Chronic exposure to nitrate significantly reduces growth and affects the health status of juvenile Nile tilapia (Oreochromis niloticus L.) in recirculating aquaculture systems
Abstract
Studies on chronic or acute toxicity of nitrogen species on fish in recirculating aquaculture systems (RAS) usually focused on adverse effects of total ammonia nitrogen (TAN: sum of NH3 + NH4+) and nitrite ( ), while underestimating the potential effects of high nitrate accumulation on growth and health status of fish. In our study, Nile tilapia (Oreochromis niloticus) were exposed to five different nitrate concentrations (0, 10, 100, 500 and 1000 mg L−1
), while underestimating the potential effects of high nitrate accumulation on growth and health status of fish. In our study, Nile tilapia (Oreochromis niloticus) were exposed to five different nitrate concentrations (0, 10, 100, 500 and 1000 mg L−1 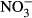 -N) over 30 days. Growth parameters (feed conversion ratio (FCR), specific growth rate (SGR), hepatosomatic index (HSI)), blood samples (concentrations of haemoglobin, methaemoglobin, plasma
-N) over 30 days. Growth parameters (feed conversion ratio (FCR), specific growth rate (SGR), hepatosomatic index (HSI)), blood samples (concentrations of haemoglobin, methaemoglobin, plasma 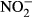 /
/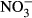 ) and the histology of the gills were studied to evaluate growth and health status of the fish. At the highest nitrate concentration, the fish showed significantly reduced growth and impaired health status (SGR, FCR, plasma
) and the histology of the gills were studied to evaluate growth and health status of the fish. At the highest nitrate concentration, the fish showed significantly reduced growth and impaired health status (SGR, FCR, plasma 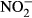 /
/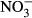 , haemoglobin and methaemoglobin concentration), demonstrating that too high nitrate concentrations can negatively influence tilapia production in RAS. Here, we recommend not exceeding concentrations of 500 mg L−1
, haemoglobin and methaemoglobin concentration), demonstrating that too high nitrate concentrations can negatively influence tilapia production in RAS. Here, we recommend not exceeding concentrations of 500 mg L−1 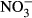 -N in juvenile tilapia culture to ensure an optimal health and growth status of the fish, as below that concentration no effects on the tilapia have been observed.
-N in juvenile tilapia culture to ensure an optimal health and growth status of the fish, as below that concentration no effects on the tilapia have been observed.
Introduction
Recirculating aquaculture systems (RAS) have been rapidly evolving over the last two decades and are envisioned a great potential with regard to a sustainable aquaculture development due to the efficient use of water and space as well as minor environmental impact (Gutierrez-Wing & Malone 2006). However, a major drawback of RAS is the accumulation of waste products such as nitrate after biofiltration. As a consequence of improved recirculation technology and subsequently decreasing water exchange, waste products such as nutrients are accumulating in the process water (Van Rijn 2013). Compared to open aquaculture systems such as ponds, net cages or semi-closed systems where these products are of minor relevance to the cultured species due to high water exchange, concentrations may exceed critical levels impacting welfare as well as performance of the fish. This is particularly relevant for aquaponics, where high nitrate concentrations originating from a RAS-based fish production are desirable to fertilize the plants in the hydroponic unit. Here, nitrate concentrations in the range of 150–230 mg L−1 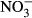 -N are recommended, for example, for the hydroponic production of tomatoes, cucumbers and peppers (Lattauschke 2004).
-N are recommended, for example, for the hydroponic production of tomatoes, cucumbers and peppers (Lattauschke 2004).
Biofiltration in RAS is necessary to convert toxic total ammonia nitrogen (TAN) via nitrite to nitrate (Timmons, Holder & Ebeling 2006). Based on the experience in open systems and the respective concentrations, nitrate has been considered harmless to the fish (Rakocy, Masser & Losordo 2006), and only limited attention was directed to the adverse effects of nitrate in the past. However, in contrast to ponds and other open systems, nitrate can accumulate to concentrations of up to 1000 mg L−1 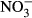 -N in RAS (Van Rijn 2010). Therefore, potential chronic effects on growth and health of fish become more likely. Furthermore, problems interfering with the production efficiency may emerge due to reduced growth performance caused by high nitrate concentrations.
-N in RAS (Van Rijn 2010). Therefore, potential chronic effects on growth and health of fish become more likely. Furthermore, problems interfering with the production efficiency may emerge due to reduced growth performance caused by high nitrate concentrations.
The conversion of haemoglobin to methaemoglobin has been reported as the main mechanism of nitrate toxicity on aquatic animals (Jensen 1996; Scott & Crunkilton 2000; Cheng & Chen 2002), but alternative modes of action (MOA) have been discussed including pathological impairment of the gills, immune suppression and endocrine effects on the thyroid system as well as on androgens and estrogens (Camargo, Alonso & Salamanca 2005; Hamlin, Moore, Edwards, Larkin, Boggs, High, Main & Guillette 2008; Davidson, Good, Welsh & Summerfelt 2014; Freitag, Thayer, Leonetti, Stapleton & Hamlin 2015). In a 30-day trial, nitrate modulated the conversion of steroids at 57 mg L−1 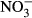 -N, affecting key players – testosterone, 11-ketotestosterone and estradiol – in the endocrine regulation of growth and reproduction (Hamlin et al. 2008) and concentrations as low as 10 mg L−1
-N, affecting key players – testosterone, 11-ketotestosterone and estradiol – in the endocrine regulation of growth and reproduction (Hamlin et al. 2008) and concentrations as low as 10 mg L−1 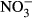 -N raised testosterone in Atlantic salmon (Freitag et al. 2015). In mosquitofish, embryonal dry weight was reduced and reproductive behaviour of mature females was affected at minimal concentrations of 5 mg L−1
-N raised testosterone in Atlantic salmon (Freitag et al. 2015). In mosquitofish, embryonal dry weight was reduced and reproductive behaviour of mature females was affected at minimal concentrations of 5 mg L−1 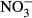 -N (Edwards, Miller & Guillette 2006). Moreover, elevated nitrate concentrations up to 110 mg L−1
-N (Edwards, Miller & Guillette 2006). Moreover, elevated nitrate concentrations up to 110 mg L−1 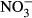 -N lead to a decrease in the thyroid hormones T3 and T4 in rats (Eskiocak, Dundar, Basoglu & Altaner 2005). The impact on swimming performance and survival in juvenile rainbow trout has already been reported at 91 mg L−1
-N lead to a decrease in the thyroid hormones T3 and T4 in rats (Eskiocak, Dundar, Basoglu & Altaner 2005). The impact on swimming performance and survival in juvenile rainbow trout has already been reported at 91 mg L−1 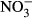 -N (Davidson et al. 2014). Still, substantially reduced growth performance might be the most relevant for the farmer in terms of economic impact. At increasing nitrate concentrations, linear decrease in specific growth rate (SGR) was observed in turbot (Scophthalmus maximus) resulting in a dramatically reduced SGR (30 %) at 500 mg L−1
-N (Davidson et al. 2014). Still, substantially reduced growth performance might be the most relevant for the farmer in terms of economic impact. At increasing nitrate concentrations, linear decrease in specific growth rate (SGR) was observed in turbot (Scophthalmus maximus) resulting in a dramatically reduced SGR (30 %) at 500 mg L−1 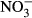 -N (Van Bussel, Schroeder, Wuertz & Schulz 2012). Similarly, Schram, Roques, Abbink, Yokohama, Spanings, De Vries, Bierman, Van De Vis and Flik (2014) observed reduced growth performance in African catfish (Clarias gariepinus) at nitrate concentrations >140 mg L−1
-N (Van Bussel, Schroeder, Wuertz & Schulz 2012). Similarly, Schram, Roques, Abbink, Yokohama, Spanings, De Vries, Bierman, Van De Vis and Flik (2014) observed reduced growth performance in African catfish (Clarias gariepinus) at nitrate concentrations >140 mg L−1 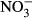 -N. Consequently, adverse effects need to be evaluated for one of the most important species in intensive aquaculture, where concentrations above 100 mg L−1
-N. Consequently, adverse effects need to be evaluated for one of the most important species in intensive aquaculture, where concentrations above 100 mg L−1 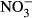 -N are regularly observed and thus may be relevant upon chronic exposure.
-N are regularly observed and thus may be relevant upon chronic exposure.
In contrast, acute toxicity of nitrate in fish is often observed at extreme concentrations, where 96-h LC50 were observed between 1250 mg L−1 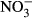 -N and 1,400 mg L−1
-N and 1,400 mg L−1 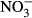 -N, for example, in rainbow trout (Oncorhynchus mykiss), channel catfish (Ictalurus punctatus) and Chinook salmon (Oncorhynchus tshawytscha) in separate studies (Westin 1974; Colt & Tchobanoglous 1976; Tomasso & Carmichael 1986). Despite the importance of tilapia aquaculture globally (FAO 2014), no data on chronic effects of nitrate exposure and safe threshold concentrations have been published so far. In addition, the uptake of nitrate in fish is not yet comprehensively described, but essential to understand nitrate toxicity in fish. Compared to NH3 or
-N, for example, in rainbow trout (Oncorhynchus mykiss), channel catfish (Ictalurus punctatus) and Chinook salmon (Oncorhynchus tshawytscha) in separate studies (Westin 1974; Colt & Tchobanoglous 1976; Tomasso & Carmichael 1986). Despite the importance of tilapia aquaculture globally (FAO 2014), no data on chronic effects of nitrate exposure and safe threshold concentrations have been published so far. In addition, the uptake of nitrate in fish is not yet comprehensively described, but essential to understand nitrate toxicity in fish. Compared to NH3 or 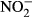 , nitrate uptake is presumably low as a result of low branchial permeability towards nitrate (Stormer, Jensen & Rankin 1996). Still, relatively high plasma concentrations of NOx (sum of
, nitrate uptake is presumably low as a result of low branchial permeability towards nitrate (Stormer, Jensen & Rankin 1996). Still, relatively high plasma concentrations of NOx (sum of 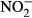 and
and 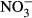 ) have been reported upon nitrate exposure (Stormer et al. 1996; Schram, Roques, Van Kuijk, Abbink, Van De Heul, De Vries, Bierman, Van De Vis & Flik 2014; Schram, Roques, Abbink, et al. 2014). Consequently, alternative uptake routes and sites may be involved.
) have been reported upon nitrate exposure (Stormer et al. 1996; Schram, Roques, Van Kuijk, Abbink, Van De Heul, De Vries, Bierman, Van De Vis & Flik 2014; Schram, Roques, Abbink, et al. 2014). Consequently, alternative uptake routes and sites may be involved.
The objective of this study was to identify potential effects of high nitrate concentrations on growth and health status of juvenile Nile tilapia. Therefore, an exposure experiment was conducted with juvenile Nile tilapia to assess the impact of nitrate in intensive aquaculture. Based on the results, we give a recommendation for safe levels of nitrate in the production of juvenile Nile tilapia. In a second experiment, the reduction of nitrate to nitrite in the stomach juice was studied in vitro over time to clarify whether nitrate conversion and subsequent nitrite uptake is an alternative uptake route to direct uptake of nitrate, considering the plasma concentrations of nitrite and nitrate observed in vivo.
Material and methods
Experimental set-up
We conducted an experimental  exposure of juvenile tilapia (total length 8.8 ± 0.48 cm, wet weight 13.5 ± 2.5 g) at concentrations of 0, 10, 100, 500 and 1000 mg L−1 NO3-N (0, 0.7, 7, 36, 70 mM) over a 30-d period in a continuous flow-through system. Tilapia were individually stocked to forty 9-L glass aquaria (30 × 20 × 14.5 cm) with an overflow providing 7 L of rearing volume (flow rate 50 L per day). All aquaria were placed in a water bath and aerated, assuring a constant temperature of 27.3°± 0.3°C (min 26.0°C, max 28.9°C) and 7.8 ± 0.3 mg/L O2 (100 % O2). Fish were fed a commercial food (Aller Futura Ex; Emsland-Aller Aqua, Germany) at 1.5% of their body weight per day.
exposure of juvenile tilapia (total length 8.8 ± 0.48 cm, wet weight 13.5 ± 2.5 g) at concentrations of 0, 10, 100, 500 and 1000 mg L−1 NO3-N (0, 0.7, 7, 36, 70 mM) over a 30-d period in a continuous flow-through system. Tilapia were individually stocked to forty 9-L glass aquaria (30 × 20 × 14.5 cm) with an overflow providing 7 L of rearing volume (flow rate 50 L per day). All aquaria were placed in a water bath and aerated, assuring a constant temperature of 27.3°± 0.3°C (min 26.0°C, max 28.9°C) and 7.8 ± 0.3 mg/L O2 (100 % O2). Fish were fed a commercial food (Aller Futura Ex; Emsland-Aller Aqua, Germany) at 1.5% of their body weight per day.
After acclimatization for 1 week, respective concentrations were established by flow-controlled assembly consisting of a peristaltic pump, a rotameter flow gauge, a needle valve and a mixing chamber, diluting a 100-fold stock solution with prefiltered, temperature-conditioned tap water (Lutz, Kloas, Springer, Holden, Wolf, Krueger & Hosmer 2008). The stock solution was formulated with NaNO3 and KNO3 at Na+/K+ weight ratio of 6.2 : 1 considering the mean ratio in the Nile (Komy & El-Samahy 1995; Dekov, Komy, Araujo, Van Put & Van Grieken 1997; Zimmermann-Timm 2011) to avoid disturbances in cellular homoeostasis (Van Bussel et al. 2012). NaNO3 and KNO3 were of food quality grade (CHEM-DIS, Eisenberg, Germany). Each mixing chamber supplied four aquaria, referred to as cluster. For each treatment, there were two clusters assessing eight fish in total. Flow rates of nitrate stock solutions were controlled and adjusted twice a day, and flow rates of tap water were controlled on a weekly basis. Temperature, pH and oxygen concentration were determined daily with a portable multimeter (HQ40d multi; Hach Lange GmbH, Berlin, Germany). Salinity was measured three times over the experimental period with a portable meter (WTW LF92; WTW GmbH, Weilheim, Germany). The experiment was conducted in compliance with the local animal welfare committee (LAGESO G0367/12).
Concentrations (mg L−1-N) of TAN, 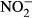 and
and 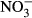 in the water were determined every second day by the cadmium reduction method, the diazotization method and the ammonia salicylate method using a spectrophotometer DR3900 (Hach Lange GmbH).
in the water were determined every second day by the cadmium reduction method, the diazotization method and the ammonia salicylate method using a spectrophotometer DR3900 (Hach Lange GmbH).
Sampling
After 30 days, fish were killed and blood samples were taken from the caudal vein with heparinized syringes. Samples for the determination of haemoglobin were kept on ice and analysed within 3 h. For methaemoglobin, whole-blood samples were shock-frozen and stored at −80°C. Blood plasma was obtained by centrifugation (5000 g, 2 min), shock-frozen and stored at −80°C. Fish were weighed to the nearest 0.1 g, and length was recorded to the nearest of 1 mm, liver to the nearest of 1 mg. The HSI was calculated as HSI = (liver weight/final weight of fish) × 100. For histology, the fourth right gill arch was dissected and fixed in 10% phosphate-buffered formaldehyde solution (Histofix; Carl Roth, Germany).
Plasma concentrations of 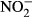 and
and 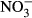
We measured the sum of nitrite and nitrate (NOx) as well as nitrite in the plasma using the nitrate/nitrite colorimetric assay kit (Cayman, USA) according to the user's manual. Briefly, for NOx and 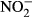 determination, plasma was diluted 1:20 prior to measurement. Absorbtion was determined at 530 nm with an Infinite M200 microplate reader (Tecan Trading AG, Männedorf, Switzerland). All samples were analysed in duplicate. The
determination, plasma was diluted 1:20 prior to measurement. Absorbtion was determined at 530 nm with an Infinite M200 microplate reader (Tecan Trading AG, Männedorf, Switzerland). All samples were analysed in duplicate. The 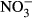 concentration was then calculated as NOx – NO2.
concentration was then calculated as NOx – NO2.
Haemoglobin and methaemoglobin determination
Total haemoglobin was determined within three hours upon sampling with a diagnostic haemoglobin kit (DiaSys Diagnostic Systems, Germany) and calculated from a standard dilution series (12 g/dL haemoglobin standard, HEM QS; Diaglobal, Berlin, Germany) as described in Wuertz, Schulze, Eberhardt, Schulz and Schroeder (2013). For the methaemoglobin concentration, the ratio of Meth-Hb and total-Hb was determined according to Hegesh, Gruener, Cohen, Bochkovsky and Shuval (1970). Briefly, 20 μL blood was incubated (15 min) in 1 mL pure water. After the addition of 600 μL saponin solution (1% saponin, 14 mM Na2HPO4, 42 mM KH2PO4, pH 6.6) and vortexing, cell debris were separated by centrifugation (10 min, 3000 g). Samples were analysed in duplicate, measuring the absorption at 633 nm in (A1) 250 μL supernatant, (A2) after the addition of 5 μL 1% KCN and incubation for 10 min, in (A3) 250 μL supernatant after the addition of 5 μL K4[Fe(CN)6], followed by an addition of 5 μL 1% KCN and incubation for 10 min (A4). Total-Hb:Met-Hb was calculated as (A1–A2)/(A3–A4).
Gill histology
After fixation in phosphate-buffered formalin for approximately 24 h at 4°C, samples were transferred to embedding cassettes and washed three times with 0.1 M phosphate buffer [0.1 M NaH2PO4, 0.1 M Na2HPO4, pH 7.3]. The last washing step was carried out overnight. Samples were dehydrated with successive washes of EtOH (70%, 96%, 100%, 100%) for 1 h each. Preinfiltration was carried out with a 1:1 ethanol Technovit 7100 solution for one hour, followed by infiltration in 100 mL Technovit 7100 with 1 g hardener (dissolved within 5 min) on a shaker overnight (approx. 12 h). Samples were then transferred to Histoform S and orientated, and the polymerization was initiated with 1 mL hardener 2 in 15 mL solution and embedded within five minutes. After the polymerization, blocking of the embedded specimen was carried out with Technovit 3040. Samples were cut into 2-μm slices with a rotary microtome (Jung RM 2065; Leica, Germany), transferred to microscope slides and haematoxylin–eosin (HE)-stained.
Gills were analysed at 400× magnification with the PALM Robo Imaging Software and a Zeiss Axio Observer microscope attached to a CCD camera (Carl Zeiss Microscopy GmbH, Jena, Germany). Within five primary filaments per sample, a total of 100 secondary lamellae were considered for each fish and histopathological changes were recorded. Dorsal and ventral secondary lamellae were considered in same amounts. Histopathological changes of the secondary lamellae and interlamellar spaces of the primary filament in-between were recorded according to Monteiro, Rocha, Fontainhas-Fernandes and Sousa (2008).
Conversion of nitrate in stomach content of tilapia
To examine the potential conversion of nitrate in vitro, the stomach content (1.5 mL per fish) of adult tilapia (550–650 g, n = 20) was collected after killing. After centrifugation (16000 g for 2 min), nitrate stock solution (3.035 g NaNO3 in 10 mL) was added to the supernatant (gastric juice) to reach a target concentration of 1000 mg L−1 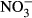 -N. Samples (gastric juice and solids) were mixed gently with the tip of the pipette and incubated at room temperature for 5, 45, 90 and 150 min respectively. After incubation, samples were centrifuged (16 000 g for 5 min) and supernatant was analysed for
-N. Samples (gastric juice and solids) were mixed gently with the tip of the pipette and incubated at room temperature for 5, 45, 90 and 150 min respectively. After incubation, samples were centrifuged (16 000 g for 5 min) and supernatant was analysed for 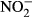 and
and 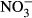 (mg L−1-N) as described earlier.
(mg L−1-N) as described earlier.
Statistical analysis
Data are presented as means ± standard deviation (SD) of n samples. Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). Data were tested for normality (Shapiro–Wilk test) and equal variance (Kruskal–Wallis test). Multiple comparisons were carried out by nonparametric Dunn's test (P < 0.05). Results for gill histology were expressed in per cent and, prior to statistics, transformed with an arcsine-square root transformation.
Results
Survival and growth performance
During the experiment, mortality was only observed in the highest treatment group (1000 mg L−1 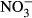 -N), where three fish died. No further analyses were carried out on these fish. There was a general decrease in the specific growth rate (SGR) observed with increasing
-N), where three fish died. No further analyses were carried out on these fish. There was a general decrease in the specific growth rate (SGR) observed with increasing 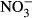 concentration (Fig. 1). Lowest SGR (1.1% per day ±0.1) was recorded at 1000 mg L−1
concentration (Fig. 1). Lowest SGR (1.1% per day ±0.1) was recorded at 1000 mg L−1 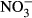 -N, which was significantly lower compared to the control group (P < 0.01, nonparametric Dunn's test). The SGR already decreased at 100 mg L−1
-N, which was significantly lower compared to the control group (P < 0.01, nonparametric Dunn's test). The SGR already decreased at 100 mg L−1 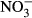 -N group, although not significantly different from the control fish. The feed conversion ratio (FCR) increased with increasing nitrate concentration (Fig. 2). Again, only the FCR at 1000 mg L−1
-N group, although not significantly different from the control fish. The feed conversion ratio (FCR) increased with increasing nitrate concentration (Fig. 2). Again, only the FCR at 1000 mg L−1 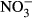 -N was significantly increased at 1.1 g g−1 ± 0.2 compared to the control (P < 0.01, nonparametric Dunn's test).
-N was significantly increased at 1.1 g g−1 ± 0.2 compared to the control (P < 0.01, nonparametric Dunn's test).
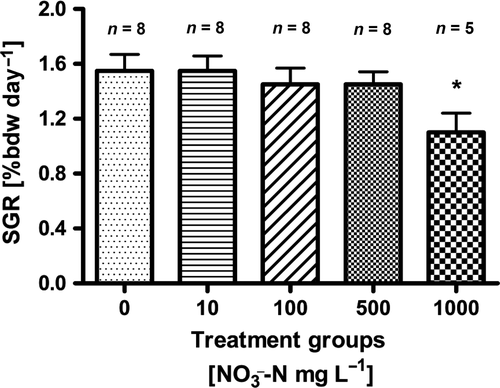
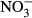 -N. Significant differences to the control are indicated by an asterisk (P < 0.01, nonparametric Dunn's test). The number of samples is indicated on the top of each column. SGR = (ln final weight−ln start weight)/days × 100.
-N. Significant differences to the control are indicated by an asterisk (P < 0.01, nonparametric Dunn's test). The number of samples is indicated on the top of each column. SGR = (ln final weight−ln start weight)/days × 100.
 -N. Significant differences to the control are indicated by an asterisk (P < 0.01, nonparametric Dunn's test). The number of samples is indicated on the top of each column. FCR = dry weight feed/(final wet weight – initial wet weight).
-N. Significant differences to the control are indicated by an asterisk (P < 0.01, nonparametric Dunn's test). The number of samples is indicated on the top of each column. FCR = dry weight feed/(final wet weight – initial wet weight).Blood parameters
There was an increase in the  and
and 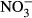 plasma concentrations with increasing nitrate concentration (Fig. 3). The maximum increase in plasma concentration of
plasma concentrations with increasing nitrate concentration (Fig. 3). The maximum increase in plasma concentration of 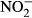 (516 μM
(516 μM 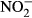 ±284) and
±284) and 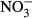 (22 μM ± 2.8) was found at an exposure of 1000 mg L−1
(22 μM ± 2.8) was found at an exposure of 1000 mg L−1 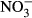 -N (P < 0.01, nonparametric Dunn's test), but no statistical analysis was carried out due to low n in the highest treatment group.
-N (P < 0.01, nonparametric Dunn's test), but no statistical analysis was carried out due to low n in the highest treatment group.
 and
and  (mean ± SD) in juvenile Nile tilapia Oreochromis niloticus after 30 days of exposure to 0, 10, 100, 500 and 1000 mg L−1
(mean ± SD) in juvenile Nile tilapia Oreochromis niloticus after 30 days of exposure to 0, 10, 100, 500 and 1000 mg L−1 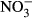 -N. Significant differences to the control are indicated by an asterisk (P < 0.01, nonparametric Dunn's test). The number of samples is indicated on the top of each column. No statistical analysis was conducted in the highest treatment group for plasma
-N. Significant differences to the control are indicated by an asterisk (P < 0.01, nonparametric Dunn's test). The number of samples is indicated on the top of each column. No statistical analysis was conducted in the highest treatment group for plasma 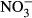 due to a low number of replicates.
due to a low number of replicates.Total haemoglobin concentration decreased with increasing  concentration (Fig. 4), lowest (3.5 g/dL ± 0.8) in the 1000 mg L−1
concentration (Fig. 4), lowest (3.5 g/dL ± 0.8) in the 1000 mg L−1 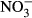 -N group (P < 0.05, nonparametric Dunn's test). Congruently, an increase in methaemoglobin with increasing
-N group (P < 0.05, nonparametric Dunn's test). Congruently, an increase in methaemoglobin with increasing 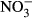 concentration (Fig. 4) was observed. The highest methaemoglobin concentration (44% ± 9) was recorded in the treatment group exposed to 1000 mg L−1
concentration (Fig. 4) was observed. The highest methaemoglobin concentration (44% ± 9) was recorded in the treatment group exposed to 1000 mg L−1 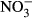 -N (P < 0.05, nonparametric Dunn's test).
-N (P < 0.05, nonparametric Dunn's test).
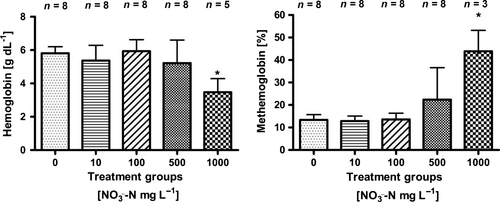
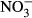 -N. Significant differences to the control are indicated by an asterisk (P < 0.05, nonparametric Dunn's test). The number of samples is indicated on the top of each column.
-N. Significant differences to the control are indicated by an asterisk (P < 0.05, nonparametric Dunn's test). The number of samples is indicated on the top of each column.Hepatosomatic index (HSI)
We observed an increase in HSI with increasing 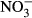 concentrations (Fig. 5). The highest HSI (1.5 ± 0.5) was recorded at 1000 mg L−1
concentrations (Fig. 5). The highest HSI (1.5 ± 0.5) was recorded at 1000 mg L−1 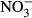 -N, but no significant differences were detected (P < 0.05, nonparametric Dunn's test).
-N, but no significant differences were detected (P < 0.05, nonparametric Dunn's test).
 -N. No significant differences were detected (P < 0.05, nonparametric Dunn's test). The number of samples is indicated on the top of each column. HSI = (liver weight/final weight of fish) × 100.
-N. No significant differences were detected (P < 0.05, nonparametric Dunn's test). The number of samples is indicated on the top of each column. HSI = (liver weight/final weight of fish) × 100.Gill histology
Major abnormalities observed here were hyperplasia of epithelial cells, hyperplasia in cells between the lamellae, hypertrophy of pillar cells, clubbing, hypertrophy of epithelial cells, hypertrophy of mucus cells, fusion of secondary lamella and epithelial lifting (Table 1). No significant differences were analysed between treatments, but, as a trend, most abnormalities increased with increasing  concentrations (Table 1). Congruently, occurrence of undamaged secondary filaments decreased with increasing nitrate concentrations. Above 100 mg L−1
concentrations (Table 1). Congruently, occurrence of undamaged secondary filaments decreased with increasing nitrate concentrations. Above 100 mg L−1 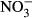 -N, <50% of the lamellae were undamaged compared to 62% in the control. A strong increase of hyperplasia in epithelial cells as well as secondary lamella was recorded, particularly in the treatment group exposed to 1000 mg L−1
-N, <50% of the lamellae were undamaged compared to 62% in the control. A strong increase of hyperplasia in epithelial cells as well as secondary lamella was recorded, particularly in the treatment group exposed to 1000 mg L−1 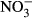 -N. Hypertrophy of pillar cells was frequently observed (between 20% at 1000 mg L−1
-N. Hypertrophy of pillar cells was frequently observed (between 20% at 1000 mg L−1 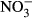 -N and 56% at 500 mg L−1
-N and 56% at 500 mg L−1 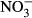 -N), but revealed high individual variability. In contrast, hypertrophy of mucus and epithelial cell was very low (<5 %), again irrespective of treatment. Clubbing was equally low (<10%) irrespective of treatment. Other abnormalities encompassed <5% of the total damages.
-N), but revealed high individual variability. In contrast, hypertrophy of mucus and epithelial cell was very low (<5 %), again irrespective of treatment. Clubbing was equally low (<10%) irrespective of treatment. Other abnormalities encompassed <5% of the total damages.
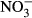 -N
-NTreatment (mgL−1 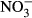 N) N) |
Number of secondary lamella (n) | Undamaged (%) | Hyperplasia of epithelial cells (%) | Hyperplasia between secondary lamella (%) | Hypertrophy of pillar cells (%) | Hypertrophy with clubbing (%) | Hypertrophy of epithelial cells (%) | Hypertrophy of mucus cells (%) | Fusion of lamella (%) | Epithelial lifting (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 700 | 62 ± 17 | 5 ± 6 | 4 ± 5 | 29 ± 16 | 4 ± 5 | 1 ± 3 | 0 | 0 | 1 ± 1 |
| 10 | 700 | 53 ± 12 | 3 ± 5 | 4 ± 4 | 37 ± 8 | 10 ± 6 | 1 ± 3 | 0 | 0 | 2 ± 3 |
| 100 | 600 | 44 ± 18 | 3 ± 3 | 5 ± 5 | 48 ± 16 | 9 ± 10 | 0 | 0 | 1 ± 1 | 2 ± 1 |
| 500 | 600 | 38 ± 13 | 3 ± 4 | 4 ± 3 | 56 ± 13 | 6 ± 5 | 0 | 0 | 0 | 0 |
| 1000 | 400 | 42 ± 11 | 23 ± 17 | 19 ± 12 | 20 ± 20 | 1 ± 1 | 0 | 2 ± 2 | 0 | 2 ± 1 |
- The major histopathological categories analysed were as follows: (a) undamaged, (b) hyperplasia of epithelial cells, (c) hyperplasia in cells between the lamellae, (d) hypertrophy of pillar cells, (e) clubbing, (f) hypertrophy of epithelial cells, (g) hypertrophy of mucus cells, (h) fusion of secondary lamella and (i) epithelial lifting. No significant differences were detected between treatment groups (P < 0.05, nonparametric Dunn's test).
Conversion of nitrate in the stomach of tilapia
We observed a significant conversion of nitrate in the stomach content of Nile tilapia (P < 0.01, nonparametric Dunn's test, n = 5). Nitrite already increased after 45 min, but not significantly different compared to 14 μM 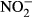 (± 2) after 5 min. After 90 min, a significant increase up to 74 μM
(± 2) after 5 min. After 90 min, a significant increase up to 74 μM 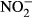 (±14) was observed (P < 0.01, nonparametric Dunn's test, n = 5). No further increase in nitrite was observed after 150 min (Fig. 6).
(±14) was observed (P < 0.01, nonparametric Dunn's test, n = 5). No further increase in nitrite was observed after 150 min (Fig. 6).
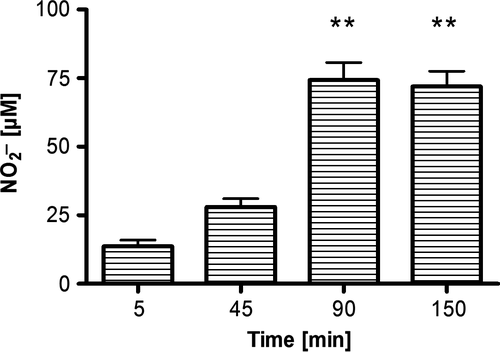
 -N) to nitrite in the gastric juice of Nile tilapia after incubation at room temperature. Presented are the means (±SD, n = 5). Significant differences to the start of the incubation (after 5 min) are indicated by asterisks (P < 0.01, nonparametric Dunn's test).
-N) to nitrite in the gastric juice of Nile tilapia after incubation at room temperature. Presented are the means (±SD, n = 5). Significant differences to the start of the incubation (after 5 min) are indicated by asterisks (P < 0.01, nonparametric Dunn's test).Discussion
The aim of this study was to investigate whether chronic exposure to realistic nitrate concentrations observed in RAS (10–1000 mg L−1  -N) induces adverse effects on growth performance, feed conversion or health status in juvenile Nile tilapia and to provide data on safe nitrate concentrations in intensive RAS-based tilapia culture. Mortalities only occurred in the highest treatment group, confirming that the range of concentrations chosen was adequate. Due to coagulation, we did not consider these fish for blood analysis. Directly after sampling, brown-coloured blood was recorded in fish of the highest treatment group confirming methaemoglobinaemia in these fish.
-N) induces adverse effects on growth performance, feed conversion or health status in juvenile Nile tilapia and to provide data on safe nitrate concentrations in intensive RAS-based tilapia culture. Mortalities only occurred in the highest treatment group, confirming that the range of concentrations chosen was adequate. Due to coagulation, we did not consider these fish for blood analysis. Directly after sampling, brown-coloured blood was recorded in fish of the highest treatment group confirming methaemoglobinaemia in these fish.
Both decreasing SGR and increasing FCR were observed with increasing ambient nitrate concentrations. Still, significant differences to the control were only observed at 1000 mg L−1  -N. In several studies, reduced growth performance was indicative of inadequate water quality in tilapia. For example, Shaw and Handy (2006) evaluated chronic copper toxicity in Nile tilapia, reporting depression of SGR from 1.58 (control) to 1.2. More pronounced, El-Sherif and El-Feky (2009) observed a drastic decrease in SGR from 1.16 (control) to 0.53 in tilapia fingerlings during an experiment at pH 6. Although there are no data on chronic nitrate toxicity in tilapia, reduced growth as well as increased feed conversion has been observed in other species. For example, Van Bussel et al. (2012) reported a significant decrease in SGR from 1.6 to 0.45 with increasing nitrate concentration, as well as a significant increase in FCR from 1.07 to 3.80 in juvenile turbot (Scophthalmus maximus). In comparison with turbot (Van Bussel et al. 2012), pikeperch (Schram, Roques, Van Kuijk, et al. (2014) and catfish (Schram, Roques, Abbink, et al. 2014), results of our study suggest that tilapia is less sensitive, not surprisingly with regard to the habitat of the respective species. Here, a low feeding rate was chosen to assure an optimal water quality. Still, the decrease in SGR observed here is moderate and thus unexpectedly good with regard to the control. Congruently, feed conversion was significantly reduced at 1000 mg L−1
-N. In several studies, reduced growth performance was indicative of inadequate water quality in tilapia. For example, Shaw and Handy (2006) evaluated chronic copper toxicity in Nile tilapia, reporting depression of SGR from 1.58 (control) to 1.2. More pronounced, El-Sherif and El-Feky (2009) observed a drastic decrease in SGR from 1.16 (control) to 0.53 in tilapia fingerlings during an experiment at pH 6. Although there are no data on chronic nitrate toxicity in tilapia, reduced growth as well as increased feed conversion has been observed in other species. For example, Van Bussel et al. (2012) reported a significant decrease in SGR from 1.6 to 0.45 with increasing nitrate concentration, as well as a significant increase in FCR from 1.07 to 3.80 in juvenile turbot (Scophthalmus maximus). In comparison with turbot (Van Bussel et al. 2012), pikeperch (Schram, Roques, Van Kuijk, et al. (2014) and catfish (Schram, Roques, Abbink, et al. 2014), results of our study suggest that tilapia is less sensitive, not surprisingly with regard to the habitat of the respective species. Here, a low feeding rate was chosen to assure an optimal water quality. Still, the decrease in SGR observed here is moderate and thus unexpectedly good with regard to the control. Congruently, feed conversion was significantly reduced at 1000 mg L−1 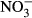 -N with an FCR of 1.13 compared to 0.72 in the control. In a study on deleterious sublethal ammonia exposure (0.4 mg L−1 NH3-N) to juvenile Nile tilapia, FCR increased from 1.5 (control) to 8 (El-Shafai, El-Gohary, Nasr, Van Der Steen & Gijzen 2004). Here, at an exposure of up to 500 mg L−1
-N with an FCR of 1.13 compared to 0.72 in the control. In a study on deleterious sublethal ammonia exposure (0.4 mg L−1 NH3-N) to juvenile Nile tilapia, FCR increased from 1.5 (control) to 8 (El-Shafai, El-Gohary, Nasr, Van Der Steen & Gijzen 2004). Here, at an exposure of up to 500 mg L−1 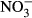 -N, neither SGR nor FCR was affected. Congruently, no effects on FCR and SGR were reported in pikeperch (Sander lucioperca) at nitrate concentrations up to 358 mg L−1
-N, neither SGR nor FCR was affected. Congruently, no effects on FCR and SGR were reported in pikeperch (Sander lucioperca) at nitrate concentrations up to 358 mg L−1 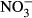 -N (Schram, Roques, Van Kuijk, et al. 2014).
-N (Schram, Roques, Van Kuijk, et al. 2014).
As a conclusion, reduced growth performance and feed conversion could be a consequence of increased energy expenditure required to counteract adverse effects, for example conversion of methaemoglobin as discussed later on. Alternatively, growth depression could also arise from nitrate-mediated modulation of the thyroid axis, as nitrate competes with the uptake of iodide in the thyroid (Ward, Kilfoy, Weyer, Anderson, Folsom & Cerhan 2010). Thereby, formation of thyroid hormones T3 and T4 would be reduced, which in turn leads to reduced growth. Still, plasma nitrate observed was low and nitrite much higher, supporting the conclusion that the formation of Met-Hb and the subsequent energy expenditure is the primary cause of reduced growth and feed conversion observed here.
The concentration of nitrate in the plasma samples was well below the concentrations in ambient water. Nitrite and nitrate concentrations increased with ambient nitrate concentrations of the rearing water, but, in contrast to Schram, Roques, Abbink, et al. (2014), Schram, Roques, Van Kuijk, et al. (2014), nitrite exceeded the nitrate concentrations in the plasma about 27-fold. Therefore, it seems that there was an uptake of nitrate, whether active or passive, followed by a reduction of nitrate to nitrite within the body of tilapia.
Until today, the uptake of nitrate is still poorly understood, mainly due to the fact that most tissues represent a barrier preventing the passage of the large hydrated nitrate ion. In their study on nitrate toxicity to African catfish (Clarias gariepinus), Schram, Roques, Abbink, et al. (2014) concluded that the integument of the fish forms a significant barrier to waterborne nitrate. As a consequence, alternative routes for nitrate uptake are limited and uptake via the gills seems most plausible with regard to the direct contact with the ambient water as well as the importance in osmoregulation and ion uptake (Hwang 2009). However, a low permeability for nitrate through the gills was discussed in trout (Stormer et al. 1996) and has been reported in freshwater crayfish (Jensen 1996). In contrast, nitrite uptake has been described for the gills as well as the intestinal wall. For example, Grosell and Jensen (2000) documented nitrite passage over the intestinal/stomach wall of the European flounder and nitrite uptake in the stomach is very fast in rats (Bryan, Fernandez, Bauer, Garcia-Saura, Milsom, Rassaf, Maloney, Bharti, Rodriguez & Feelisch 2005). Additionally, nitrite and chloride compete for the active branchial chloride uptake mechanism in freshwater fish (Williams & Eddy 1986), and as the chloride concentration in freshwater is low, the presence of nitrite can lead to massive nitrite accumulation in the plasma (Grosell & Jensen 2000). Furthermore, low stability of nitrite suggests rather acetic conditions to prevent fast oxidation.
Consequently, we hypothesized that uptake involves a reduction of nitrate to nitrite in the stomach, prior to the actual passage of the intestinal wall. Such route would result in high plasma nitrite, similar to those observed here. Therefore, we assessed the reduction of nitrate to nitrite in stomach juice in an in vitro experiment. We demonstrate that nitrate is rapidly converted into nitrite reaching a maximum of 74 μM 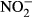 after 90 min. Our findings strongly indicate that conversion of nitrate to nitrite in the gastrointestinal system of tilapia represents the most probable uptake route. As a consequence, nitrate toxicity in tilapia is mainly a result of nitrate reduction to nitrite and irreversible oxidation of haemoglobin to methaemoglobin. Nevertheless, nitrate is quite stable (~8 h; Webb, Patel, Loukogeorgakis, Okorie, Aboud, Misra, Rashid, Miall, Deanfield, Benjamin, Macallister, Hobbs & Ahluwalia 2008), and anaerobic conversion of nitrate to nitrite in the gut needs to be considered (Fanning 2000; Speijers & Van Den Brandt 2003; Webb et al. 2008).
after 90 min. Our findings strongly indicate that conversion of nitrate to nitrite in the gastrointestinal system of tilapia represents the most probable uptake route. As a consequence, nitrate toxicity in tilapia is mainly a result of nitrate reduction to nitrite and irreversible oxidation of haemoglobin to methaemoglobin. Nevertheless, nitrate is quite stable (~8 h; Webb, Patel, Loukogeorgakis, Okorie, Aboud, Misra, Rashid, Miall, Deanfield, Benjamin, Macallister, Hobbs & Ahluwalia 2008), and anaerobic conversion of nitrate to nitrite in the gut needs to be considered (Fanning 2000; Speijers & Van Den Brandt 2003; Webb et al. 2008).
In this experiment, observations, which are typically attributed to nitrite toxicity, furthermore confirm nitrite-mediated intoxication. At 500 and 1000 mg L−1 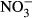 -N, formation of methaemoglobin was 22.5 % (±14.1) and 43.9 % (±9.3) respectively. At lower concentrations, methaemoglobin was low, ranging between 8.9 % and 16.5 %. Considering the actual nitrite concentrations from 23.9 μM (0 mg L−1
-N, formation of methaemoglobin was 22.5 % (±14.1) and 43.9 % (±9.3) respectively. At lower concentrations, methaemoglobin was low, ranging between 8.9 % and 16.5 %. Considering the actual nitrite concentrations from 23.9 μM (0 mg L−1 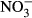 -N) to 65.3 μM (100 mg L−1
-N) to 65.3 μM (100 mg L−1 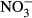 -N) in the plasma, counteracting mechanisms seem to restore homoeostasis until an ambient concentration of at least 100 mg L−1
-N) in the plasma, counteracting mechanisms seem to restore homoeostasis until an ambient concentration of at least 100 mg L−1 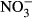 -N. Here, methaemoglobin reductase converts methaemoglobin to haemoglobin and restores functionality of red blood cells, but also represents a substantial energy expenditure (Choury, Leroux & Kaplan 1981). Therefore, a decrease in SGR is most likely a result of increasing methaemoglobin formation and its energy-demanding recycling. The presence of around 10% methaemoglobin in the blood as observed between 0 mg L−1
-N. Here, methaemoglobin reductase converts methaemoglobin to haemoglobin and restores functionality of red blood cells, but also represents a substantial energy expenditure (Choury, Leroux & Kaplan 1981). Therefore, a decrease in SGR is most likely a result of increasing methaemoglobin formation and its energy-demanding recycling. The presence of around 10% methaemoglobin in the blood as observed between 0 mg L−1 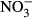 -N and 100 mg L−1
-N and 100 mg L−1 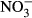 -N is within the range reported as basic level in other species (Kroupova, Machova & Svobodova 2005; Wuertz et al. 2013). A visible indicator for severe methaemoglobinaemia is the formation of brown-coloured blood, which in Nile tilapia is first observed at approximately 20% of methaemoglobin with no other symptoms of toxicity (Svobodova, Machova, Poleszczuk, Huda, Hamackova & Kroupova 2005). Here, brown colour was observed during sampling of the highest treatment group at 33.9–52.2 % methaemoglobin. Levels above 50% methaemoglobin are considered threatening to fish (Bowser, Falls, Vanzandt, Collier & Phillips 1983), which clearly identifies
-N is within the range reported as basic level in other species (Kroupova, Machova & Svobodova 2005; Wuertz et al. 2013). A visible indicator for severe methaemoglobinaemia is the formation of brown-coloured blood, which in Nile tilapia is first observed at approximately 20% of methaemoglobin with no other symptoms of toxicity (Svobodova, Machova, Poleszczuk, Huda, Hamackova & Kroupova 2005). Here, brown colour was observed during sampling of the highest treatment group at 33.9–52.2 % methaemoglobin. Levels above 50% methaemoglobin are considered threatening to fish (Bowser, Falls, Vanzandt, Collier & Phillips 1983), which clearly identifies 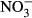 -N ≥ 1000 mg L−1 as intolerable for the rearing of juvenile Nile tilapia. We further recorded a significantly elevated HSI (Fig. 5) at 1000 mg L−1
-N ≥ 1000 mg L−1 as intolerable for the rearing of juvenile Nile tilapia. We further recorded a significantly elevated HSI (Fig. 5) at 1000 mg L−1 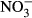 -N, which indicates other adverse effects on the liver. As nitrite is an oxidizing agent, this finding may indicate increased oxidative stress, but further studies are needed. Still, detoxification mechanisms to cope with oxidative stress as well as elevated nitrite include enhanced turnover by catalase and cytochrome c oxidase (summarized by Kroupova et al. 2005), which often lead to increased liver metabolism and, subsequently, liver size. These processes are energy-demanding and will hence further reduce growth performance and increase FCR.
-N, which indicates other adverse effects on the liver. As nitrite is an oxidizing agent, this finding may indicate increased oxidative stress, but further studies are needed. Still, detoxification mechanisms to cope with oxidative stress as well as elevated nitrite include enhanced turnover by catalase and cytochrome c oxidase (summarized by Kroupova et al. 2005), which often lead to increased liver metabolism and, subsequently, liver size. These processes are energy-demanding and will hence further reduce growth performance and increase FCR.
As gills comprise the largest surface in direct contact with the surrounding water (Evans, Piermarini & Choe 2005) and subsequently represent the organ most heavily exposed, abnormalities such as fusion of the secondary lamellae have been regarded as defence mechanism limiting the uptake of toxins (Reiser, Schroeder, Wuertz, Kloas & Hanel 2010). Although some histopathological changes have been recorded in the gills, high individual variation was observed here. With regard to the low brachial permeability of nitrate, such lower incidence of gill abnormalities seems plausible. Nevertheless, a decreasing trend of undamaged secondary filaments from the control group to the highest treatment group was recorded (Table 1). We also observed increased hyperplasia of the epithelial cells as well as cells of the secondary lamella in the highest treatment group, which are typically regarded as mild responses to increase the diffusion barrier towards toxins in the water, compared to strong ones such as fusion of the lamella.
To our knowledge, this investigation is the first one demonstrating that high nitrate concentrations, realistic for commercial RAS, impact juvenile tilapia at high concentrations of 500 mg L−1 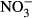 -N and 1000 mg L−1
-N and 1000 mg L−1 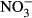 -N. Thus, tilapia is relatively robust towards nitrate and subsequent nitrite toxification. Here, no significant impacts on growth performance, feed conversion and health status were observed between 10 mg L−1
-N. Thus, tilapia is relatively robust towards nitrate and subsequent nitrite toxification. Here, no significant impacts on growth performance, feed conversion and health status were observed between 10 mg L−1 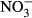 -N and 500 mg L−1
-N and 500 mg L−1 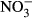 -N. Once more, it has been shown that tilapia is well suited for intensive RAS-based aquaculture, but nutrient management such as decoupled aquaponics can improve animal health and welfare and production effectiveness.
-N. Once more, it has been shown that tilapia is well suited for intensive RAS-based aquaculture, but nutrient management such as decoupled aquaponics can improve animal health and welfare and production effectiveness.
Acknowledgments
We would like to thank Susanne and Petra for their help during the study. Thomas Mehner provided helpful advice on the first draft of the manuscript. Hendrik Monsees was funded with a scholarship of the Elsa-Neumann-Stiftung (Berlin, Germany).




