Growth performance, digestive and immune enzyme activities, and the environmental effects of the ivory shell, Babylonia areolata (Link 1807) in integrated Multi-Trophic aquaculture systems
Abstract
This study was conducted to evaluate the growth performance and the digestive and immune enzyme activities of the ivory shell (Babylonia areolata, Link 1807) in an integrated multi-trophic aquaculture (IMTA) system. The impact of environmental factors on the ivory shell was also investigated. The ivory shell B. areolata, sea cucumber Holothuria leucospilota and seaweed Gracilaria tenuistipitata comprised the IMTA group in this study, while ivory shell in monoculture was used as the control group. After 150 days rearing experiment, a higher survival rate, farming yield, and feed efficiency was found in the IMTA ponds. Seaweed and sea cucumber have a good purification effect on ammonia nitrogen, nitrite nitrogen in water and total nitrogen, phosphorus and organic carbon in the sediment. In addition, Vibrio as a conditional pathogen showed a significant decrease in sediment and in ivory shell intestines from the IMTA group. The digestive and immune enzyme activities of ivory shell in the IMTA group were higher than in the control group, suggesting the IMTA system may promote digestive and immunity capabilities of ivory shell. Results from the present study suggest that the IMTA system is an important method to develop the sustainable aquaculture of the ivory shell industry by using G. tenuistipitata and H. leucospilota in an integrated system. By applying such a method, the aquaculture environment can be improved, and the digestion and immunity of ivory shell can be enhanced, leading to the healthy growth of the ivory shell.
1 INTRODUCTION
The ivory shell, Babylonia areolata (Link 1807), is an important economical marine gastropod mollusc that is intensively cultured in the provinces of Hainan, Fujian, and Guangdong in China as well as in some Southeast Asian countries including Thailand and Vietnam (Kritsanapuntu et al., 2006). With its delicate taste and nutritiousness especially unsaturated fatty acid, ivory shell is becoming more and more popular with consumers. From an aquaculture perspective, the ivory shell has many biological attributes, as well as production and market characteristics that are necessary for it to become a profitable aquaculture venture. Consequently, it is considered a promising candidate species for the land-based aquaculture industry (Chaitanawisuti & Kritsanapuntu, 1997). The ivory shell has been cultured in southern China for over 20 years and has made a great contribution to the local economy. However, in recent years, with the rapid development of farming, outbreaks of diseases, such as reverse back syndrome, proboscis intumescence disease, and shell-flesh separating syndrome have affected ivory shell aquaculture, causing heavy economic losses in China (Wang et al., 2013; Zhang et al., 2010; Zhao et al., 2020). A variety of microorganisms have been considered to be the pathogens causing disease in ivory shell, among which Vibrio was the most common pathogen (Wang et al., 2013; Zhao et al., 2020). The presence of Vibrio in ivory shell aquaculture systems was affected by water quality and substrate factors (Ling et al., 2018). According to a previous study, the occurrence of disease in B. areolata was closely related to the deterioration of the breeding environment (Ruangsri et al., 2018). Therefore, it is possible to maintain a relative stability aquaculture environment to prevent diseaseoutbreaksk in B. areolata.
At present, the main method of B. areolata industrial aquaculture in China is spreading a sand layer at the bottom of cement ponds (Ye et al., 2016). In such a system, the bait residue must be removed; otherwise, the water and sand will be contaminated in the system and the breeding environment will be deteriorated (Yang et al., 2019). The deterioration of aquaculture environment, frequent occurrence of aquaculture diseases, and increasing potential safety risks of obsolete aquatic products in aquaculture facilities pose challenges to a healthy and sustainable development of aquaculture (Khor et al., 2019). Therefore, monoculture systems must be transformed and improved. It is of great significance for the healthy, efficient and sustainable development of aquaculture to develop a green, environmentally friendly and energy-saving eco-farming model (Troell et al., 2009).
Integrated multi-trophic aquaculture (IMTA) is a sustainable form of aquaculture developed in the past decades. It is an integrated aquaculture system composed of organisms on different trophic levels. The excretion of some organisms becomes a source of nutrients for other organisms, such as seaweeds and sea cucumber in the system, and the nutrients are used efficiently while protecting the aquaculture environment (Chopin et al., 2001). In the case of a salmon IMTA system, additional income was generated from an increase in the production of salmon, mussels and seaweed, and economic losses were reduced, which provided better economic resilience (Ridler et al., 2007). Because seafood produced in IMTA systems is safer and healthier, it is considered better for the environment and animal welfare. Consequently, IMTA gained social acceptability over traditional fish monoculture in the USA and Canada (Barrington et al., 2008). Furthermore, the mariculture model reform and breeding technology innovation that took place in China from the 1970s onwards have established some IMTA systems, such as fish–shellfish, fish–shrimp, fish–seaweed, shrimp–shellfish, shrimp–seaweed, shell–seaweed, fish–shell–seaweed, and sea cucumber–seaweed among others (Jiang et al., 2012; Niu et al., 2006; Shpigel et al., 2017; Su et al., 2015; Tian et al., 2001; Wang et al., 2015; Wu et al., 2017). This caused a major change, from simply pursuing the highest yield in aquaculture to comprehensively optimizing the variety and structure as well as improving the quality of products. Consequently, the level of intensive, large-scale, and modernized aquaculture has gradually been improved (Ma et al., 2016). Seaweed is used in IMTA systems as biofilters of fishpond effluents, saving on the cost of water treatment while being a reliable food source (Neori et al., 2004; Neori & Shpigel, 1999). Sea cucumbers, the deposit feeders, ingest substantial amounts of organic wastes from the surface layer of bottom sediments and convert them into inorganic nutrients, thereby reducing the content of organic wastes in sediments (Michio et al., 2003).
In this study, a farming system was established based on B. areolata, Holothuria leucospilota and Gracilaria tenuistipitata to evaluate the significance of IMTA application in ivory shell aquaculture. We intended to assess the potential of sea cucumber and seaweed-driven ivory shell aquaculture in terms of growth performance, digestion and immune enzyme activities, and environmental effects. The results of this study will provide a theoretical basis for the sustainable development of ivory shell aquaculture, and can be applied to other similar species.
2 MATERIALS AND METHODS
2.1 Experiment design and operation
The study was conducted at the Farming Base of the ivory shell Sanya Comprehensive Experimental Station of China Agriculture Research System (Wanning, Hainan). The ivory shells were cultured in six outdoor cement ponds (5.74 × 2.87 × 1.0 m). A 10 cm high perforated plastic board covered the bottom of the pond, and a sieve with 0.3 mm holes was set on the plastic board. Subsequently, the sieve and the plastic board were buried under 4–6 cm of sand particles (1–3 mm in size) to limit the movement of ivory shells along the bottom of the pond. The bait residue was removed daily to maintain the oxygen content of the sand layer because the water flows through the sand layer. This assists in reducing the blackening of the sand layer. A pond aeration blower was used to provide oxygen to the ponds and the dissolved oxygen was maintained above 5 mg L−1. The water inlet and outlet were placed at opposite ends of the pond, at the greatest possible distance from each other to facilitate water exchange. Natural seawater was filtrated through sand and precipitated before being added to the ponds. Before the start of the experiment, the breeding facilities were cleaned with chlorine-containing disinfectants.
The ponds were designated either IMTA (3) or control (3) ponds. In the IMTA ponds, H. leucospilota and G. tenuistipitata were placed at a density of 2 ind. m−2 and 500 g m−2, respectively. The control ponds remained unstocked for a week, after which, self-breeding juvenile ivory shell of the same size from this breeding base was stocked into the IMTA and control ponds at a density of 1200 ind. m−2. The water exchange rate of all ponds was adjusted synchronously according to the breeding time, 100% in the first month, 200% in the second to third months, and 300% in the fourth to fifth months. The salinity of the ponds was maintained at 27–33‰, the water temperature at 25–32°C and pH at 7.5–8.3. The light intensity was 5000–10,000 Lx. The ivory shells were daily fed 3%–10% of their total weight and their primary bait was fresh Trachurus japonicus. The specific feeding amount was estimated by the amount of food intake on the previous day. Feeding was discontinued if <3% of provided bait was consumed. There was no need to feed the sea cucumbers, and no nutrients were added for seaweeds. The experiment lasted for 150 days during which no other drugs were used.
2.2 Sample collection and processing
The weight of ivory shell, seaweed, and sea cucumber was determined at the beginning of the experiments and again at the termination (after 150 days) to evaluate growth performance. At the termination of the experiment, a circular sampling frame with an area of 0.05 m2 was used to evaluate a total of nine sample points in the front, middle and back of each pond. Subsequently, the number of ivory shells was counted, and their weight was estimated to calculate the survival rate and average weight. A number of ivory shells were randomly selected from each aquaculture pond at the end of the experiments for subsequent testing.
The activity of immune and digestive enzymes of ivory shell in different groups was determined. In brief, use clean pliers to carefully crush the shell, the digestive tract was removed using sterile forceps for the detection of digestive enzymes including pepsin, lipase and amylase. The hepatopancreas was removed for the detection of immune enzymes, including catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), acid phosphatase (ACP), alkaline phosphatase (AKP) and lysozyme (LZM). All enzyme activities are measured using the reagent kit from Nanjing Jiancheng Bioengineering Institute.
The Vibrio content in the intestines of the ivory shells, the water and the sand layer were measured following the thiosulphate–citrate–bile salts–sucrose agar (TCBS) plate culture method (Ling et al., 2019) every 30 days. To determine the Vibrio content in the intestines, three shells of similar size were randomly collected, and the visceral mass dissected out and placed into a sterile petri dish with an aseptic dissection tool. The mass was subsequently cut into pieces and mixed. A weight of approximately 0.3 g of tissue was then placed into an aseptic tissue homogenizer (Omni International) and homogenized with 1 ml of sterile physiological saline. This solution was diluted in a 10-fold gradient (10−1, 10−2, 10−3) and 100 μl of each gradient dilution was evenly spread on a TCBS plate. To determine the Vibrio content of the pond water, a sample from each pond was collected, diluted 10 times and spread on the TCBS plates. To calculate the content of Vibrio in the sand layer, a sample was taken from the front, middle and back of each pond and mixed. Furthermore, approximately, 1 g of sand from these samples was washed with sterile seawater. The eluate was collected, diluted 10 times with sterile seawater, and spread on TCBS plates. All TCBS plates were incubated at 28°C for 48 h. The number of Vibrio was estimated using a counting method from National Food Safety Standard (GB 4789.2–2016).
Ammonia nitrogen (NH4-N) levels in the water were determined using indophenol blue spectrophotometry from the Specification for Marine Monitoring—Part 4: Seawater analysis (GB 17378.4–2007), nitrous nitrogen (NO2-N) concentration was determined using naphthalene ethylenediamine spectrophotometry (GB 17378.4–2007). Total nitrogen (TN) and total phosphorus (TP) in the sand were determined by the Specification for Marine Monitoring—Part 5: Sediment Analysis (GB 17378.5–2007).
2.3 Statistics and analysis
Data were analysed using analysis of variance and T tests using the software SPSS 19.0 (IBM SPSS). The differences between groups of the results were expressed as mean ± standard deviation. Mean values of growth performance, digestive and immune enzyme activities and the environmental factors were compared, p < 0.05 was considered a significant difference, and p < 0.01 as a highly significant difference. The principal component was determined by the standard with an eigenvalue >1 (Ling et al., 2019). Origin 9.0 was used for mapping.
3 RESULTS
3.1 Growth performance of B. areolata in IMTA
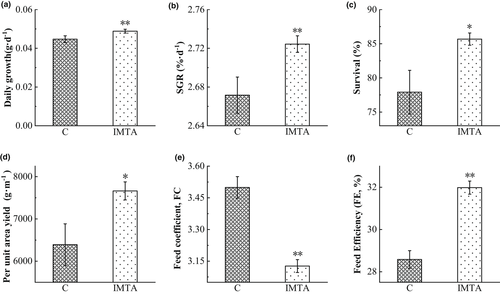
There were significant differences in growth between the two groups after 150 days of cultivation (Figure 1), The average daily growth of the IMTA group was 0.049 g day−1, which was significantly higher than the control group (p < 0.01, Figure 1a). The SGR of B. areolata in the IMTA group was significantly increased compared with the data observed in the control group (p < 0.01, Figure 1b). At the end of the experiment, the survival rate of the IMTA group was 7.77% higher than that of the control group, and difference between the groups reached a significant level (p < 0.05). The yield per unit area of the IMTA ponds was 19.90% higher than that of the control group, and the difference between the groups reached a significant level (p < 0.05). The FC of the IMTA group was significantly decreased than that of the control group (p < 0.01). The feed efficiency was 3.21% higher than that of the control groups (p < 0.01).
3.2 Digestive and immune enzyme activities of B. areolata in IMTA systems
The effects of antioxidant enzyme activity in IMTA showed that there was no significant difference in SOD activity between the IMTA group and the control group (p > 0.05). But the activity of CAT and POD of the IMTA group was significantly higher than that of the control group (p < 0.05) during the experiment period, the activities of CAT and POD were increased by 65.91% and 51.06% respectively (Figure 2a–c). The ACP activity of B. areolata from the IMTA group was significantly 75.15% (p < 0.01) higher than that of the control group. The AKP and LZM activities of B. areolata from the IMTA group were not significantly different from the control group (p > 0.05, Figure 2d–f).

The digestive enzyme activity of B. areolata in the control group and the IMTA group showed that the lipase activity varied between groups (p < 0.05). The activity of lipase of B. areolata from the IMTA group was significantly higher than that of the control group and exceeded the control group by 27.3% (p < 0.05). As the experiment progressed, the activity of amylase in B. areolata from the IMTA group showed an increasing trend, but there was no significant difference between the two groups (p > 0.05). The pepsin activity of the IMTA group was significantly higher than that of the control group, and the difference between the two was highly significant (p < 0.01, Figure 2g–i).
3.3 Environmental effects on B. areolata in IMTA systems
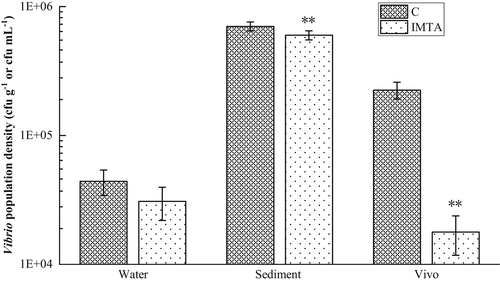
The content of Vibrio in water collected from the ponds was between 3.08 × 104–4.41 × 104 cfu ml−1, but there was no significant difference between the IMTA ponds and control ponds. The content of Vibrio in the sediment of the IMTA group was 6.00 × 105 cfu g−1, which was significantly lower than the control group (p < 0.01). The Vibrio content of the intestines of ivory shells in the IMTA group was significantly decreased compared with the data observed in the control group (p < 0.01) (Figure 3).
Water and sediment quality differed between the two aquaculture systems (Table 1). In the IMTA system, the concentrations of nitrite nitrogen and ammonia nitrogen were significantly reduced compared with the control group (p < 0.05), and the removal rate of the two reached 52.95% and 57.93%, respectively. Similarly, total phosphorus, total nitrogen and organic carbon in the sediment of the IMTA group were significantly lower than that of the control group (p < 0.05). Compared with the control group, the sediment organic carbon, total nitrogen and total phosphorus removal rate of the IMTA group reached 49.10%, 50.30% and 53.88%, respectively.
| Major environmental factors | Control group | Removal rate (%) of IMTA |
|---|---|---|
| Water nitrite nitrogen (mg L−1) | 0.87 ± 0.08 | 52.95 ± 4.15** |
| Water ammonia nitrogen (mg L−1) | 0.89 ± 0.09 | 57.93 ± 4.37** |
| Sediment organic carbon (mg g−1) | 6.54 ± 0.48 | 49.10 ± 3.92** |
| Sediment total nitrogen (μg g−1) | 423.03 ± 41.40 | 50.30 ± 4.71** |
| Sediment total phosphorus (μg g−1) | 107.88 ± 10.67 | 53.88 ± 4.43** |
- Note: “*” represents significant difference (p < 0.05); “**” represents highly significant difference (p < 0.01). Data were expressed as mean ± SD (n = 3).
3.4 Principal component analysis of B. areolata aquaculture system factor
There were three principal components, and the cumulative variance contribution rate was 89.606% (Table 2). The first principal component was primarily characterized by sediment organic carbon, sediment total nitrogen, vivo Vibrio, sediment total phosphorus and ammonia nitrogen and nitrite nitrogen in the water. Of these, sediment organic carbon and sediment total nitrogen were the main environmental factors that affected the levels of Vibrio present in the intestines of B. areolata. The second principal component was primarily characterized by LZM, pepsin and SOD, of which LZM accounted for a larger weight coefficient. The third principal component was primarily characterized by Vibrio present in the water, ACP, AKP and lipase, of which ACP and AKP occupied a larger weighting coefficient, and their role cannot be underestimated.
| Factors | Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Sediment organic carbon (mg g−1) | 0.998 | 0.019 | −0.036 |
| Sediment total nitrogen (μg g−1) | 0.988 | 0.112 | −0.037 |
| Vivo Vibrio (cfu g−1) | 0.955 | 0.213 | −0.169 |
| Sediment total phosphorus (μg g−1) | 0.947 | −0.25 | 0.037 |
| Water ammonia nitrogen (mg L−1) | 0.943 | −0.175 | −0.202 |
| Weight (g) | −0.928 | 0.308 | 0.068 |
| Water nitrite nitrogen (mg L−1) | 0.921 | 0.223 | −0.195 |
| Sediment Vibrio (cfu g−1) | 0.88 | 0.115 | 0.184 |
| Peroxidase, POD (U mgprot−1) | −0.806 | −0.429 | −0.187 |
| Lipase (U mgprot−1) | −0.783 | 0.109 | −0.5 |
| Catalase, CAT (U mgprot−1) | −0.773 | 0.135 | −0.218 |
| Water Vibrio (cfu ml−1) | 0.756 | −0.107 | 0.614 |
| Acid phosphatase, ACP (U gprot−1) | −0.731 | −0.213 | 0.631 |
| Alkaline phosphatase, AKP (U gprot−1) | 0.677 | −0.325 | 0.509 |
| Lysozyme, LZM (U mg−1) | −0.34 | 0.905 | 0.237 |
| Pepsin (U mgprot−1) | −0.499 | −0.756 | 0.075 |
| Superoxide dismutase, SOD (U mgprot−1) | 0.063 | 0.713 | 0.202 |
| Amylase (U mgprot−1) | −0.675 | 0.117 | 0.715 |
| Eigenvalue | 11.403 | 2.597 | 2.129 |
| Percentage of variance (%) | 63.351 | 14.425 | 11.83 |
| Cumulative (%) | 63.351 | 77.776 | 89.606 |
4 DISCUSSION
4.1 Effects of IMTA on growth performance of B. areolata
IMTA is a healthy and sustainable form of aquaculture developed in the past decades. It is an integrated culture system composed of organisms on different trophic levels. In this system, the nutrients and energy input into the farming system are fully utilized (e.g., the excretion of some organisms becomes a source of nutrients for others) to reduce the loss of nutrients and the potential economic loss to a minimum. Hence, the system has a higher capacity of breeding and sustainable food production (Shpigel et al., 2018; Zhang & Kitazawa, 2016). It has the advantages of high resource utilization, environmental protection and diversified products (Ma et al., 2016). IMTA has been shown to be successful with many different species. For example, the IMTA group consisting of silver carp, mrigal and stinging catfish has the highest survival, weight gain and the highest yields. Another example is the production of snails and water spinach in IMTA ponds, which contributed to the bio mitigation process of organic and inorganic waste, keeping the water quality within suitable conditions for fish culture (Kibria & Haque, 2018).
Seaweed is an important part of IMTA. Sun et al. (2015) found that as a result of the co-culture of G. lemaneiformis with the bay scallop (Argopecten irradias), the yield of the bay scallop and G. lemaneiformis increased by 1020 kg hm−2 and 9000 kg hm−2, respectively. Seaweed can absorb and concentrate nutrients and other elements in water; they have ability to curb the breeding of other harmful seaweed to stop mud from releasing nutrients to the water and to increase the oxygen in the water, which is more conducive to the ecological balance in an aquaculture system (Yang et al., 2015). The sea cucumber, H. leucospilota, mainly feeds on bacteria, organic debris and benthic diatoms in the bottom mud, and acts as a sand transporter and scavenger (Džeroski & Drumm, 2003). Utilizing the characteristics of sea cucumbers to purify the bottom soil quality of the ponds, there have been studies on the sea cucumbers participating in ecological polyculture with other aquatic animals (Michio et al., 2003; Paltzat et al., 2008; Zhou et al., 2006). Yu et al. (2012) found that SGR and survival rate of Litopenaeus vannamei increased significantly after co-culture with sea cucumbers. Similar results were obtained in this study. The study evaluated the effects of IMTA systems consisting of sea cucumber, gracilaria and ivory shell on the growth performance of B. areolata. The weight gain and SGR of the IMTA group were significantly higher than that in the control group. Furthermore, the ivory shells in the IMTA ponds had higher rates of survival, production, bait coefficient and efficiency of bait than the control group. It may be due to the effect of H. leucospilota and G. tenuistipitata on the environment. Firstly, the co-culture of sea cucumber and seaweed can reduce the toxic stress of ammonia nitrogen, nitrite nitrogen and other harmful substances on ivory shells, maintaining them in good physiological condition and saving more energy to promote growth. Secondly, the co-culture with sea cucumber and seaweed creates a relatively stable physical and chemical environment. The contents of pathogenic Vibrio in the culture system have remained relatively stable and at low levels. This could be the primary reasons for the high survival rate observed in the present study. In addition, the extract of seaweed has antibacterial activity and can significantly improve the immunity of farmed animals to pathogens (Li et al., 2018), which may also be an important factor for the higher survival rate of the ivory shells cultures in IMTA ponds than the control. In this study, it is believed that the use of IMTA in culture has a significant effect on the growth performance of ivory shells.
4.2 Effects of digestive and immune enzyme activities of B. areolata in IMTA systems
CAT, SOD and POD are important antioxidant enzymes in the body. CAT and SOD work together to generate and scavenge free radicals, maintain the body's dynamic balance of free radicals, and reduce the body's oxidative damage to the body. POD mainly catalyses the decomposition of oxides and peroxides in the body (Martínez-Álvarez et al., 2005). The results showed that the activities of CAT, SOD, POD and other antioxidant enzymes are related to environmental factors, diet and physiological conditions (Zhao et al., 2020). In addition, the level of antioxidant enzyme activity under normal conditions reflects the immune function of farmed animals (Martínez-Álvarez et al., 2005). In this study, it was found that compared with the control group, the IMTA system could significantly increase the activity of CAT and POD. Thus, IMTA can significantly enhance the immunity of farmed animals, which is an important reason for improving survival.
Phosphatase is a kind of orthophosphate monoesterase. It is divided into AKP and ACP based on the difference of optimum pH. It is an important part of lysosomal enzymes and has the function of removing, hydrolysing and digesting foreign bodies. Phosphatase is an important part of the detoxification system in animals, directly involved in the metabolism and transfer of phosphate groups, and is related to the metabolism of DNA, RNA, proteins and lipids (Yang et al., 2017). Studies on two species of abalone showed that the phagocytic activity of abalone was easily affected by salinity, and thus affected its immune defence ability (Martello et al., 2000). The results showed that when the salinity of the environment was changed, the number of blood cells decreased. Subsequently, the activity of phenol oxidase, phagocytosis and the ability to clear Vibrio parahaemolyticus decreased (Cheng et al., 2004). The results of this experiment showed that in the IMTA group, the activity of AKP and LZM did not change much, and the activity of ACP was significantly higher than that of the control group. This is similar to the initial immune response of the body when the disease occurs. When the pathogen invades, ACP can change or modify the surface structure of the foreign matter to improve the recognition rate (Wang et al., 2011), thereby increasing the speed of phagocytic phagocytosis and removal of the pathogen. The results of this study indicate that IMTA culture could improve the immune response of B. areolata.
The activity of digestive enzymes, which determines the ability of animals to digest and absorb nutrients, is an important index reflecting the physiological function of aquatic animals (Natalia et al., 2004). Studies have shown that the activity of digestive enzymes is affected by many factors, which can be different due to species, physiological conditions, water factors, bait, specifications and breeding methods (Hoseinifar et al., 2017; Zhou et al., 2016). In IMTA farming, the lipase and pepsin levels of ivory shells were significantly higher than those of the control group. This indicates that the ivory shells in the IMTA ponds have a better digestion capacity for fat and protein than those in the control. Therefore, carnivorous ivory shells can better ingest and digest fish bait, which in turn promotes growth.
4.3 Environmental factors affecting B. areolata in IMTA systems
Many factors in the culture ponds influence each other, including water factors, sediment factors, microorganisms and the cultured organisms to form a relatively independent aquaculture ecosystem. The aquaculture environment plays a key role in the success of ecological aquaculture, whereas water and sediment factors affect the healthy growth of aquatic animals; this may be achieved by influencing the microorganisms in the cultured animals, especially pathogenic bacteria (Schets et al., 2011) showed that the density of bacteria was closely related to the variation of environmental factors. This was confirmed in the present study. IMTA can effectively reduce the content of Vibrio in the sediments of the culture ponds and the intestines of the ivory shells. In the correlation analysis, there was a certain correlation between the sediment organic carbon and sediment total nitrogen with the number of Vibrio in B. areolata. This illustrates the indirect effect that the environment has on the level of Vibrio present in the ivory shells.
Ammonia nitrogen and nitrite nitrogen are important water quality factors in the aquaculture, and its effective control is of great importance to the growth and survival of aquatic animals. Seaweed grows and absorbs a large amount of nutrients, thereby reducing the deposition of nutrients, such as nitrogen and phosphorus. For example, after G. conferta was added to the culture ponds of Haliotis discus, the levels of ammonia nitrogen, nitrite nitrogen and phosphate in the pond were significantly reduced. Ulva lactuca had the highest uptake of ammonia nitrogen in an IMTA system, but the absorption rates of nitrite nitrogen and phosphate by Gracilaria asiatica were higher than those of other groups. However, Gracilaria chorda absorbed more nitrate nitrogen than that by other three types of algae (Shpigel et al., 2018). In an IMTA system stocked with Gracilaria lichenoides and shrimp L. vannamei, the content of ammonia nitrogen and phosphate was far lower than the control group (Xu et al., 2008). Degradation of the residual bait, other organic residues, and faecal sediment produces ammonia nitrogen, nitrite nitrogen, and other substances harmful in the breeding of B. areolata. However, ammonia nitrogen and nitrite nitrogen were absorbed by in the IMTA group. The absorption rate of these substances is mainly related to the species and quantity of organisms present in the breeding system, as well as the light and the concentration of ammonia nitrogen and nitrite nitrogen. Therefore, when B. areolata is cultivated in an IMTA system, stoked with G. tenuistipitata, it can significantly reduce harmful substances such as ammonia nitrogen and nitrite nitrogen in the water and improve water quality.
Total nitrogen, total phosphorus and organic carbon are usually used as important evaluation indicators for sediment pollution in aquaculture (Cai et al., 2013). Sea cucumber, H. leucospilota, improves the sediment owing to its biological disturbance and feeding effect (Ye et al., 2016), and it effectively reduced the contents of nitrogen, phosphorus and organic carbon in sediments of the IMTA system in this study. Sea cucumbers improve the sediment in several ways, absorbing residual bait, faeces, benthic diatoms and other organic matter deposited in the sediment of aquaculture through feeding and digestion, and significantly improve substrate environmental factors. Furthermore, the activities of sea cucumbers have biodisturbing effects on the sediment, and consequently some organic particles are resuspended into the water, which reduces the content of organic matter in the sediment.
5 CONCLUSION
In summary, a IMTA system was established based on Babylonia areolata, Holothuria leucospilota and Gracilaria tenuistipitata. IMTA can significantly improve the water quality and bottom quality, enhance the resistance of the ivory shell, and promote the growth and survival. IMTA is a feasible way for healthy breeding of ivory shell. Protocol developed in the present study can also be adopted to other similar species.
AUTHOR CONTRIBUTIONS
Wang Zhao conceived and designed the project, wrote the manuscript. Xinmei Huang and Zhenghua Deng collected the data. Yang Rui, Weigeng Wen and Wei Fang cultured the ivory shell. Wang Zhao, Zhongming Zheng and Gang Yu collected the samples and carried out analysis. All listed authors have read, edited and approved the final manuscript.
ACKNOWLEDGEMENTS
This research was financially supported by the National Key R & D Program of China (2020YFD0900201), Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO.2020TD55), Project of Academy Locality Science and Technology Cooperation of Sanya (2018YD19, 2019YD21), China Agriculture Research System of MOF and MARA, Financial Fund of Ministry of Agriculture and Rural affairs of China (NHYYSWZZZYKZX2020), and the K.C. Wong Magna Fund in Ningbo University. The authors would like to thank the staffs of Sanya aquaculture base who provided the assistance for samples.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL STATEMENT
The whole experiment was conducted according to the guidelines established by the National Institutes of Health.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




