Fish oil–based finishing diets strongly increase long-chain polyunsaturated fatty acid concentrations in farm-raised common carp (Cyprinus carpio L.)
Abstract
This study investigated effects of linseed or fish oil–enriched finishing diets on the polyunsaturated fatty acids (PUFA) composition in dorsal muscle tissues of pond-cultured common carp (Cyprinus carpio). After 180 days of dietary exposure to cereal diet containing vegetable oil (1%), carp were exposed to 7% linseed (LO) or 7% fish oil–enriched (FO) finishing diets for 30 days. FO supplied 17 and 20 mg fish−1 day−1, respectively, of the long-chain n-3 fatty acids eicosapentaenoic and docosahexaenoic acid for 30 days and doubled long-chain PUFA concentrations in carp of the FO pond. The increased supply of short-chain PUFA in LO resulted in higher short chain, but not long-chain PUFA, showing that there was very little PUFA conversion. Thus, dietary short-chain PUFA could not compensate for the low levels of dietary long-chain PUFA in LO. However, moderate supply of dietary long-chain PUFA in finishing diets for 30 days is very efficient in increasing nutritionally important long-chain PUFA concentrations in carp.
Introduction
Today's aquaculture still highly depends on marine fish oils (FO). FO-enriched feeds used for the annual production of approximately 60 million tons of farmed fish, crustaceans and mollusks (Turchini, Torstensen & Ng 2009) currently account for up to 87% of the global supply of FO as a lipid source (Tacon, Hasan & Subasinghe 2006). The continuously increasing demand for FO, frequently used in aquaculture, has brought several global fish stocks close to extinction (Pauly, Christensen, Guenette, Pitcher, Sumaila, Walters, Watson & Zeller 2002). Therefore, the identification of alternative and sustainable lipid sources for farm-raised fish species are a major challenge for current aquaculture research. With a consumption of 5.5% of the total FO used in aquaculture worldwide (Tacon et al. 2006), carp farming is among the aquaculture sectors where the need for a reduction in FO use is particularly urgent.
Fish oils used in aquaculture feeds are, in comparison with terrestrial vegetable oils (VO), highly enriched in beneficial omega-3 polyunsaturated fatty acids (n-3 PUFA) (Olsen 1999). In particular, dietary long-chain (C20–22) PUFA such as eicosapentaenoic (EPA; 20:5n-3), docosahexaenoic (DHA; 22:6n-3) and arachidonic (ARA; 20:4n-6) acids are required by fish to support somatic growth and survival (Cowey, Sargent, Russell & Yonge 1972; Harel, Gavasso, Leshin, Gubernatis & Place 2001; Copeman, Parrish, Brown & Harel 2002; Trenzado, Morales & de la Higuera 2008), stress resistance (Bell, Tocher, Farndale & Sargent 1998) and successful ontogeny (Mourente, Tocher & Sargent 1991; Villalta, Estevez, Bransden & Bell 2005; Benitez-Santana, Masuda, Carrillo, Ganuza, Valencia, Hernandez-Cruz & Izquierdo 2007).
Several studies have shown that, up to a certain extent, supplying compound feeds based on VO can replace FO without apparent detrimental effects on growth and survival of various fish species (e.g. Glencross, Hawkins & Curnow 2003; Tocher, Dick, MacGlaughlin & Bell 2006; Kowalska, Zakes, Jankowska & Siwicki 2010). However, the low n-3 and high n-6 PUFA amounts in VO, in particular linoleic acid (LA), render fatty acid profiles of farmed fish less favourable from a human nutrition perspective (Bell, McEvoy, Tocher, McGhee, Campbell & Sargent 2001; Kowalska et al. 2010; Senadheera, Turchini, Thanuthong & Francis 2010). Lindseed oil (LO) is generally rich (>50%) in alpha-linolenic acid (ALA; 18:3n-3) (Turchini et al. 2009) and therefore often considered as a potential (terrestrial) n-3 PUFA-rich substitute for FO in aquaculture nutrition as most freshwater and some marine fish species are capable to enzymatically bioconvert, at various degrees, ALA to long-chain n-3 PUFA, such as EPA and DHA (e.g. Tocher & Dick 1999; Bell et al. 2001). However, the bioconversion rates of the essential precursors ALA and LA to C20–22 PUFA are in many cases insufficient to fully cover physiological needs of fish (Bell et al. 2001; Tocher et al. 2006). The latter might lead to some physiological disturbances (e.g. decreased stress resistance or immunity) (Bell et al. 1998; Copeman et al. 2002; Benitez-Santana et al. 2007) and to lower concentrations of C20–22 PUFA that subsequently reduce the nutritional quality of fish for humans (Calder & Yaqoob 2009).
Several feeding strategies have been developed to enhance fish performance and nutritional quality for humans. In recent years, FO was applied as short-term finishing diet after VO-based grow-out to increase n-3 PUFA concentrations in several fish species. In particular, piscivorous species such as Atlantic salmon (Salmo salar L.), rainbow trout (Oncorhynchus mykiss Walbaum) and murray cod (Maccullochella peeliipeelii Mitchell) attracted worldwide attention (e.g. Bell, Henderson, Tocher & Sargent 2004; Turchini, Francis & De Silva 2007; Thanuthong, Francis, Senadheera, Jones & Turchini 2011). However, supply of finishing diets in these studies lasted between 5 and 24 weeks and the supplementation of FO finishing diets had only limited success in fatty acid recovery (max. 80% of n-3 PUFA) when compared with a FO supplement throughout the grow-out period. Additional studies showed that in omnivorous species such as common carp (Cyprinus carpio L.) and also piscivorous rainbow trout the steady supplementation of C18 PUFA-rich diets subsequently increases C20–22 PUFA concentrations in muscle tissues (Thanuthong, Francis, Senadheera, Jones & Turchini 2011; Mráz, Máchová, Kozák & Pickova 2012). Based on these findings, this study investigates how LO (rich in C18 PUFA) and FO (rich in C20–22 PUFA) compound feeds supplemented as finishing diet for a very short period (30 days) after VO-based grow-out (180 days) affected total lipids and PUFA profiles of omnivorous common carp cultivated in pond production systems. In these natural pond systems, it is impossible to conduct normalized feeding trials because of the occurrence of natural food (i.e. zooplankton). We therefore used mixing models of stable isotope (δ13C and δ15N) signatures of natural food and supplemented feeds to assess the contribution of these two diet sources to carp in different ponds. Results of this study will help to identify nutritional supplements and alternative feeding strategies to keep a high dietary supply of long-chain PUFA for human nutrition by common carp and possibly other farmed fish.
Methods
Experimental design and sampling procedures
The experiments were conducted in temperate lower Austria (N 48.815049, E 15.297321). Common carp from the same stock were initially introduced into two ponds of equal size at similar stocking densities (pond sizes 1.4 ha, 7000 carp pond−1, stocking density 5000 carp ha−1). Carp of these ponds were feeding on natural pond zooplankton and a supplemented cereal diet enriched with 1% milk thistle (Silybum marianum L.) oil (VO, total supply 264 g feed fish−1) over a period of 180 days, representing the traditional cultivation procedure (Mráz et al. 2012). Subsequently, VO was replaced by commercial pellet feeds enriched with either 7% LO (pond A) or 7% FO (pond B; Fig. 1). These pellet feeds (total supply 75 g feed fish−1) were fed for additional 30 days (finishing period). Total feed supply (VO plus LO or FO) for 210 days in both ponds was 339 g fish−1. Commercial pellet feeds were mostly composed of soybean, pumpkin and rapeseed meal and wheat (Table 1). This experimental set-up was chosen to test how a short-term finishing period affects fatty acid profiles of carp under ‘real world’ aquaculture conditions. We did not include replicate ponds as this study evaluates changes in fatty acid compositions of common carp before and after a diet shift to n-3 PUFA-rich compound feeds. Individual analyses of 7–10 fish per sampling date and pond were considered to provide the required replication (Turchini, Francis & De Silva 2006).
| LO | FO | |
|---|---|---|
| Ingredients | ||
| Soybean meal | 305.0 | 305.0 |
| Pumpkinseed press cake | 150.0 | 150.0 |
| Wheat | 148.0 | 148.0 |
| Rapeseed press cake | 125.0 | 125.0 |
| Wheat flour | 120.0 | 120.0 |
| Crop groats | 50.0 | 50.0 |
| Fish oil (sprayed on) | – | 70.0 |
| Linseed oil (sprayed on) | 70.0 | – |
| Monocalciumphosphate | 20.0 | 20.0 |
| Composition | ||
| Crude protein | 330.0 | 330.0 |
| Total lipids | 114.0 | 114.0 |
| Fiber | 40.0 | 40.0 |
| Ash | 75.0 | 75.0 |
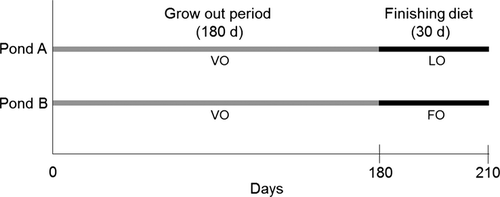
Carp (n = 7–10 pond−1) were sampled, respectively, after 180 days of equal exposure to diet VO and after a subsequent 30 days finishing period (total cultivation time 210 days) during which they were exposed to FO or LO compound feeds. Every fish was measured (±0.1 cm standard length), weighed (±0.1 g wet weight) and kept frozen (−80°C) to limit possible lipolytic degradation.
Zooplankton represents the main natural food source for farm-raised common carp (Schlott 2007). Therefore, zooplankton (>250 μm) was collected from both ponds after 180 days (VO grow-out) and subsequently after 30 days (finishing period) by vertical plankton net hauls (n = 6, respectively) and immediately frozen at −80°C. In both ponds, Daphnia longispina (Müller) and Bosmina longirostis (Müller) were the dominant zooplankton species, followed by cyclopoid (Eucyclops sp. Claus) and, to a lesser extent, calanoid copepods (Eudiaptomus sp. Kiefer). No benthic invertebrates were found in sediments or in carp gut contents.
Lipid and fatty acid methyl ester analysis
Fish feeds, zooplankton and skinless, dorsal muscle tissue of carp were freeze-dried and subsequently homogenized. Lipids were extracted using chloroform/methanol as described elsewhere (Heissenberger, Watzke & Kainz 2010). In brief, total lipids of muscle tissues, diet samples and zooplankton were extracted and gravimetrically measured. Subsequently, total lipids (1 mg) were methylated to obtain fatty acid methyl esters (FAME), using sulphuric acid and methanol. FAME were separated [Supelco™ column SP™-2560 (Sigma Aldrich Corporation, St. Louis, MO, USA); 100 m × 0.25 mm ID, 0.20 μm film thickness] and analysed using a gas chromatograph (Thermo Scientific TRACEGC Ultra™; Thermo Fisher Scientific, Waltham, MA, USA), equipped with a flame ionization detector (FID).
Stable isotopes analysis and δ13C lipid correction
As the experiment was conducted in a semi-intensive carp aquaculture farm, it was logistically difficult to control the feeding conditions between ponds because of the unpredictable presence of natural food (zooplankton). Therefore, stable isotopes and subsequent mixing model calculations were used to estimate the contribution of potential food sources (supplementary feeds and zooplankton) to carp biomass and control for the homogeneity of feeding conditions between ponds (e.g. Phillips 2001). It was assumed that substantial interpond differences in the availability of the two food sources for carp would result in marked differences in the mixing model results.
Signatures of stable carbon and nitrogen isotopes (δ13C and δ15N, respectively) of freeze-dried and homogenized fish muscle tissues, zooplankton and supplementary feed samples (≈1 mg) were analysed using an elemental analyser (EA 1110; CE Instruments, Milan, Italy) interfaced via a ConFlo II device (Finnigan MAT, Bremen, Germany) to a continuous flow stable isotope ratio mass spectrometer (Delta Plus, Finnigan MAT). Nitrogen (Air Liquide™; Air Liquide, Paris, France) and CO2 were used as reference gases. Calibration was performed by using the at air international standard using IAEA-N-1, IAEA-N-2 and IAEA-NO-3 for nitrogen and IAEA-CH-6, IAEA-CH-7 (IAEA, Vienna, Austria) for carbon.
Lipid correction for δ13C values, as suggested by Kiljunen, Grey, Sinisalo, Harrod, Immonen and Jones (2006), was applied based on the model of McConnaughey and McRoy (1979). The model normalized δ13C values of samples presenting large differences in their lipid concentrations (e.g. fish and zooplankton) by using C/N ratios as a proxy for total lipid contents. The proportional lipid content (L) and lipid-normalized δ13C values were defined as
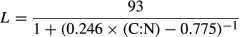 (1)
(1)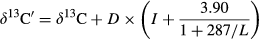 (2)
(2)Where δ13C′ is the lipid-normalized and δ13C the measured value of the sample. L represents the proportional lipid content of the sample and C/N is the proportion of carbon and nitrogen in the sample. The protein/lipid discrimination (D) was set to 7‰ (Sweeting, Barry, Polunin & Jennings 2007). I defines the intersection on the x-axis (Kiljunen et al. 2006) and was assigned a value of −0.207.
All stable isotope values were reported using the δ notation, Where δ13C or δ15N = ([Rsample/Rstandard] − 1) × 1000 were13C:12C or 15N:14N.
The dietary contribution of zooplankton and added feeds to the carp diets was assessed using SIAR (Stable Isotope Analysis in R). Fractionation factors of 13C and 15N between trophic levels (i.e. potential food sources and carp) were set at 1.1 ± 0.3‰ and 2.8 ± 0.4‰ for C and N respectively (McCutchan, Lewis, Kendall & McGrath 2003). Zooplankton and the additional fish feeds were used as the only potential food sources in the mixing models as zooplankton was identified (analyses of gut content and sediments) as the major natural food source for carp in all ponds.
Statistical analysis
Differences between means of standard length, fish weight, total lipids and individual fatty acids were analysed using paired t-tests as samples were obtained from the same ponds at different sampling dates. Thus, individual fish and zooplankton samples were not independent units of observation. Differences between means of total lipids and individual fatty acids in supplementary feeds were analysed using one-way anova followed by Tukey′s HSD post hoc test. T-tests were used to assess the effect of different specific growth rates (SGR) in carp exposed to LO and FO within the 30 days finishing period. All data were ln-transformed to obtain normal distribution. Analyses were performed using spss (15.0), and the level of significance was set at P < 0.05.
Results
Lipid composition of food sources
However, there were significant differences, total lipid and fatty acid concentrations of zooplankton varied little within both ponds after 180 (grow-out) and 210 days (finishing period; Table 2). After 210 days, zooplankton in pond A had significantly lower LA and ALA than at the beginning of the finishing period. Moreover, C20–22 PUFA concentrations were similar after 180 days and 210 days except for DHA, which increased significantly within the 30 days finishing period. In general, total PUFA, total n-3 PUFA and total n-6 PUFA were not different after 180 days and 210 days in pond A. LA and ALA concentrations of zooplankton in pond B did not change significantly from beginning to the end of the finishing period. Stearidonic acid (18:4n-3, SDA), EPA and DHA were significantly increased during the 30 days finishing period resulting in significantly increased total PUFA and total n-3 PUFA concentrations at the end of the trial. The highest n-3/n-6 PUFA ratio (5.8) was found in zooplankton of pond B after 210 days, whereas n-3/n-6 PUFA ratios were generally similar (3.6–4.3) between ponds and sampling dates. Nevertheless, n-3/n-6 PUFA ratios increased significantly within the finishing period in both ponds.
| Fatty acids | Pond A | Pond B | ||
|---|---|---|---|---|
| After 180 days | After 210 days | After 180 days | After 210 days | |
| 16:0 | 18.6 ± 4.1 | 15.1 ± 3.8* | 16.0 ± 3.1 | 17.2 ± 3.3 |
| 16:1n-7 | 6.5 ± 3.0 | 3.8 ± 2.2* | 3.4 ± 1.1 | 5.9 ± 2.1* |
| 18:0 | 4.2 ± 0.4 | 4.6 ± 0.4* | 3.8 ± 0.6 | 3.6 ± 0.2 |
| 18:1n-9 | 9.2 ± 1.1 | 7.6 ± 0.9* | 11.5 ± 2.7 | 9.1 ± 3.2* |
| 18:2n-6 | 8.9 ± 0.6 | 7.9 ± 0.7* | 6.0 ± 1.0 | 5.4 ± 1.2 |
| 18:3n-6 | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.8 ± 0.2 | 1.0 ± 0.1* |
| 20:1n-9 | 0.2 ± 0.1 | 0.3 ± 0.1* | 0.5 ± 0.3 | 0.4 ± 0.1 |
| 18:3n-3 | 22.2 ± 3.9 | 18.0 ± 4.1* | 13.1 ± 3.9 | 13.9 ± 3.8 |
| 18:4n-3 | 4.7 ± 0.9 | 6.5 ± 3.3 | 8.7 ± 2.2 | 11.7 ± 1.7* |
| 20:4n-6 | 2.9 ± 0.8 | 2.9 ± 1.2 | 3.2 ± 0.6 | 2.9 ± 0.7 |
| 20:5n-3 | 10.1 ± 2.1 | 11.9 ± 5.0 | 12.3 ± 2.5 | 16.7 ± 3.6* |
| 22:6n-3 | 7.2 ± 4.5 | 12.1 ± 5.8* | 5.8 ± 3.1 | 10.8 ± 4.5* |
| ∑SAFA | 30.0 ± 4.6 | 25.4 ± 4.3* | 25.2 ± 3.7 | 28.5 ± 4.2* |
| ∑MUFA | 16.7 ± 3.9 | 12.5 ± 3.0* | 16.3 ± 3.3 | 16.3 ± 5.1 |
| ∑PUFA | 58.3 ± 4.4 | 61.8 ± 9.3 | 51.4 ± 7.8 | 64.3 ± 7.8* |
| ∑n-3 PUFA | 45.5 ± 2.9 | 50.2 ± 7.1 | 41.2 ± 6.1 | 54.7 ± 5.9* |
| ∑n-6 PUFA | 12.8 ± 1.5 | 11.7 ± 2.3 | 10.2 ± 1.8 | 9.7 ± 2.1 |
| n-3/n-6 ratio | 3.6 ± 0.2 | 4.3 ± 0.3* | 4.1 ± 0.1 | 5.8 ± 0.8* |
| Total lipids | 181.2 ± 19.0 | 172.7 ± 22.3* | 168.3 ± 19.7 | 181.7 ± 11.5 |
- ∑SAFA = 12:0, 13:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 21:0, 22:0, 23:0, 24:0; ∑MUFA = 14:1n-5, 15:1n-5, 16:1n-7, 17:1n-7, 18:1n-9, 20:1n-9, 22:1n-9, 24:1n-9; ∑PUFA = 18:2n-6, 18:3n-6, 18:3n-3, 18:4n-3, 20:3n-6, 20:3n-3, 20:4n-6, 20:4n-3, 20:5n-3, 22:6n-3.
Compound feeds (LO and FO) used as finishing diet had significantly higher total lipid concentrations than the VO cereal feed used for grow-out (Table 3). Docosahexaenoic acid and EPA were the main n-3 PUFA in FO, whereas ALA was almost the only n-3 PUFA in LO. Linoleic, oleic and palmitic acid represented the major fatty acids of the supplementary VO feed. Except for n-6 PUFA, VO feed had generally significantly lower concentrations of all fatty acids and significantly lower n-3/n-6 PUFA ratios with respect to LO and FO (Table 3).
| Fatty acid | VO | LO | FO |
|---|---|---|---|
| 16:0 | 6.4 ± 0.2a | 9.1 ± 0.2b | 14.7 ± 1.4c |
| 16:1n-7 | 0.1 ± 0.0a | 0.2 ± 0.0b | 4.4 ± 0.4c |
| 18:0 | 2.2 ± 0.1a | 3.5 ± 0.1b | 3.2 ± 0.2b |
| 18:1n-9 | 11.0 ± 0.3a | 25.3 ± 0.5b | 26.9 ± 2.3b |
| 18:2n-6 | 28.7 ± 0.1b | 29.0 ± 0.8b | 22.5 ± 1.9a |
| 18:3n-6 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 20:1n-9 | 0.7 ± 0.1b | 0.4 ± 0.0a | 3.0 ± 0.3c |
| 18:3n-3 | 2.4 ± 0.2a | 21.0 ± 1.0c | 4.0 ± 0.8b |
| 18:4n-3 | 0.1 ± 0.0a | 0.1 ± 0.0a | 1.8 ± 0.2b |
| 20:4n-6 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.4 ± 0.0b |
| 20:5n-3 | 0.1 ± 0.0a | 0.1 ± 0.0b | 5.9 ± 0.6c |
| 22:6n-3 | 0.1 ± 0.0a | 0.1 ± 0.0b | 7.4 ± 0.6c |
| ∑SAFA | 12.2 ± 0.2a | 13.6 ± 0.4a | 22.5 ± 2.0b |
| ∑MUFA | 12.1 ± 0.3a | 26.0 ± 0.5b | 35.1 ± 3.1c |
| ∑PUFA | 31.3 ± 0.3a | 50.4 ± 0.2b | 43.5 ± 4.1b |
| ∑n-3 PUFA | 2.6 ± 0.2a | 21.3 ± 1.0b | 19.8 ± 2.2b |
| ∑n-6 PUFA | 28.7 ± 0.1b | 29.1 ± 0.8b | 23.7 ± 2.0a |
| n-3/n-6 ratio | 0.1 ± 0.0a | 0.7 ± 0.1b | 0.9 ± 0.0c |
| Total lipids | 71.5 ± 12.2a | 137.6 ± 0.3b | 147. 0 ± 10.3b |
- ∑SAFA = 12:0, 13:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 21:0, 22:0, 23:0, 24:0; ∑MUFA = 14:1n-5, 15:1n-5, 16:1n-7, 17:1n-7, 18:1n-9, 20:1n-9, 22:1n-9, 24:1n-9; ∑PUFA = 18:2n-6, 18:3n-6, 18:3n-3, 18:4n-3, 20:3n-6, 20:3n-3, 20:4n-6, 20:4n-3, 20:5n-3, 22:6n-3.
Growth and lipid composition of carp
Specific growth rates were similar in carp exposed to LO and FO during the 30 days finishing period (P = 0.839; Table 4). During the 30 days exposure to LO and FO, the relative biomass gain of carp was not significantly different (P = 0.596) between carp exposed to LO and FO (40.7 ± 11.8% and 30.4 ± 10.6% of biomass gain for LO and FO carp, respectively).
| Diet | C. carpio | SGR (%) Day 180–day 210 | ||
|---|---|---|---|---|
| Length (cm) | Weight (g) | Length | Weight | |
| Initial carp (0 day) | 9.0 ± 1.5 | 10.7 ± 5.3 | ||
| Pond A | ||||
| After 180 days VO diet | 23.3 ± 1.4 | 401.7 ± 112.0 | 9.6 ± 2.9 | 40.7 ± 11.8 |
| After LO finishing diet | 25.5 ± 2.1* | 565.2 ± 149.5* | ||
| Pond B | ||||
| After 180 days VO diet | 18.5 ± 2.0 | 215.7 ± 66.8 | 13.0 ± 2.9 | 30.4 ± 10.6 |
| After FO finishing diet | 20.9 ± 1.7* | 281.3 ± 72.0* | ||
Total lipid concentrations (40.7 and 44.2 mg g−1 d.w., respectively) and PUFA concentrations of carp in ponds A and B were similar after 180 days grow-out (Table 5). However, supplementation for 30 days of LO and FO finishing diets significantly increased total lipid concentrations by 60% (70.8 mg g−1 d.w.) in carp exposed to FO and 34% (54.6 mg g−1 d.w.) in carp exposed to LO (Table 5).
| Fatty acids | Pond A | Pond B | ||
|---|---|---|---|---|
| After 180 days VO diet | After LO finishing diet | After 180 days VO diet | After FO finishing diet | |
| 16:0 | 4.6 ± 0.6 | 5.6 ± 1.7 | 5.2 ± 0.2 | 10.3 ± 5.0* |
| 16:1n-7 | 0.6 ± 0.2 | 1.1 ± 0.4* | 0.6 ± 0.1 | 2.1 ± 1.2* |
| 18:0 | 1.7 ± 0.1 | 2.0 ± 0.5 | 2.0 ± 0.1 | 3.5 ± 1.7* |
| 18:1n-9 | 2.8 ± 0.7 | 5.9 ± 3.2* | 3.3 ± 0.2 | 10.7 ± 6.4* |
| 18:2n-6 | 2.5 ± 0.8 | 5.0 ± 2.9* | 2.9 ± 0.8 | 7.7 ± 5.0* |
| 18:3n-6 | 0.1 ± 0.0 | 0.1 ± 0.0* | 0.1 ± 0.0 | 0.1 ± 0.1 |
| 20:1n-9 | 0.2 ± 0.0 | 0.4 ± 0.2* | 0.2 ± 0.0 | 1.2 ± 0.7* |
| 18:3n-3 | 0.5 ± 0.1 | 3.1 ± 2.4* | 0.4 ± 0.1 | 1.9 ± 1.2* |
| 18:4n-3 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.8 ± 0.5* |
| 20:4n-6 | 1.7 ± 0.2 | 1.7 ± 0.2 | 2.1 ± 0.2 | 3.3 ± 1.5 |
| 20:5n-3 | 1.4 ± 0.1 | 1.7 ± 0.2* | 1.6 ± 0.1 | 3.8 ± 1.6* |
| 22:6n-3 | 3.8 ± 0.3 | 4.4 ± 0.4* | 3.9 ± 0.6 | 10.1 ± 4.1* |
| ∑SAFA | 6.8 ± 0.8 | 8.1 ± 2.5 | 7.7 ± 0.4 | 15.0 ± 7.4* |
| ∑MUFA | 3.7 ± 1.0 | 7.5 ± 3.8* | 4.2 ± 0.3 | 14.4 ± 8.5* |
| ∑PUFA | 10.7 ± 1.4 | 17.2 ± 5.8* | 11.9 ± 1.1 | 29.2 ± 13.8* |
| ∑n-3 PUFA | 6.2 ± 0.4 | 10.0 ± 2.8* | 6.4 ± 0.6 | 17.3 ± 7.3* |
| ∑n-6 PUFA | 4.5 ± 1.1 | 7.3 ± 3.1* | 5.5 ± 1.0 | 11.9 ± 6.7* |
| n-3/n-6 ratio | 1.4 ± 0.3 | 1.5 ± 0.3 | 1.2 ± 0.3 | 1.6 ± 0.3* |
| Total lipids | 40.7 ± 6.1 | 54.6 ± 15.1* | 44.2 ± 3.4 | 70.8 ± 17.8* |
- ∑SAFA = 12:0, 13:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 21:0, 22:0, 23:0, 24:0; ∑MUFA = 14:1n-5, 15:1n-5, 16:1n-7, 17:1n-7, 18:1n-9, 20:1n-9, 22:1n-9, 24:1n-9; ∑PUFA = 18:2n-6, 18:3n-6, 18:3n-3, 18:4n-3, 20:3n-6, 20:3n-3, 20:4n-6, 20:4n-3, 20:5n-3, 22:6n-3.
The 30 days exposure to LO and FO generally increased fatty acid concentrations in carp (Table 5): palmitoleic acid (16:1n-7), oleic acid (18:0), total MUFA and total PUFA concentrations increased significantly and LA and ALA concentrations in carp even doubled in both ponds (Table 5). Concentrations of EPA and DHA were significantly increased and ~2× higher in carp exposed to FO compared with carp exposed to LO after 210 days (Table 5). In contrast, carp exposed to LO diet for 30 days increased only slightly, yet significantly, its EPA and DHA concentrations (Table 5). However, ALA concentrations were strongly increased in carp exposed to LO. Ratios of n-3/n-6 PUFA (1.2–1.6) remained generally unaffected in carp of both ponds and sampling dates. However, n-3/n-6 PUFA ratio of carp exposed to FO increased significantly.
Relative contribution of zooplankton and feeds to carp diet
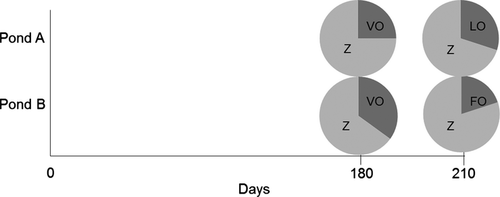
Delta-13C and δ15N signatures of all feeds varied little (−23.7 to −22.4 ‰ and 2.6–3.4 ‰, respectively; Table 6). Stable isotope signatures of zooplankton ranged between −34.2 to −32.6 ‰ for δ13C and 7.6–9.3‰ for δ15N. After the 30 days finishing period, δ13C signatures were more enriched in carp exposed to FO and more depleted in carp exposed to LO, whereas δ15N signatures generally tended to be more depleted. Signatures of carp in both ponds ranged between −29.7 and −28.3 ‰ for δ13C, and 9.6 and 10.7 ‰ for δ15N. Stable isotope mixing models indicated that carp clearly retained more zooplankton (>65%) than supplementary feeds in all ponds and treatments (Fig. 2). After feeding for 180 days on pond zooplankton and milk thistle oil–enriched feeds (VO) carp had very similar retention patterns of zooplankton (70–75%) and supplementary feeds (25–30%). After 30 days exposure to different finishing diets, carp retained up to 35% of the LO-feeds, whereas only 20% of FO-feeds were recovered in carp dorsal muscle tissues.
| Dietary pond treatments | Carp | Zooplankton | Supplementary feeds | |||
|---|---|---|---|---|---|---|
| δ13C | δ15N | δ13C | δ15N | δ13C | δ15N | |
| Pond A | ||||||
| After 180 days | −28.3 ± 1.1 | 10.7 ± 0.1 | −34.2 ± 0.3 | 8.5 ± 0.5 | −22.4 ± 0.4 | 3.4 ± 0.7 |
| After LO-finishing diet | −28.4 ± 0.4 | 9.7 ± 0.2 | −32.9 ± 0.2 | 7.6 ± 0.7 | −23.7 ± 0.0 | 3.0 ± 0.0 |
| Pond B | ||||||
| After 180 days | −29.7 ± 0.8 | 10.2 ± 0.3 | −33.6 ± 0.2 | 9.3 ± 0.5 | −22.4 ± 0.4 | 3.4 ± 0.7 |
| After FO-finishing diet | −29.4 ± 0.4 | 9.6 ± 0.2 | −32.6 ± 0.4 | 7.6 ± 0.4 | −23.2 ± 0.0 | 2.6 ± 0.0 |
Discussion
In this study, it is shown that, irrespective of any initial weight difference, a 30 days supplementation of PUFA-rich compound feeds resulted in a similar relative increase in biomass of carp exposed to either LO- or FO-based finishing diets. Testing the effect of these finishing diets in natural fish ponds allows to examine their dietary role for PUFA retention in carp under ‘real world’ conditions. Results of the carbon and nitrogen stable isotope mixing model revealed that the new biomass in carp exposed to LO and FO finishing diets consisted of <35% supplementary feeds and >65% natural pond zooplankton. These results enable us to compare the effects of LO and FO finishing diets on the fatty acid profile of carp despite the inability to control for the food intake or other naturally occurring physicochemical and biochemical differences in these ponds and their planktonic food webs respectively.
The 30 days supplementation of PUFA-rich compound feeds at the end of the cultivation period markedly increased total lipids and PUFA concentrations of common carp. The diet shift from supplementary milk thistle oil–enriched cereal VO-feed, poor in total lipids and PUFA, to the lipid- and PUFA-rich compound feeds LO and FO indicates that common carp very efficiently retained high-quality supplementary feeds within such a short period of time. Despite the similar amount of supplemented dietary lipids, the significantly higher increase in total lipid concentrations in carp exposed to FO compared with LO suggest more efficient assimilation and/or higher retention of long-chain PUFA compared with short-chain PUFA as recently reported in other fish species (Olsen, Henderson & Ringø 1998; Caballero, Obach, Rosenlund, Montero, Gisvold & Izquierdo 2002; Pratoomyot, Bendiksen, Bell & Tocher 2010).
Despite the lack of C20–22 PUFA in LO feeds, carp exposed to these feeds slightly increased ARA, EPA and DHA concentrations within the finishing period, indicating that carp either bioconverted precursors to these target PUFA and/or received an increased supply of ARA, EPA and DHA from zooplankton. Such PUFA conversions are known to occur in a variety of freshwater fish species (e.g. Buzzi, Henderson & Sargent 1996, 1997; Tocher et al. 2006), including common carp (Farkas, Csengeri, Majoros & Oláh 1980; Tocher & Dick 1999). It was also demonstrated that dietary LA and ALA stimulate gene expression of desaturases and elongases (Zheng, Torstensen, Tocher, Dick, Henderson & Bell 2005). However, for many freshwater fish species, the conversion of C18 PUFA to long-chain PUFA seems to occur at a very slow rate (Tocher et al. 2006) because of limiting delta-6 and delta-5 desaturation steps (Buzzi et al. 1996, 1997; Tocher et al. 2006; Vagner & Santigosa 2011). This also seems to be the case in this study given that the very important increase in LA and ALA concentrations of carp exposed to LO feeds (100% and 500% increase, respectively) was followed only by a moderate increase in EPA and DHA concentrations (~20%). Hence, dietary supply of EPA and DHA from zooplankton rather than bioconversion of C18 PUFA in supplementary feeds is more likely the main dietary source of EPA and DHA in carp exposed to LO-feeds, although ALA and LA were supplemented at high concentrations (52.5 mg and 72.5 mg LA fish−1 day−1, respectively). This argument is supported by the relatively high dietary contribution of zooplankton to carp in both ponds, as assessed by stable isotope mixing models (65–80%). Therefore, feeding supplementary fish feeds containing C18 PUFA-rich LO to common carp is unlikely to result in considerable conversion to long-chain PUFA-enriched fish within such a short finishing period.
Although previous fatty acid studies on common carp suggest that, compared with C18-PUFA, dietary C20–22 PUFA requirements for carp are low (Radunz-Neto, Corraze, Bergot & Kaushik 1996; Glencross 2009), this study shows a strong increase in C20–22 PUFA in carp exposed to FO within only 30 days. The additional supply of 17 mg EPA and 20 mg DHA fish−1 day−1 for 30 days from FO finishing diet to common carp doubled EPA and DHA concentrations in muscle tissues of carp compared with those exposed for 30 days to the LO finishing diet. In addition to the high accumulation of C20–22 PUFA in carp, it is evident that short-term supplementation of FO generally results in significantly higher PUFA concentrations compared with those fed on VO-enriched cereals. However, it is noteworthy that C20–22 PUFA accumulation may not necessarily be related to physiological requirements of carp. Absorption efficiency of dietary fatty acids in fish is known to increase with the degree of fatty acid unsaturation, chain length and the position of the first double bond (n-3 > n-6 > n-9 fatty acids; Francis, Turchini, Jones & De Silva 2007; Olsen et al. 1998). Therefore, we suggest that the lower accumulation of C20–22 n-3 PUFA in the LO treatment is primarily the result of limited dietary C20–22 n-3 PUFA supply along with low endogenous bioconversion rates of C18 PUFA, whereas higher dietary supply and potentially highly efficient uptake of dietary EPA and DHA supplied by FO feeds accounts for the rapid increase in EPA and DHA within the 30 days finishing period.
Total fatty acid concentrations were similar in carp of both ponds after being exposed for 180 days to the same supplementary VO-enriched cereal feeds, irrespective of carp body size or weight. When carp were exposed to LO or FO finishing diets for 30 days, fatty acid profiles of carp reacted selectively to their corresponding dietary fatty acids suggesting that increasing PUFA concentrations were strongly affected by diet quality. In particular, results of stable isotope analysis demonstrate that carp after being exposed to FO for 30 days retained less of this supplementary diet than of VO diet after 180 days, which strongly supports the argument that diet quality rather than quantity determines long-chain PUFA retention. We thus argue that the high PUFA concentrations of carp exposed to FO indicate that carp are able to rapidly increase their PUFA concentrations within a short period of time (30 days) after being reared on a natural pond diet mixed with supplementary VO-enriched cereals. Finally, these results suggest that such strong increase in highly desirable PUFA may potentially lead to increased fish performance (Bell et al. 1998), subsequently to higher fish quality with respect to enhanced fish production yields (Copeman et al. 2002), and to increased nutritional values for human consumption.
In conclusion, inducing a dietary shift from supplementary cereal-based feeds to long-chain PUFA-rich (e.g. FO based) feeds supplied as finishing diet strongly increases long-chain PUFA concentrations in common carp. Beneficial (from fish fitness and human nutrition perspectives) C20–22 PUFA are highly increased compared with carp exposed to a traditional VO-enriched cereal diet with a subsequent C18 PUFA-enriched (e.g. LO based) finishing diet. In contrast, using LO-based compound feeds, often considered as suitable alternatives to reduce use of marine resources in aquaculture nutrition, as a finishing diet mostly results in increased ALA concentrations in common carp. Finally, other omnivorous species used in today's aquaculture such as tilapia species may similarly benefit from these findings that short-term access to high-quality dietary lipids translates into higher lipid quality for farmed fish and subsequently fish consumption. Further research is required to keep a high and steady dietary supply of long-chain PUFA for human nutrition by farmed fish fed via sustainable supplements and/or feeding strategies.
Acknowledgments
We thank M. Watzka (University of Vienna) for stable isotopes analyses, Teichwirtschaft Kainz (Waidhofen/Thaya; Austria) for logistical help and cultivation of carp, and GarantTiernahrung™ (Pöchlarn; Austria) for providing compound feeds. This work was supported by the Austrian Science Fund (FWF project L516-B17) to MJK. We also thank the editor and two anonymous reviewers for their constructive comments, which helped us to improve the manuscript.




