Effect of dietary supplementation of periphyton on growth, immune response and metabolic enzyme activities in Penaeus monodon
Abstract
A 60-day indoor trial was conducted to study the effect of periphyton supplementation on metabolic and immune responses in tiger shrimp, Penaeus monodon. Periphyton developed over bamboo substrate in outdoor tanks (15 m2) was used as dietary supplement for P. monodon (2.02 ± 0.04 g) reared in 1000 L FRP tanks. Graded levels of periphyton were included in shrimp basal diets: 0% (P0), 3% (P3), 6% (P6), 9% (P9) and P0 diet with natural periphyton (NP) over bamboo substrate. At the end of the trial, P6 and NP showed significantly higher (P < 0.01) growth rate, 23.9% and 20%, respectively, compared with control, P0. Comparatively, lower level of metabolic enzymes, such as lactate dehydrogenase, malate dehydrogenase, aspartate aminotransferase and alanine aminotransferase, was recorded in treatments P3, P6 and NP compared with control, P0. The periphyton-supplemented group, P3 had significantly higher (P < 0.05) superoxide dismutase (15.83 ± 0.96) and catalase activity (15.73 ± 0.69) compared to 6.88 ± 2.84 and 9.15 ± 0.67 unit mg−1 protein min−1, respectively, in P0. Similarly, higher total haemocyte counts, 32.58 ± 1.30, 28.51 ± 3.12 and 27.26 ± 4.43 × 106 cells mL−1, were recorded in P6, NP and P3, respectively, compared to P0, 23.57 ± 1.80 × 106 cells mL−1. After challenge with Vibrio harveyi, P3 recorded the highest relative percentage survival 67% followed by NP (58%) and P6 (42%) compared with control. However, treatment with highest periphyton inclusion (P9) did not differ significantly with P0 on growth and immunological parameters. This study indicates that periphyton supplementation at 3–6% level improves growth, immune response and metabolic activities in P. monodon.
Introduction
In the light of disease outbreaks, increasing price of quality feed ingredients and environmental degradation in shrimp farming, there is an urgent need to explore sustainable farming methods in shrimp culture. Provision of immobile substrates for development of periphyton is an eco-friendly approach in aquaculture. Periphyton refers to the entire complex of microalgae, heterotrophic bacteria, benthic organisms and detritus developed over submerged substrate in aquatic systems (Azim, Asaeda, Verdegem, van Dam & Beveridge 2005). Periphyton-based aquaculture is widely practised in fin fish like major carps (Keshavanath, Gangadhar, Ramesh, Van Rooij, Beveridge, Baird, Verdegem & Van Dam 2001), tilapia (Azim et al. 2005) and giant fresh water prawn (Asaduzzaman, Rahman, Azim, Islam, Wahab, Verdegem & Verreth 2010) to augment aquaculture production and efficient nutrient utilization. Similarly, better growth performance and improved feed conversion ratio (FCR) were observed in substrate-based penaeid shrimp culture (Audelo-Naranjo, Martinez-Cordova, Voltolina & Gomez-Jimenez 2011; Anand, Kumar, Panigrahi, Ghoshal, Dayal, Biswas, Sundaray, De, Raja, Deo, Pillai & Ravichandran 2013a). Provision of substrate improves water quality parameters and mitigates negative effects of overcrowding (Azim et al. 2005; Arnold, Coman, Jackson & Groves 2009).
Immunostimulants are used in shrimp culture to improve the shrimp health through the induction of non-specific immune system (Sakai 1999). Widely used dietary immunostimulants are β-glucan and yeast (Pacheco, Ascencio, Zarain, Gomez & Campa 2011), microalgae and carotenoids (Supamattaya, Kiriratnikom, Boonyaratpalin & Borowitzka 2005) and probiotics (Li, Tan & Mai 2009). Dietary immunostimulants affect the physiological status of aquatic organisms (Akhtar, Pal, Sahu, Alexander, Gupta, Choudhary, Jha & Rajan 2010). The metabolic enzymes like aspartate aminotransferase (AST), alanine aminotransferase (ALT), malate dehydrogenase (MDH) and lactate dehydrogenase (LDH) are valuable indicators of physiological and health status of aquatic organisms (Pan, Chien & Hunter 2003). The enzymes like superoxide dismutase (SOD) and catalase help to control the reactive oxygen radicals (Campa-Córdova, Hernández-Saavedra & Ascencio 2002). It has been reported that periphyton developed over the submerged substrate improves non-specific immune response in Litopenaeus vannamei (Zhang, Lin, Wang & Xu 2010). The biofilm developed over substrate are capable to elicit immune response in fish and shrimps (Verdegem, Van Dam, Azim & Beveridge 2005). However, there is a dearth of information about the role of periphyton as a dietary immunostimulants and its role on physiological status of shrimp. Recently, we have reported that periphyton as a dietary ingredient enhances growth performance and digestive enzyme activities in shrimp (Anand, Kohli, Dam Roy, Sundaray, Kumar, Sinha, Pailan & Sukham 2013b). In this study, we aim to evaluate the effect of dietary supplementation of periphyton on immune response and metabolic enzyme activities of P. monodon juveniles.
Materials and methods
Experimental design
Periphyton produced in outdoor tanks was used as dietary supplement for shrimp feed over a 60-day indoor growth trial. A control diet without periphyton was compared against three experimental diets with graded levels of periphyton inclusion. Treatments were also compared with a positive control, provided with bamboo substrates for natural growth of periphyton. The experiment was conducted at Kakdwip Research Centre, Central Institute of Brackishwater Aquaculture, Kakdwip (21° 51′ N and 88°11′ E), West Bengal, India.
Production of periphyton
Periphyton production trial was carried out in four cemented cistern outdoor tanks (5 × 3 × 1.7 m; bottom area 15 m2) for 45 days during March to May, 2011. Tanks were filled with water from the nearby brackishwater source. Fine meshed filter bag (<60 μm) was used to prevent entry of unwanted materials into the tanks and kept for 2 days to settle the suspended particles. Split bamboos (150 cm × 2.5 × 1 cm) were suspended vertically in the tanks at the rate of 100 numbers per tank for development of periphytic algae on the submerged substrate. This generated a bamboo surface area of 7.5 sq. m per tank for growth of periphyton. Agricultural lime, CaCO3 (200 Kg ha−1) was applied to all the tanks followed by vermicompost (3000 Kg ha−1). Inorganic fertilizers like urea (150 Kg ha−1) and single super phosphate (150 Kg ha−1) were applied for the development of periphytic algae on submerged substrate. Developed periphyton was manually collected at fortnight interval by scrapping out periphyton from each bamboo poles using blunt end of a scalpel. Due care was taken to remove the periphytic algae and to minimize the chance of scraping out bamboo surface. Afterwards, the poles were reinstalled in their position. Inorganic fertilizers were applied at fortnight interval, and round the clock aeration was provided to meet the oxygen demand. Collected periphyton samples were dried under shade followed by overnight drying in hot air oven at 45°C. The dried samples were ground into fine powder (<200 μm) and stored in airtight containers in refrigerator until further use.
Experimental diets
Four isonitrogenous and isoenergetic experimental diets were formulated and their composition is presented in Table 1. A control diet, without periphyton supplementation (P0) was compared against three test diets formulated with 3% (P3), 6% (P6) and 9% (P9) graded levels of periphyton. Ingredients like wheat flour, fish meal, soyabean meal, shrimp meal, guar gum and lecithin were mixed with water to make dough. The dough was steam cooked for 20 min in a pressure cooker at 15 psi. After cooling, periphyton powder (only in test diets) and additives (cholesterol, butylated hydroxytoluene, oil and vitamin-mineral mixture) were mixed with the dough. The dough was pressed through a pelletizer with 2 mm die and then dried overnight at 60°C. The experimental feeds were stored at 4°C until further use.
| Ingredients | Experimental diets | |||
|---|---|---|---|---|
| P3 | P6 | P9 | P0 | |
| Fish meal | 380 | 380 | 380 | 380 |
| Shrimp meal | 150 | 150 | 150 | 150 |
| Soyabean meal | 195.3 | 183 | 170.7 | 207.6 |
| Wheat flour | 155.2 | 137.5 | 119.8 | 172.9 |
| Dried periphyton powder | 30 | 60 | 90 | 0 |
| Soya oil | 15 | 15 | 15 | 15 |
| Cod liver oil | 20 | 20 | 20 | 20 |
| Lecithin | 10 | 10 | 10 | 10 |
| Cholesterol | 1 | 1 | 1 | 1 |
| Vitamin and mineral mixa | 23 | 23 | 23 | 23 |
| Butylated hydroxytoluene | 0.5 | 0.5 | 0.5 | 0.5 |
| Guar gum | 20 | 20 | 20 | 20 |
| Total | 1000 | 1000 | 1000 | 1000 |
- a Composition of vitamin-mineral mix (Supplevite-M; quantity kg−1): Vitamin A, 20 00 000 IU; Vitamin D3, 400 000 IU; Vitamin B2, 800 mg; Vitamin E, 300 unit; Vitamin K, 400 mg; Vitamin B6, 400 mg; Vitamin B12, 2.4 mcg; Calcium Pantothenate, 1000 mg; Nicotinamide, 4 g; Choline Chloride, 60 g; Mn, 10 800 mg; Iodine, 400 mg; Fe, 3000 mg; Zn, 6 g; Cu, 800 mg; Co, 180 g; Vitamin C, 1000 mg.
- Periphyton in shrimp basal diets at 0% (P0), 3% (P3), 6% (P6) and 9% (P9).
Experimental system and feeding
Healthy juvenile shrimps, P. monodon, tested negative for white spot syndrome virus by polymerase chain reaction were obtained from a scientific shrimp farm (South 24 Parganas, West Bengal, India). Shrimps acclimatized for 14 days and fed control diet (40% crude protein) three times daily. Split feeding was done to reduce the wastage of feed. The experiment was conducted in triplicate in 15 circular 1000 L fibreglass reinforced tanks (bottom area 2 m2) filled with chlorine free brackishwater. Three hundred and sixty P. monodon juveniles (2.2 ± 0.04 g) distributed in the five experimental groups at 12 nos m−2 in each FRP tank following a completely randomized design. In fifth treatment group (natural periphyton, NP), split bamboos (5 × 2 ×1 cm) were suspended in the water column at 27 nos per tank for periphyton growth, and arranged in three horizontal rows and three vertical columns at 10 cm apart.
The daily feeding was done at 7% of body weight at the start of the experiment, and declined gradually to 4.5% of body weight at the end of the experiment. Daily feed ration was divided into two parts, 40% of the total feed quantity was given in the morning (10.00 h) and 60% in the evening (18.00 h). Leftover feed and faecal matters were removed daily and 20% water was exchanged every third day.
Proximate composition of experimental diets
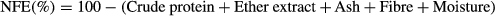
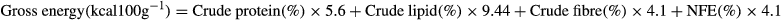
Sample preparation for enzyme assay
After completion of the feeding experiment, 18 inter-moult shrimps from each treatment group (six shrimps from each replicate) were anaesthetized before collecting haemolymph and tissue with 50 μL of clove oil (HiMedia, Mumbai, India) added to 1 L of brackishwater (Bressler & Ron 2004). The moult stage was determined by the setal development of the uropod using stereomicroscope (Dall, Hill, Rothlisberg & Sharples 1990). The muscle and hepatopancreas of shrimps were dissected out, weighed, and separately homogenized with 0.25 M chilled sucrose prepared in distilled water (pH 7, 1:10 w/v) in a hand-held glass homogenizer in ice-cold condition. The homogenate was centrifuged at 6000 rpm (2400 g) for 20 min at 4°C (Centrifuge 5417R; Eppendorf, Hamburg, Germany). After centrifugation, the floating top lipid layer was removed and supernatant was kept as aliquot in centrifuge tubes. The samples were stored at −40°C until further analysis.
For analysis of immunological parameters, haemolymph was withdrawn by 26 Gauze 1-mL tuberculin syringe from the ventral sinus of each shrimp. A 100 μL haemolymph was mixed with 900 μL cooled anticoagulant (30 mM Tri-sodium citrate, 388 mM sodium chloride, 0.12 M glucose, 10 mM EDTA, 780 mOsm kg−1 osmolality and pH 7.55). To collect serum, haemolymph without anticoagulant was allowed to clot overnight in refrigerator, and centrifuged at 600 g for 5 min at 4°C (Centrifuge 5417R; Eppendorf).
Metabolic enzymes assay
Lactate dehydrogenase activity (E.C.1.1.1.27) was assayed in 100 mM phosphate buffer (pH 7.5) using 0.1 mM NADH. The reaction was initiated by adding substrate, 0.02 M sodium pyruvate and OD was recorded at 340 nm at 30 s interval for 3 min. The enzyme activity was expressed as micromoles of NAD released mg−1 protein min−1 (Wroblewski & Ladue 1955). Malate dehydrogenase (E.C. 1.1.1.37) activity was estimated following Ochoa (1955). The reaction mixture comprised of 0.1 M phosphate buffer (pH 7.5), 0.1 mM NADH solution, tissue homogenate and 0.02 M oxaloacetate. The OD was recorded at 340 nm at 30 s interval for 3 min. Aspartate aminotransferase (E.C. 2.6.1.1.) was assayed in 0.05 M phosphate buffer (pH 7.4) using 0.2 M D, L-aspartic acid and 2 mM α-ketoglutarate as substrate. Enzyme source (homogenate) and substrate were incubated for 1 h at 37°C, and the reaction was stopped by the addition of 2, 4-dinitrophenyl hydrazine. After 20 min, 0.4 N NaOH was added and OD was recorded at 540 nm. A control and a standard (sodium pyruvate) were run along with the samples (Wootton 1964). The enzyme activity was expressed as nanomoles oxaloacetate formed min−1 mg−1 protein at 37°C. Alanine aminotransferase (E.C. 2.6.1.2.) activity was assayed by same procedure as for AST except the substrate L-alanine instead of aspartic acid. Total protein content was analysed from the supernatant (Lowry, Rosebrough, Farr & Randall 1951) for calculating enzyme activities. All the colorimetric assays were carried out using UV–VIS spectrophotometer (model UV2310; Techcomp, China).
Antioxidant enzymes and serum parameters
Superoxide dismutase activity was assayed by the method of Misra and Fridovich (1972). The reaction mixture consisted of 0.5 mL sample, 0.5 mL EDTA (0.6 mM) solution in 1 mL carbonate bicarbonate buffer (0.1 M; pH 10.2). The reaction was initiated by the addition of 0.5 mL substrate (1.8 mM Epinephrine). The increase in OD was recorded at 480 nm at every 30 s for 3 min. The values were expressed as 50% inhibition of epinephrine auto oxidation min−1 mg−1 protein.
Catalase assay was carried out following the method of Takahara, Hamilton, Neel, Kobara, Ogura and Nishimura (1960). A 0.05 mL sample was added to 1.2 mL of phosphate buffer. Catalase reaction was initiated by addition of 1 mL H2O2 substrate (0.03 M in phosphate buffer). The decrease in OD was recorded at 240 nm at every 30 s for 3 min and expressed as μmoles of H2O2 decomposed min−1 mg−1 protein. Serum and muscle protein was estimated by Lowry's method (Lowry et al. 1951) using bovine serum albumin as standard. Serum albumin was estimated using bromocresol green binding method (Doumas, Ard Watson & Biggs 1971).
Haemocyte count
A 50 μL of haemolymph–anticoagulant solution (1:10) was mixed with 50 μL of Rose Bengal solution (1.2% Rose Bengal in 50% ethanol) immediately after haemolymph collection. The stained haemolymph was counted in improved Neubauer bright-line chamber under 40× objective (CarlZeiss, Jena, Germany). The cells were differentiated into granulocyte and agranulocyte based upon the granular content (Le Moullac 2000) and expressed as total haemocyte count mL−1 (THC mL−1), total granulocyte count mL−1 (TGC mL−1) and total agranulocyte count mL−1 (TAC mL−1).
Challenge
After 60 days of the feeding experiment, 15 shrimps from each treatment group were challenged with virulent Vibrio harveyi collected from vibriosis infected shrimp. A 20 μL V. harveyi suspension (107 cfu mL−1) was injected intra-muscularly by 1 mL tuberculin syringe into five shrimps per replicate. Due care was taken to avoid stress and injury during injection. All the challenged shrimps were released into their respective tank and observed for mortality for 10 days. During challenge test, no water was exchanged and the shrimp survival was counted every day. The relative percentage survival (RPS) in different treatment groups was calculated by the following formula (Burrells, Williams & Forno 2001).

Statistical analysis
The data were analysed by statistical package SPSS version 17.0 (SPSS, Chicago, IL, USA). Before all analysis, data were checked for normality by probability plots and for homogeneity of variances by Levene's test. One way anova was used to determine the significance of each parameter among different treatments. If a main effect was significant, the anova was followed by Tukey's test. Level of significance was made at 95% probability level.
Result
Experimental diets
Experimental diets did not show significant difference (P > 0.05) in crude protein, crude lipid, crude fibre and NFE content (Table 2). However, the total ash content was significantly higher (P < 0.01) in experimental diets P6 and P9 compared with control and P3. The crude protein content ranged from 37.97 ± 0.25% to 38.52 ± 0.18% and crude lipid from 7.88 ± 0.03% to 8.50 ± 0.06%.
| Nutrients | P0 | P3 | P6 | P9 | Level of significance |
|---|---|---|---|---|---|
| Organic mattera | 81.79 ± 0.03a | 81.50 ± 0.07a | 80.75 ± 0.14b | 80.55 ± 0.10b | ** |
| Moisture | 8.30 ± 0.14 | 8.05 ± 0.30 | 7.90 ± 0.19 | 7.82 ± 0.18 | NS |
| Crude protein | 37.97 ± 0.25 | 38.25 ± 0.42 | 38.52 ± 0.18 | 38.29 ± 0.09 | NS |
| Crude lipid | 7.88 ± 0.03 | 8.15 ± 0.28 | 8.39 ± 0.04 | 8.50 ± 0.06 | NS |
| Ash | 18.21 ± 0.03a | 18.50 ± 0.07a | 19.25 ± 0.14b | 19.45 ± 0.10b | ** |
| Crude fibre | 2.60 ± 0.08 | 2.80 ± 0.07 | 2.90 ± 0.04 | 2.93 ± 0.04 | NS |
| NFEb | 25.05 ± 0.47 | 24.25 ± 1.15 | 23.07 ± 0.23 | 23.03 ± 0.28 | NS |
| Gross energy (kcal per 100 g)c | 402.36 ± 0.46 | 402.04 ± 0.64 | 401.33 ± 0.15 | 400.98 ± 0.13 | NS |
- *P < 0.05; **P < 0.01; NS, Non-significant.
- a Organic matter = 100 − Ash (%).
- b NFE = 100 − (CP + EE + CF + ash + moisture).
- c Gross energy (GE) = (CP × 5.6) + (EE × 9.44) + (Hastings & Dupree are 1969) (CF × 4.1) + (NFE × 4.1) Kcal per 100 g (NRC 1993).
- The means with no superscript letter in common per factor indicate significant difference. If the effects were significant, anova was followed by Tukey test.
- Periphyton in shrimp basal diets at 0% (P0), 3% (P3), 6% (P6), 9% (P9).
Growth
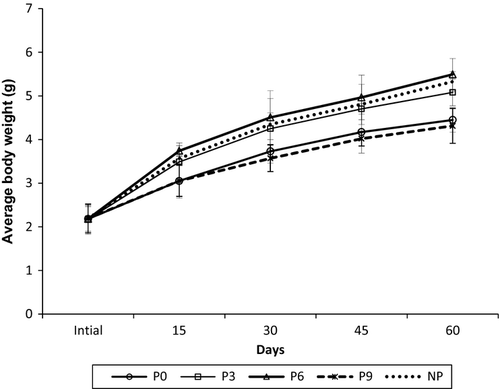
Growth performance of P. monodon juveniles over the time period is presented in Fig. 1. About 23.9% and 20% significantly higher (P < 0.01) final body weight was noticed in P6 and NP, respectively, compared with control. However, no significant difference in final body weight was noticed between shrimp fed 9% inclusion level of periphyton, P9 and control P0.
Metabolic enzymes
The activity of metabolic enzymes in hepatopancreas and muscle is presented in Table 3. In hepatopancreas, the AST activity showed non-significant difference (P > 0.05) while ALT activity was significantly (P < 0.05) different among treatments. The highest ALT and AST activities were observed in control and the lowest activity in P6. As for LDH activity, control exhibited non-significantly (P > 0.05) higher activity in both hepatopancreas and muscle compared with treatment groups. The MDH activity in muscle was significantly higher (P < 0.05) in control compared with P6. Similarly, control showed non-significantly (P > 0.05) higher MDH activity in hepatopancreas compared with other treatments. However, metabolic enzymes did not show significant difference in shrimp fed periphyton-supplemented diets and treatment provided with natural bamboo substrate, NP.
| Experimental groups | LDHb | MDHc | ALTd | ASTe | ||
|---|---|---|---|---|---|---|
| Muscle | Hepatopancreas | Muscle | Hepatopancreas | Hepatopancreas | Hepatopancreas | |
| P0 | 0.12 ± 0.02 | 0.010 ± 0.001 | 21.32 ± 0.84b | 2.50 ± 0.41 | 1.47 ± 0.14a | 2.37 ± 0.29 |
| P3 | 0.06 ± 0.01 | 0.005 ± 0.001 | 21.11 ± 0.50b | 1.02 ± 0.42 | 0.98 ± 0.02b | 1.68 ± 0.07 |
| P6 | 0.06 ± 0.02 | 0.006 ± 0.003 | 14.00 ± 1.01a | 1.02 ± 0.01 | 0.80 ± 0.14b | 2.01 ± 0.05 |
| P9 | 0.12 ± 0.07 | 0.005 ± 0.0001 | 15.48 ± 1.62ab | 1.12 ± 0.27 | 1.39 ± 0.23a | 1.72 ± 0.16 |
| NP | 0.06 ± 0.01 | 0.009 ± 0.002 | 18.39 ± 1.69ab | 1.32 ± 0.28 | 1.30 ± 0.11a | 2.14 ± 0.15 |
| Level of significance | NS | NS | a | NS | a | NS |
- a P < 0.05; NS, Non-significant.
- b Lactate dehydrogenase activity (LDH) expressed as micromoles of NAD released mg−1 protein min−1.
- c Malate dehydrogenase (MDH) activity (MDH) expressed as micromoles of NAD released mg−1 protein min−1.
- d Alanine aminotransferase activity (ALT) expressed as nanomoles of pyruvate formed mg−1 protein min−1.
- e Aspartine aminotransferase activity (AST) expressed as nanomoles of oxaloacetate formed mg−1 protein min−1.
- Periphyton in shrimp basal diets: 0% (P0), 3% (P3), 6% (P6), 9% (P9) and P0 diet with natural periphyton (NP) over bamboo substrate.
- The means with no superscript letter in common per factor indicate significant difference.
- If the effects were significant, anova was followed by Tukey test.
- Values are presented as mean ± SE.
Antioxidant enzymes
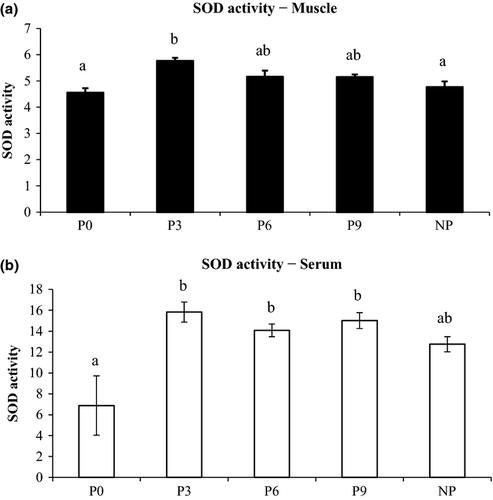
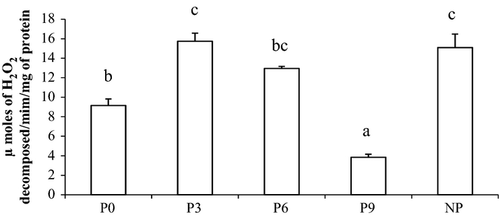
Superoxide dismutase activity in muscle and serum is presented in Fig. 2. Shrimp fed periphyton-supplemented diets showed 130.2%, 118.4% and 104.7% significant increase in serum SOD in P3, P9 and P6, respectively, compared with control. Similarly, the highest SOD level in muscle was noticed in P3 which was 26.5% higher (P < 0.01) compared with control. The catalase activity in muscle, P3 showed 308.4% and 71.8% significantly higher (P < 0.01) activity compared with P9 and control respectively (Fig. 3). Among serum parameters, NP showed significantly higher serum protein and serum albumin compared with P3 and P9, respectively, while no difference was noticed among other experimental groups (Table 4).
| Treatment | P0 | P3 | P6 | P9 | NP | Level of significance |
|---|---|---|---|---|---|---|
| Serum protein (mg mL−1) | 11.99 ± 0.19ab | 11.52 ± 0.71a | 13.63 ± 0.38ab | 12.56 ± 0.75ab | 14.65 ± 0.93b | * |
| Serum albumin (mg mL−1) | 4.36 ± 0.09b | 5.42 ± 0.36ab | 5.81 ± 0.12b | 4.61 ± 0.26a | 5.74 ± 0.13b | ** |
| Muscle protein (mg g−1) | 28.41 ± 1.07a | 32.25 ± 0.73a | 37.36 ± 2.56ab | 32.39 ± 2.48a | 41.79 ± 2.11b | * |
- *P < 0.05; **P < 0.01; NS, Non-significant; Values are presented as mean ± SE and expressed as mg protein mL−1 serum.
- The means with no superscript letter in common per factor indicate significant difference.
- If the effects were significant, anova was followed by Tukey test.
- Periphyton in shrimp basal diets: 0% (P0), 3% (P3), 6% (P6), 9% (P9) and P0 diet with natural periphyton (NP) over bamboo substrate.
Total haemocyte count
Total haemocyte, granulocyte and total agranulocyte count (cells mL−1) are presented in Table 5. The highest total haemocyte count (THC) was noticed in P6 (32.58 ± 1.3 × 106 cells mL−1) which differed significantly from P9 (17.12 ± 3.60 × 106 cells mL−1). Furthermore, there was 38%, 20.9% and 15.7% non-significant increase in THC in P6, NP and P3 compared with control. Among the differential count, significantly higher (P < 0.05) granulocytes were noticed in NP compared with P9 while no significant difference was noticed among other treatments.
| Haemocyte count | P0 | P3 | P6 | P9 | NP | Level of significance |
|---|---|---|---|---|---|---|
| Total haemocyte count (×106 cells mL−1) | 23.57 ± 1.80ab | 27.26 ± 4.43ab | 32.58 ± 1.30b | 17.11 ± 3.60a | 28.51 ± 3.12ab | a |
| Total granulocyte count (×106 cells mL−1) | 8.33 ± 0.63ab | 11.39 ± 1.82ab | 8.21 ± 0.55ab | 5.67 ± 1.62a | 12.32 ± 0.98b | a |
| Total agranulocyte count (×106 cells mL−1) | 15.24 ± 1.85ab | 15.88 ± 2.6ab | 24.36 ± 1.31b | 11.44 ± 2.78a | 16.19 ± 2.27ab | a |
- a P < 0.05; P < 0.01; NS, Non-significant.
- The means with no superscript letter in common per factor indicate significant difference.
- If the effects were significant, anova was followed by Tukey test.
- Values are presented as mean ± SE.
- Periphyton in shrimp basal diets: 0% (P0), 3% (P3), 6% (P6), 9% (P9) and P0 diet with natural periphyton (NP) over bamboo substrate.
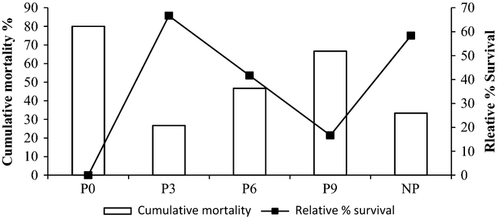
Relative percentage survival
Relative percentage survival and cumulative mortality in the different treatment groups are presented in Fig. 4. After challenge with V. harveyi, the cumulative mortality in the control was 80% while P3 recorded the lowest mortality (27%) and the highest RPS (67%). Similarly, P6 and NP treatment groups had better RPS of 42% and 58% compared with control.
Discussion
Algal proteins are considered as a good quality protein (Oser 1959). The consumption of periphyton enhances the growth performance and overall productivity in penaeid shrimps (Audelo-Naranjo et al. 2011; Anand et al. 2013a), fin fish like carps, tilapia (Azim et al. 2005) and giant freshwater prawns (Asaduzzaman et al. 2010). In the present study, dietary periphyton supplementation at 6% level significantly enhanced (P < 0.01) the final body weight of tiger shrimp compared with control. Similarly, better growth performance was recorded in shrimps fed microalgae-supplemented diet (Ju, Forster & Dominy 2009; Nonwachai, Purivirojkul, Limsuwan, Chuchird, Velasco & Dhar 2010). Since, there was no variation in macronutrients level in the experimental diets, better growth recorded in shrimp fed periphyton-supplemented diets might be attributed to unknown growth promoters or higher digestive enzymes secretion in gut and hepatopancreas (Anand et al. 2013b).
The growth performance of shrimp did not increase proportionately with the increased periphyton supplementation. Inclusion of periphyton at higher level, 9% (P9) showed a lower weight gain compared with other treatments. Earlier, addition of higher level of intact algal cells in P. monodon diets recorded a lower growth rate and higher FCR (Liao, Nur-E-Borhan, Okada, Matsui & Yamaguichi 1993). It is reported that inclusion of algal cells at higher level can cause imbalance in amino acid or minerals composition (Tangeras & Slinde 1994). However, amino acid or mineral profile and feed digestibility were not studied in the present experiment; the present findings suggest that inclusion of periphyton at higher level may lead to imbalances in microelements, leading to reduced palatability of the feed. Furthermore, ash content in the experimental diets increased as periphyton inclusion level increased (P6 and P9). Similarly, higher ash content was noticed in microalgae incorporated diets than the control diet (Ju et al. 2009). Better growth rate observed in P6 reflect that ash and acid soluble ash level recorded in the diet up to 6% periphyton inclusion level was within the limit of shrimp assimilation capacity. This is further supported from the fact that the presence of detritus up to 30% in gut content of shrimp juvenile does not have a negative effect on growth and physiology (Bombeo-Tuburan, Guanzon & Schroeder 1993).
Metabolic enzymes reflect the physiological status of living organisms. Enzymes in carbohydrate metabolism, LDH and Kreb's cycle enzyme, MDH level indicate energy demand in crustaceans (Rosas, Cuzon, Gaxiola, Le Priol, Pascual, Rossignyol, Contreras, Sanchez & Van Wormhoudt 2001). The level of LDH and MDH enzymes increases under different stress conditions like high temperature (Gupta, Pal, Sahu, Dalvi, Akhtar, Jha & Baruah 2010) and high density transportation (Chatterjee, Pal, Das, Mohammed, Sarma, Venkateshwarlu & Mukherjee 2006). In the present study, comparatively lower levels of LDH and MDH were noticed in treatment groups compared with control. Similar results were noticed when fish fed dietary supplements like neutraceuticals and microbial products (Akhtar et al. 2010; Gupta et al. 2010). These suggest that periphyton has positive effect on the physiological condition of shrimp.
The level of AST and ALT activities in hepatopancreas indicate the health status of shrimp (Pan et al. 2003). Their levels increase during hepatopancreatic damage (Mohankumar & Ramasamy 2006). The AST and ALT activity in the present study was lower in P6 and P3 compared with control, P0. It has been reported that dietary supplementation of astaxanthin rich red yeast, Phaffia rhodozyma reduced the oxidative stress, and AST and ALT enzymes in liver of rainbow trout (Nakano, Kanmuri, Sato & Takeuchi 1999). The present findings are also in agreement with the earlier reports which recorded a lower AST and ALT activities after dietary supplementation of immunostimulants and neutraceuticals in rohu fingerlings (Choudhury, Pal, Sahu, Kumar, Das & Mukherjee 2005; Gupta et al. 2010). So, it can be inferred that dietary supplementation of periphyton helped to maintain better physiological status in shrimp.
Algal products and their cell wall components are widely used to elicit non-specific defence mechanism of crustaceans (Ringø, Jose, Vecino, Wadsworth & Song 2012). Quantitative assessment of haemocyte populations is a marker for cellular immunological response in crustaceans (Bachere 2000). In the present study, dietary supplementation of periphyton elicited a higher haemocyte counts in treatments groups compared with control. Similarly, higher THC was noticed when shrimps fed dietary supplements like probiotics (Rengpipat, Rukpratanporn, Piyatiratitivorakul & Menasaveta 2000; Li et al. 2009), macroalgae- and β-carotene-(Supamattaya et al. 2005) supplemented diets. The SOD and catalase are the major anti-oxidative enzymes related to immunity in crustacean (Holmblad & Soderhall 1999; Mohankumar & Ramasamy 2006). In the present study, higher SOD and catalase activities were noticed in muscle and serum of shrimp fed periphyton-incorporated diets compared with control. This agrees with the earlier reports where higher immune response in terms of SOD and catalase activities were recorded in shrimps fed β-glucan, carotene (Pacheco et al. 2011), microalgae (Madhumathi & Rengasamy 2011) and macro algal supplements (Felix & Murugan 2003). These suggest that dietary supplementation of periphyton can elicit the anti-oxidative enzymes in shrimp and enhance the defence potential against oxidative stress. However, its immunostimulatory properties do not increase proportionally with dietary inclusion level (P9). This indicates that inclusion of periphyton at higher level has an inherent limit.
In this study, the serum biochemical parameters like total serum protein and serum albumin were higher in treatments, P6 and NP compared with control. The present results are in line with earlier findings where immunostimulants like levan (Maqsood, Samoon & Singh 2009) and β-glucan (Misra, Das, Mukherjee & Pattnaik 2006) increased the serum protein and albumin level in fish (Sahoo & Mukherjee 2002). Although, no parallel report is available in shrimp, the result suggests that periphyton has positive effect on nutritional condition of shrimp. Relative per cent survival indicated that periphyton as dietary supplement significantly improved the survival of shrimp after challenge with V. harveyi. Overall, shrimp fed 3% and 6% periphyton supplement showed better immune response with higher resistance to Vibrio infection compared with control. This is comparable to the findings of Nonwachai et al. (2010) who reported better resistance in L. vannamei upon challenge with V. harveyi when fed algal meal-incorporated diet compared with control. This further confirms the potential role of periphyton in eliciting non-specific immune responses of shrimps.
Conclusion
The present findings elucidate the potential of periphyton as a novel dietary immunostimulant in P. monodon. Periphyton supplementation up to 6% level enhanced the growth, haemocyte count, anti-oxidative enzyme level and reduced the susceptibility to bacterial infection. Since, dietary supplementation at 6% did not perform significantly better compared with 3% level; the lower level can be recommended due to cost-effectiveness. However, supplementation at higher level (9%) did not improve immune response proportionately indicating that higher inclusion level can lead to immune suppression. Application of periphyton in field condition requires detail study related to effective dose for different age groups of shrimp. Further work needs to be carried out to isolate and characterize the active principles of periphyton as well as delineate its specific properties.
Acknowledgment
The authors are grateful to Dr. A.G. Ponniah, Director, Central Institute of Brackishwater Aquaculture, Chennai and Dr. W. S. Lakra, Director, Central Institute of Fisheries education, Mumbai for providing the required facilities to conduct this study.




