentiation was found to be heparan sulfate-dependent. Intrinsic perlecan was suggested to be effective at the late stage of adipogenic differentiation by a study of perlecan-blocking performed at distinct periods, but was suggested to be effective at the early stage of osteogenic differentiation. Our results showed perlecan has contrasting effect on adipogenic and osteogenic differentiation of MSCs due to its diverse actions. Based on these outcomes, we recognized that employing extrinsic perlecan or blocking intrinsic perlecan is effective for regulating adipogenic and osteogenic differentiation of MSCs by restricting its direction.
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cells which can be isolated from adult mesenchymal tissues (Prockop 1997). They can differentiate into so many types of cells, including adipocytes, osteoblasts and chondrocytes (Jiang et al. 2002). Therefore, the multipotent cells are considered as promising agents for tissue repair and regenerative medicine. However, it is difficult to regulate their differentiation freely and unrestrictedly along with inducing differentiation into specific cell types.
Perlecan is one of the basement membrane (BM) components, and is observed adjacent to MSCs (Kruegel & Miosge 2010) and many types of differentiated mesenchymal cells, including adipocytes and osteocytes, in vivo (Murdoch et al. 1994; Thompson et al. 2010). Perlecan can interact with various molecules (Whitelock et al. 2008), such as growth factors, morphogens and extracellular matrix components, and shows diverse functions in distinct tissues or organs by modulating the function of these molecules (Knox & Whitelock 2006). Perlecan is also able to interact with molecules known to affect the differentiation of MSCs. The core protein of perlecan has low density lipoprotein (LDL) receptor-like domain, which has been shown to interact with very low density lipoprotein (VLDL) (Hummel et al. 2004), is positive for adipogenic differentiation of MSCs and preadipocytes (Chiba et al. 2003). The N-terminal domain I of perlecan core protein contains three attachment sites for heparan sulfate (HS) side chains. HS is reported to interact with various growth factors (Whitelock et al. 2008), like fibroblast growth factors and bone morphogenic proteins, known to be positive for adipogenic and osteogenic differentiation of MSCs (Hanada et al. 1997; Kakudo et al. 2007). These studies suggest the possibility that perlecan has distinct functions also in differentiation of MSCs by blocking or co-operating with these molecules, and therefore inhibition of intrinsic perlecan or supplementation of extrinsic perlecan may be useful for regulating MSC differentiation by restricting its direction. However, whether perlecan actually affects MSC differentiation has not been reported.
In this study, we examined the effect of perlecan on adipogenic and osteogenic differentiation of MSCs in vitro by adding extrinsic perlecan to culture media or blocking the function of intrinsic perlecan expressed into culture media by differentiating MSCs.
Materials and Methods
Animals
Immunization of perlecan to Japanese white rabbits and isolation of MSCs from Wistar rats (both animals from Japan Laboratory Animals, Tokyo, Japan) were performed in accordance with the ethics committee of Hokkaido University (Japan).
Purification of perlecan
Perlecan was purified from Bovine kidneys (kindly gifted from Hokkaido Research Organization) as described by Kanwar et al. (1984) with some modifications. Frozen kidneys (stored at −20°C) were minced, washed with running cold water, and extracted in 4 mol/L guanidine chloride. The extract was then precipitated by an equal volume of 95% ethanol containing 2% potassium acetate. The ethanol precipitant was sequentially washed with water, 0.5 mol/L NaCl, 4 mol/L urea, and further extracted in a solution containing both 4 mol/L urea and 0.5 mol/L NaCl. The extract was subjected to anion-exchange chromatography. Fractions rich in perlecan were adjusted to 1.33 g/mL of density by adding cesium chloride, and fractionated into five fractions by centrifugation at 100 000 × g for 48 h. The undermost fraction was again precipitated by ethanol. The precipitant was finally dissolved in PBS (pH 7.4), dialyzed against PBS, and used as purified perlecan for culturing MSCs. In order to remove HS chains, prior to ethanol precipitation, purified perlecan was treated with heparitinase I (SEIKAGAKU, Tokyo, Japan), 20 mU for 1 mg glycosaminoglycan (GAG), at 37°C for 1 h.
Purity of perlecan was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. The bands observed by Coomassie Brilliant Blue-staining were all detected by Western blotting using monoclonal antibody against bovine perlecan domain I or V (both were from Bio Porto Diagnostics, Gentofte, Denmark). Protein and GAG contents in the perlecan solution were determined by the Lowry method (Lowry et al. 1951) and carbazole reaction method of Bitter and Muir (Bitter & Muir 1962), respectively. GAG/protein ratio was determined as 0.156.
Preparation of antiserum against bovine perlecan
A Japanese white rabbit was anesthetized by sodium pentobarbital (Dainippon Sumitomo Pharma, Osaka, Japan), followed by immunization with the purified bovine kidney perlecan previously emulsified with an equal volume of Freund complete adjuvant (BD Biosciences, Franklin Lakes, NJ, USA). Six weeks later, antiserum against perlecan (Anti-Pln) was collected. Non-immune serum was also collected from the same rabbit before immunization.
Specific reactivity of Anti-Pln was confirmed by enzyme-linked immunosorbent assay, Western blotting and immunostaining of tissue sections. Anti-Pln had no cross-reactivity with laminin, type IV collagen or fibronectin. In addition, similar to commercial antibodies, Anti-Pln could also recognize perlecan bands during Western blotting.
Culture of MSCs
MSCs were isolated from bone marrow of rat femurs as described by Nakamura et al. (2009) with some modifications. Male Wistar rats of 5 weeks old were euthanized by overdose of sodium pentobarbital, and their femurs were cleaned. The tip of each femur was removed, and the marrow was harvested by flushing the bone cavities with growth medium, alpha-modified minimum essential medium (α-MEM; Life Technologies, Carlsbad, CA, USA) containing 15% fetal bovine serum (FBS) (Cell Culture Technologies, Gravesano, Switzerland), 63.3 μg/mL of penicillin G potassium (Nacalai Tesque, Kyoto, Japan), and 100 μg/mL of streptomycin sulfate (Nacalai). After culturing the harvested marrow for a day, the non-adherent cells were removed by rinsing with sphosphate buffered saline (PBS). Adherent cells were expanded in growth medium.
Adipogenic and osteogenic differentiation was induced as described by Tan et al. (2011) and Nakamura et al. (2009) with some modifications. The MSCs were cultured in adipogenic differentiation medium (ADM) and osteogenic differentiation medium (ODM). Dulbecco's modified Eagle's medium (DMEM)-high glucose (Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS, 27 μg/mL L-ascorbic acid phosphate magnesium salt n-hydrate (Wako, Osaka, Japan), 10 μg/mL insulin (Sigma), 1 mmol/L octanoate (Wako), 500 μmol/L 3-isobutyl-1-methylxanthin (IBMX; Nacalai) and 250 nmol/L dexamethasone (Nacalai) was used as ADM; on the other hand, α-MEM containing 15% FBS, 82 μg/mL L-ascorbic acid phosphate magnesium salt n-hydrate, 10 mmol/L β-glycerophosphate (Nacalai) was used as ODM. The media were exchanged at every 3 days' interval.
Perlecan or heparitinase-I treated perlecan (Pln ΔHS) was added to the media to achieve a final protein concentration of 5 μg/mL. Sodium heparin (Nacalai) solution, which contains uronic acid equivalent to the purified perlecan solution was also added. Perlecan-blocking was performed by adding 1/500 volume of Anti-Pln to the media every day, whereas non-immune serum was added as control. Alternatively, in order to know the effective period of perlecan on MSC differentiation, MSCs were cultured in ADM and ODM for 9 days in which Anti-Pln was added at 0–3, 3–6, 6–9 or 0–9 days. Non-immune serum was either added for the entire culture period, or for the remaining period of Anti-Pln treatment.
Analysis of MSC differentiation
MSCs were induced to differentiate into adipocytes and osteoblasts on 96-well cell culture plates (Thermo Fisher Scientific, Waltham, MA, USA). They were fixed in 10% formalin for 10 min, and stained with Oil Red O and Arizarin Red S. Microscopic images were obtained by using EVOS xl core AMEX-1200 (Advanced Microscopy Group, Bothell, WA, USA). To evaluate the proportion of stained area, microscopic images were obtained from central regions of each well at ×4 magnification, which covered roughly a 50% area of each well, and was analyzed by using ImageJ (downloaded from the National Institute of Health, Bethesda, MD, USA, available at http://rsb.info.nih.gov/ij).
Immunofluorescence
The formalin-fixed cells were rinsed with PBS, and then reacted with primary antibodies, anti-mouse laminin and osteocalcin antisera (both from LSL, Tokyo, Japan), and Anti-Pln. After counterstaining with fluorescein isthiocyanate (FITC)-conjugated anti-rabbit immunoglobulin G (IgG) (MP Biomedicals, Solon, OH, USA) and 4′,6-diamidino-2-phenylindole (DAPI; DOJINDO LABORATORIES, Kumamoto, Japan), fluorescent images were obtained under inverted fluorescence microscope, EVOS fl AMF-4302 (Advanced Microscopy Group).
RT-PCR
MSCs cultured in ADM were lysed in Isogen (Wako), and total RNA was isolated according to the manufacturer's instruction. The first strand complementary DNA (cDNA) was synthesized from 1 μg of total RNA by using oligo d(T) 18 primer and reverse transcriptase (Moloney murine leukemia virus, ribolunlease (RNase) H+ ; Wako). For PCR amplification, Fast Start Taq DNA Polymerase (Roche Diagnostics, Basel, Switzerland) was used according to the manufacturer's instructions. The specific primers were designed as follows: 5′-TATGCTGTTATGGGTGAAAC-3′ and 5′-TGGTAATTTCTTGTGAAGTGCTC-3′ for peroxisome proliferator-activated receptor γ2 (PPARγ2); 5′-GGTGAAGGTCGGTGTCAACGGATT-3′ and 5′-GATGCCAAAGTTGTCATGGATGACC-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The resulting PCR products were electrophoresed on 2% agarose gels, followed by staining with 0.5 μg/mL ethidium bromide (Life Technologies). The intensity of bands were evaluated by ImageJ.
Alkaline phosphatase (ALP) activity
ALP activity of MSCs cultured in ODM was measured by Lab Assay ALP (Wako). Cells in 96-well plates were rinsed with distilled water, reacted with substrate buffer at 37°C for 5 min, and enzyme activity was measured at an absorbance of 405 nm using Ultrospec visible plate reader II 96 (GE Healthcare Japan, Tokyo, Japan).
Statistical analysis
Data were shown as mean ± SD. Multiple comparison was performed for each data by Tukey's highly significant difference (HSD) test. P < 0.05 was considered as significant.
Results
Examination of MSC differentiation and perlecan expression
The MSCs cultured in ADM were stained with Oil Red O to examine the progress of adipogenic differentiation by observing lipid accumulation (Fig. 1A). Perlecan and laminin expression was also evaluated by immunostaining and is shown in Figure 1A. A small amount of lipid droplets was observed in several cells at day 3. As culture in ADM proceeded, the number of cells containing lipid droplets and the amount of lipid droplets in each adipocyte derived from MSCs increased. Many cells at 9 days of culture in ADM appeared as spherical shapes, a typical morphology of adipocytes, and were surrounded by BM components, perlecan and laminin. A spherical staining pattern was observed for laminin at day 6, but not for perlecan.

Expression of perlecan during MSC differentiation. MSCs were cultured in ADM (A) and ODM (B), and the progress of adipogenic and osteogenic differentiation was evaluated by Oil Red O-staining (OR) for lipid accumulation and Arizarin Red S-staining (AR) for calcium deposition, respectively. Perlecan (Pln), laminin (Ln) and osteocalcin (Ocn) were immunologically stained (green) at days 0, 3, 6 and 9. Nuclei were stained with DAPI (blue). Phase-contrast microscopy images were also obtained from the same field of fluorescent images for perlecan and osteocalcin, and are shown below each fluorescent image. Bars, 50 μm.
In contrast, MSCs cultured in ODM were stained with Arizarin Red S to examine the progress of osteogenic differentiation by observing calcium deposition (Fig. 1B). Perlecan and osteocalcin expression were also evaluated by immunostaining and are shown in Figure 1B. Faint Arizarin Red S staining until day 6 indicates poor calcium deposition, whereas abundant staining was observed at day 9 (Fig. 1B). During culture in ODM, under the phase-contrast microscope the round-shaped cells were observed on the layer of fibroblast-like cells. The number of the rounded cells increased as culture in ODM proceeded, and these cells developed nodules at day 9. Osteocalcin as well as perlecan was intensely stained in the rounded cells at days 3 and 6. At day 9, intense staining for perlecan and osteocalcin was also observed in several cells within the nodules. Similar to perlecan and osteocalcin, intense staining for laminin was detected in the rounded cells at days 3 and 6, and in the cells within nodules at day 9, whereas relatively weak staining on fibroblast-like cells were also observed at all time points (data not shown).
The effect of extrinsic perlecan on adipogenic and osteogenic differentiation of MSCs
The immunofluorescence study suggested perlecan expression is associated with adipogenic and osteogenic differentiation of MSCs. To assess the effect of perlecan on MSC differentiation, MSCs were induced to differentiate into adipocytes and osteoblasts in the presence of extrinsic perlecan, and compared to cells cultured in the presence of PBS, heparin or Pln ΔHS. Figure 2A shows the weak Oil Red O-staining of lipids in the cells cultured in ADM containing perlecan (Fig. 2A). When the proportion of Oil Red O-stained area was quantified using image-analyzing software, the suppressive effect of perlecan on adipogenic differentiation was significant as compared to PBS (Fig. 2B). Pln ΔHS also showed suppressive effect on adipogenic differentiation, and it was more pronounced than that of perlecan. However, heparin did not show any suppressive effect. Contrary to cells cultured in ADM, cells in ODM showed increased calcium deposition in the presence of perlecan (Fig. 2C), and this promotive effect of perlecan on osteogenic differentiation was significant compared to PBS (Fig. 2D). Neither heparin nor Pln ΔHS showed significant effect on osteogenic differentiation.
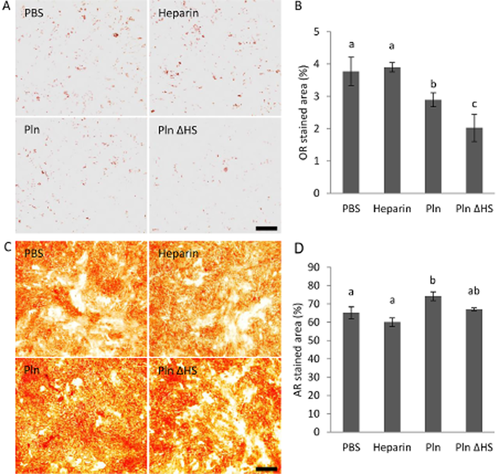
The effect of extrinsic perlecan on differentiation of MSCs. MSCs were cultured in ADM (A, B) and ODM (C, D) containing either PBS, 0.39 μg/mL uronic acid concentration of heparin, 5 μg/mL protein concentration of perlecan (Pln) or 5 μg/mL protein concentration of heparitinase I-treated perlecan (Pln ΔHS). They were stained by Oil Red O (A) and Arizarin Red S-staining (C) at day 9, and proportions of the stained areas evaluated by ImageJ are shown in B and D, respectively. Bars, 500 μm. Data represent the mean ± SD of triplicate cultures. Data denoted as different letters, a, b and c, are significantly different from each other (P < 0.05), and when ab is not significant from both data denoted as a and b. Similar results were obtained from two independent experiments.
MSCs were also cultured on a perlecan-coated 96-well plate, but the immobilized perlecan affected neither adipogenic nor osteogenic differentiation of MSCs (data not shown).
The effect of perlecan blocking on adipogenic and osteogenic differentiation of MSCs
To examine whether intrinsic perlecan secreted in medium also shows the effects observed by extrinsic perlecan, MSCs were induced to differentiate in the presence of Anti-Pln and were compared to control cells cultured with non-immune serum (Fig. 3). Increased lipid accumulation in MSCs cultured in ADM containing Anti-Pln was observed by Oil Red O-staining (Fig. 3A). The lipid accumulation was significantly higher when compared with the control (Fig. 3B). Conversely, calcium deposition in cells induced to osteogenic differentiation was reduced by Anti-Pln (Fig. 3C) and was significantly different from the control.
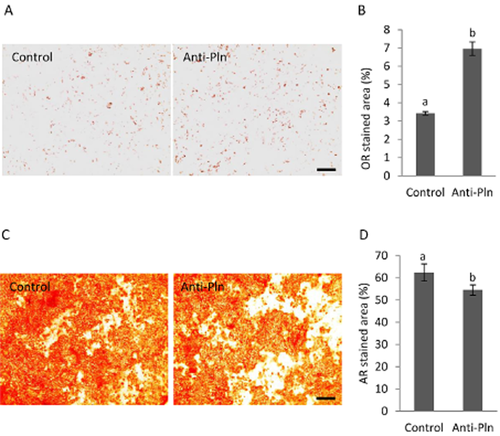
The effect of perlecan blocking on differentiation of MSCs. MSCs were cultured in ADM (A, B) and ODM (C, D) containing either non-immune serum (control) or Anti-Pln, and stained by Oil Red O (A) and Arizarin Red S-staining (C) respectively. The proportions of the areas stained by Oil Red O (B) and Arizarin Red S (D) were evaluated by ImageJ. Bars, 500 μm. Data represent the mean ± SD of triplicate cultures. Significant differences were denoted as a and b (P < 0.05). Similar results were obtained from two independent experiments.
These perlecan-blocked cells were subjected to immunological staining for perlecan, but the distribution pattern of perlecan was observed not to be altered from control cells (data not shown).
The effective period of perlecan blocking on adipogenic and osteogenic differentiation of MSCs
In order to know when perlecan affects differentiation of MSCs, Anti-Pln was added to differentiation media in distinct culture periods either at days 0–3, 3–6, 6–9 or 0–9 (Fig. 4). Perlecan blocking of cells cultured in ADM at days 0–3 and 3–6 showed slight increase of lipid accumulation as compared to control cells, which was cultured in ADM with non-immune serum the during entire period (Fig. 4A). Perlecan blocking applied at days 6–9 showed increased lipid accumulation in cells, and the level of lipid was the same even when perlecan of cells was blocked for the entire period (day 0–9). However, PPARγ2 gene expression was not altered by perlecan blocking (Fig. 4B). Perlecan blocking of cells cultured in ODM at days 3–6 but not 0–3 and 6–9 suppressed calcium deposition in cells, and the level of suppression achieved by perlecan blocking at days 3–6 was unchanged from that achieved by perlecan blocking for the entire period (days 0–9) (Fig. 4C). ALP activities of cells at days 6 and 9 were insignificantly suppressed by perlecan blocking (P = 0.133 and P = 0.114) as compared to control (Fig. 4D).
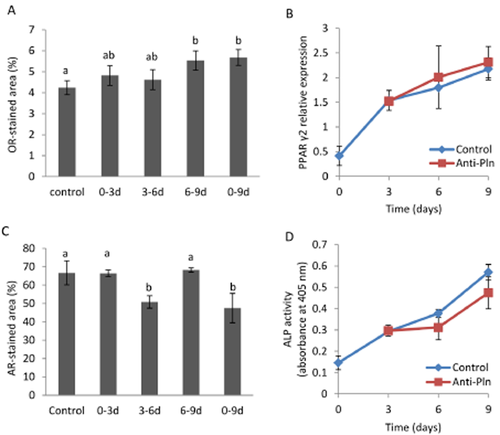
The effective period of perlecan on the differentiation of MSCs. MSCs were cultured in ADM (A, B) and ODM (C, D) for 9 days in which Anti-Pln was added at 0–3, 3–6, 6–9 or 0–9 days. Non-immune serum was either added for the entire culture period (control), or for the remaining period of Anti-Pln treatment. The proportion of stained area with Oil Red O (A) or Arizarin Red S (C) at day 9 were evaluated by Image J. mRNA expression of PPARγ2 by MSCs cultured in ADM with the addition of non-immune serum (control) or Anti-Pln at every day were analyzed by RT-PCR (B). The relative expression of PPARγ2 at days 0, 3, 6, and 9 normalized to GAPDH expression is shown. ALP activity of MSCs cultured in ODM containing non-immune serum or Anti-Pln is shown in (D). Data represent the mean ± SD of triplicate cultures (A, C and D) of three independent experiments (B). Data denoted as different letters are significantly different from each other (P < 0.05), and those as ab not significant from both data denoted as a and b. Similar results as shown in (A), (C) and (D) were obtained from two independent experiments.
Disucussion
Perlecan shows diverse functions in distinct tissues or organs (Knox & Whitelock 2006), and in this study, perlecan also showed a contrasting effect on adipogenic and osteogenic differentiation of MSCs.
The immunofluorescence study showed that BM components, including perlecan deposited around adipocytes, differentiated from MSCs. Although BM components, laminin and/or type IV collagen have been reported to facilitate adipogenic differentiation of preadipocytes (Hausman et al. 1996), our study showed extrinsic perlecan suppressed lipid accumulation in adipogenically differentiated MSCs. In addition, extrinsic Pln ΔHS, but not heparin, decreased lipid accumulation in MSCs, indicating core protein of perlecan has a suppressive effect on adipogenic differentiation of MSC. The result that perlecan-blocking promoted lipid accumulation in MSCs also indicates perlecan has a suppressive effect. We could not find any report showing the function of perlecan on adipogenic differentiation of MSCs, nor preadipocytes, but it has been reported that ribozyme-mediated perlecan knockdown causes lipid accumulation in C3H10T1/2 mesenchymal cell line (Gomes et al. 2006). A possible explanation for the suppressive effect on adipogenic differentiation of MSCs is that perlecan blocked interaction of VLDL with VLDL receptors by its core protein. Apolipoprotein E-dependent uptake of VLDL has been suggested to promote adipogenic differentiation of MSCs (Chiba et al. 2003), and expression of VLDL receptors and apolipoprotein E has been reported to increase in MSCs during adipogenic differentiation (Prawit et al. 2008).
Perlecan blocking at days 6–9 of culture in ADM was most effective for promotion of adipogenic differentiation, and PPARγ2 expression, which is prerequisite for the late events of adipogenic differentiation (Siersbaek et al. 2010), was not affected by perlecan blocking. Although expression of late differentiation markers remain to be examined, these results suggest perlecan core protein suppresses the late event of adipogenic differentiation. This seems to be consistent with the explanation for the suppressive effect of perlecan described above. Since apolipoprotein E and VLDL receptor expression can be induced by PPARγ (Galetto et al. 2001; Tao et al. 2010), expression of these genes and VLDL uptake by MSCs were probably up-regulated at the late stage of adipogenic differentiation.
The immunofluorescence study showed an analogous distribution pattern of perlecan and osteocalcin in MSCs during osteogenic differentiation. Since osteocalcin is a highly specific osteogenic differentiation marker (Nishimoto & Price 1980; Hauschka et al. 1989; Nakamura et al. 2009), this result suggests the involvement of perlecan in osteogenic differentiation. Indeed, extrinsic perlecan, not Pln ΔHS, promoted osteogenic differentiation of MSCs, indicating perlecan promoted osteogenic differentiation in a HS-dependent manner. Although the direct interaction of osteocalcin with perlecan or HS has not yet been reported, it is possible that osteocalcin and perlecan synergistically regulate osteogenic differentiation of MSCs.
Intriguingly, heparin did not show any promotive effect on osteogenic differentiation of MSCs. Heparin has been reported to promote osteogenic differentiation of C2C12 cells, when added to medium with bone morphogenetic protein 2 (BMP-2) (Takada et al. 2003), which strongly stimulates osteogenic differentiation. Our study showed the promotive effect of perlecan on osteogenic differentiation did not require BMP-2 addition. It is unclear whether perlecan modulated the function of intrinsic BMP-2, but our result suggests HS attached on perlecan is more potent in promoting osteogenic differentiation than heparin, probably due to the presence of core protein.
Perlecan blocking at days 3–6 of culture in ODM was effective for suppression of osteogenic differentiation, but calcium deposition on MSCs was very small at day 6. In addition, the ALP activity of MSCs has a tendency to decrease by perlecan blocking during osteogenic differentiation. The increase of ALP expression is known to occur at the early stage of osteogenic differentiation (Mathews et al. 2012), and is a prerequisite for calcium deposition (Golub 2009). Thereby, our results suggest perlecan expressed by MSCs mainly acts at the early stage of osteogenic differentiation, whereas further analyses are needed to confirm this.
In our study, extrinsic perlecan and Anti-Pln were added to media. Since the distribution pattern of perlecan on MSCs was not altered by perlecan blocking, Anti-Pln could be involved in blocking the function of free perlecan present in the media. In addition, immobilized extrinsic perlecan did not show any effect on adipogenic differentiation and osteogenic differentiation of MSCs. It is possible that perlecan free from extracellular matrix (ECM) shows diverse function well by freely interacting with various molecules rather than that trapped in ECM.
In conclusion, perlecan showed contrasting effects on adipogenic and osteogenic differentiation of MSCs in vitro, and these effects were due to the presence of HS chains and core protein in one molecule. Free perlecan probably modulates functions of various molecules by freely interacting with them, and shows either the suppressive effect on adipogenic differentiation with its core protein or the promotive effect on osteogenic differentiation with its HS chains. We considered using or blocking perlecan is effective for regulating osteogenic and adipogenic differentiation of MSCs by restricting its direction.
Acknowledgments
We are grateful to Md. Morshedur Rahman for advice on writing in English, and to Hokkaido Research Organization for the gift of kidney samples.




