Muscle activity as a key indicator of welfare in farmed European sea bass (Dicentrarchus labrax L. 1758)
Abstract
Groups of adult sea bass were reared at either low (10 kg m−3) or high (50 kg m−3) stocking densities respectively for 84 and 116 days. To monitor the red muscle activity, about 20 fish from both densities were surgically implanted with EMG (Electromyograms) radio transmitters, after EMG calibration during exhaustive swimming exercise (Ucrit test). Blood samples and morphometric measurements were also taken. EMG showed that the muscle activity of fish reared at 50 kg m−3 was on average twofold higher than fish kept at lower density. Cortisol was significantly more elevated at higher density and haemoglobin, haematocrit and RBCC (red blood cells count) showed the same trend, while lysozyme decreased. Patterns for glucose and lactate were less clear. The results showed that the contemporary use of functional (EMG) and physiological (haematological and biochemical) profiles could give a more comprehensive view of the fish status validating the diagnosis of fish stress induced by culture practices.
Introduction
The principles for a sustainable aquaculture industry which takes care of the farming impacts on ecosystem integrity and fish welfare, were firstly announced in the ‘Code of Conduct for Responsible Fisheries’ (FAO 1995). Thereafter, partly due to the rapid expansion of the sector, farmed fish welfare has received increasing attention as demonstrated by the number of studies and reviews on this subject (Duncan & Fraser 1997; Wendelaar Bonga 1997; FSBI 2002; Conte 2004; Turnbull, Bell, Adams, Bron & Huntingford 2005; Huntingford, Adams, Braithwait, Kadri, Pottinger, Sandøe & Turnbull 2006; Ashley 2007). The consequences of impaired animal welfare are not only important for ethical issues but also for benefits related to improved feed conversion, heightened immunity, flesh quality and ability to cope with stress (McFarlane, Cubitt, Williams, Rowsell, Moccia, Gosine & McKinley 2004; Ashley 2007).
Stress is not always synonymous with suffering or compromised welfare (Huntingford et al. 2006). On the one hand, it should be seen as an adaptive function that in the short term allows fish to preserve both single species and population (Huntingford et al. 2006). On the other hand, prolonged stress, often associated with elevated plasma cortisol, has been shown to decrease significantly the growth rates, as a result of a higher ‘cost of living’.
A common definition of animal welfare (ESFA 2009) is: ‘the welfare of an animal is its state as regards its attempts to cope with its environment’. However, measuring and establishing fish welfare is a complex issue for the difficulties in finding objective and practical indicators. Classical indicators comprise haematological (e.g. haemoglobin, haematocrit), biochemical parameters (i.e. cortisol, glucose, lactate) or behavioural indicators (i.e. aggressiveness, swimming or foraging), but their translation into fish welfare measures is quite difficult, especially if only a single indicator is considered (Huntingford et al. 2006; Ellis, Yildiz, López-Olmeda, Spedicato, Tort, Øverli & Martins 2012; Martins, Galhardo, Noble, Damsgård, Spedicato, Zupa, Beauchaud, Kulczykowska, Massabuau, Carter, Planellas & Kristiansen 2012). In contrast, the use of several non-redundant indicators might give a more comprehensive view of the fish physiological status provided that the signals from the different indicators are coherent.
Electromyograms record bio-electrical voltage changes and are proportional to the degree and the duration of muscle tension and to the energetic demand of the individual. EMG is useful to assess the amount of energy involved in swimming and living activities. In recent years, telemetry technology produced specific EMG-tag, useful for evaluating the fish activity and energy in response to the environmental conditions during free swimming under remote control (Cooke, Hinch, Wikelski, Andrews, Kuchel, Wolcott & Butler 2004). Rearing procedures at aquaculture facilities could be the possible sources of stress, responsible for enhancing the energy demand (Cooke, Chandroo, Beddow, Moccia & McKinley 2000) that, in turn, is reflected by changes in swimming behaviours (Martins et al. 2012). For this reason, data from electromyograms have been also used as a measure of swimming behaviour in captivity and as an indicator of the fish well-being (Cooke et al. 2000; McFarlane et al. 2004; Chandroo, Cooke, Mckinley & Moccia 2005; Lembo, Carbonara, Scolamacchia, Spedicato & McKinley 2007; Lembo, Carbonara, Scolamacchia, Spedicato, Bjørnsen, Holand & McKinley 2008).
In the aquaculture procedures, stocking density is still considered one of the critical and controversial points for fish welfare (Ellis, North, Scott, Bromage, Porter & Gadd 2002; Turnbull et al. 2005). The increase in stocking densities is used at aquaculture facilities to minimize production costs and maximize profits. Studies on rainbow trout have demonstrated a positive relationship between increasing densities and aggressive interactions with impairment of growing performances (Ellis et al. 2002). The impairment of the health status of farmed fish may, in turn, also affect the profitability of the aquaculture industry (Conte 2004; Ellis, Scott, North, Bromage & Turnbull 2004; Turnbull et al. 2005; North, Turnubull, Taylor, Ellis & Bromage 2006).
The main studies on density of fish welfare were primarily focused on salmonids (Ellis et al. 2002; Turnbull et al. 2005), while poor and controversial information were produced about sea bass (Di Marco, Priori, Finoia, Massari, Mandich & Marino 2008; Person-Le Ruyet & Le Bayon 2009; D'Orbcastel, Lemarie, Breuil, Petochi, Marino, Triplet, Dutto, Fivelstad, Coeurdacier & Blancheton 2010; Lupatsch, Santos, Schrama & Verreth 2010). The different stress responses of sea bass appear to be connected to the life stage and to the intensity and the duration of the stressor applied. In particular, there are evidences that sea bass larvae and fingerlings have a schooling behaviour and live at high population densities (Papoutsoglou, Tziha, Vrettos & Athanasiou 1998) up to 100 kg L−1 (D'Orbcastel et al. 2010), while in the adulthood, both confinement and high stocking density could generate a stress reaction (Vazzana, Cammarata, Cooper & Pariniello 2002; Person-Le Ruyet & Le Bayon 2009).
The aim of this study was to investigate the welfare condition of European sea bass. These were exposed to different stocking densities whose effects were assessed using a suite of physiological and haematological indicators, focusing on muscular activity, in a multi-indicator framework to diagnostic fish welfare. Indeed, a single validated welfare indicator is not available, whereas the benefits of using different indicators related to diverse aspects of animal welfare are commonly recognized (FSBI 2002). The relationships between the validated indicators (haematological, cortisol, glucose, lactate, lysozyme and muscle activity) are presented as an integrated approach for the assessment of sea bass welfare.
Materials and methods
Trials were conducted at two different densities of ~10 and ~50 kg m−3, defined respectively as low density (L) and high density (H), on a batch of adult sea bass obtained from a commercial hatchery (Panittica Pugliese S.p.A, Fasano, Italy). To avoid genetic influences the same fish were used in the density H trial and in the density L trial. Fish were individually weighed, at the beginning of the trials, and randomly allocated in three tanks (low density, about 400 fish of ~300 g) or in two tanks (high density, about 1200 fish of ~520 g) (Table 1), because fish number is not sufficient to guaranty a triplicate. Individual weight was also taken at the end of the trials. The trials were carried out in the same period (from March to June/July) of two consecutive years to avoid seasonal bias on the haematological and serological profiles (Kavadias, Castritsi-Catharios & Dessypris 2003). The specimens used for these two trials were in adulthood condition (Rodriguez, Zanuy & Carrillo 2001), thus possible differences in biological parameters can be considered as inter individual variations more than size-related effects. Lupi, Vigiani, Mecatti and Bozzi (2005), for example, did not find any significant effect of fish weight (fish size ranged between ~ 280–680 g) on glucose and haematocrit. Culture variables were kept under control, maintaining the same water exchange condition in the two experimental groups (four volumes per day). The oxygen concentration was maintained globally at saturation levels through Oxyguard 6 system that pumped pure oxygen when the concentration was reduced to the threshold limit value of 5 mg L−1. Moreover, water temperature was constant at 18°C and water salinity was at 34‰, the photoperiod was natural as fish were reared outdoors. Fish were fed 1% of the tank biomass using commercial feed (HQ MARE 18; Naturalleva, Verona, Italy) administered through two and four mechanical belt feeders per tank for density L and H respectively. Feed was supplied from 2 h after the dawn, until 2 h before the sunset. The broad feeding window and the number of feeding belts were intended to reduce the competition for feed among the fish in the tanks.
| Tanks | N° | Mean weight (g) | SE | Biomass (kg) | Density (kg/m3) | |
|---|---|---|---|---|---|---|
| Density L | ||||||
| Begin | 1 | 427 | 297.85 | 19.52 | 127.18 | 8.00 |
| 2 | 420 | 304.55 | 17.66 | 127.91 | 8.04 | |
| 3 | 411 | 311.95 | 17.85 | 128.21 | 8.06 | |
| End | 1 | 418 | 386.30 | 16.74 | 161.47 | 10.16 |
| 2 | 419 | 382.80 | 19.29 | 160.39 | 10.09 | |
| 3 | 414 | 399.00 | 23.46 | 165.19 | 10.39 | |
| Density H | ||||||
| Begin | 4 | 1187 | 547.01 | 14.68 | 649.30 | 50.43 |
| 5 | 1200 | 500.39 | 15.34 | 600.47 | 46.64 | |
| End | 4 | 1068 | 694.50 | 25.17 | 741.73 | 57.61 |
| 5 | 997 | 657.49 | 20.47 | 655.52 | 50.91 | |
For blood collection, two samplings were carried out on 10 specimens, at the beginning and at the end of the experiment for each tank and density.
EMG-tag technical features and surgical implantation
To remotely monitor fish red muscle activity, seven specimens per tank for density L and 10 fish per tank for density H were surgically implanted with CEMG-R11-25 (Lotek Wireless, Newmarket, Ontario, Canada) radio transmitters, that weighted 12 g in air (~3–4% of fish weight). The use of radio tags has some inconveniences related to the strong reduction in the radio signals in sea water. For this reason EMG signal was recorded in the following conditions: fibreglass tanks, short distances between the tanks and the receiver and shallow water (~ 1 m deep) (Cooke et al. 2000). The two gold tipped electrodes were positioned into the red muscle of the fish to decode the electromyographical (EMG) signals from each fish (Lembo et al. 2007, 2008). In the EMG-tag the voltage corresponding with muscle activity is rectified, summed and recorded over a 5 s period. Thus, the average value is transmitted every 5 s to a radio receiver in the form of a number, ranging from 0 to 50 (Cooke, Thorstad & Hinch 2004). In this way, it was possible to obtain information from each tagged fish swimming in the rearing tanks in real time. Specimens randomly selected for the surgical implantation of EMG radio tags were fasted 24 h before the surgical procedure (McFarlane et al. 2004). Fish were completely anaesthetized (stage 4: loss of reflex activity and no reaction to strong external stimuli, as reported by Iversen, Finstad, McKinley & Eliassen 2003) using 30 mg L−1 of clove oil (Lembo et al. 2007). The time to reach the stage 4 of the anaesthesia was about 5 min and during the surgery the gills were continuously irrigated with anaesthetic solution. The surgical implantation was performed according to Lembo et al. (2007). Briefly the EMG-tags were implanted into the peritoneal cavity through a 3 cm incision located 4–5 cm posterior to the pelvic girdle. The gold electrodes of the sensor were inserted in the red muscle band by mean of a hollow needle. The incision was closed with four independent sutures. The surgery lasted on average 5 min, followed by a recovery time of about 10 min. After that, all fish were recovered successfully.
Each tagged fish was treated with antibiotic injection (sodic-ampicillin–cloxacillin 1 mg kg−1 24 h−1) for 3 days after the surgery (Lembo et al. 2008). The fish resumed feeding 5 days after the surgery. Before releasing the animals, they were subjected to a critical swimming speed test (Ucrit) in a Blažka style swimming chamber (Carbonara, Scolamacchia, Spedicato, Lembo, Zupa & McKinley 2006) to calibrate the Ucrit with EMG signals (Fig. 1). Ucrit is a swimming test in which fish swim in a chamber at increasing speed step (0.1 m s−1 every 10 min) until the fatigue (Ucrit) is reached (Lembo et al. 2007). The calibration gives the possibility to correlate each single swimming level to an activity index expressed as the EMG level (McFarlane et al. 2004; Lembo et al. 2007, 2008). In particular, the EMG level at the Ucrit speed represents the threshold limit of the aerobic muscular activity (activity of red muscle) (Lembo et al. 2007). In this way, the EMG transmitter technology allows an evaluation of the activity based energetic expenditure (Cooke, Hinch et al. 2004) and consequently of the fish physiological state (Martins et al. 2012), indicating the relative cost of living for fish in its environment (Cooke, Thorstad et al. 2004). Each fish was subjected to at least two critical swimming trials to calculate the average EMG for each velocity step, in particular at the maximum critical swimming speed (Ucrit EMG). The calibration procedure was essential to evaluate the mean muscle activity of fish in the tanks, strictly connected with the activity based energetic expenditure (Cooke, Hinch et al. 2004; Lembo et al. 2007).
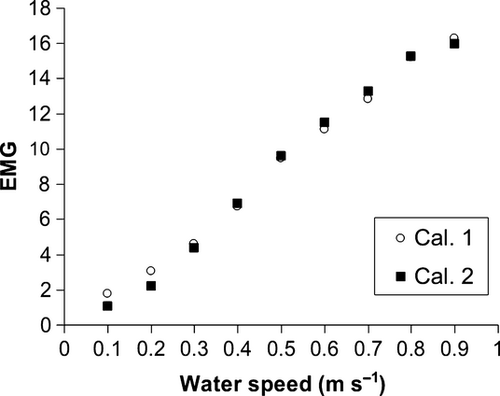
A daily monitoring was conducted for 84 days (April–June) in density L and for 116 days in density H (from April to June) due to the different duration of the tag batteries in the two experimental groups (Cooke, Hinch et al. 2004). The EMG signals were recorded using a radio receiver (SRX-400; Lotek Wireless), from 0900 a.m. until 0.5 h before the sunset. In this experiment, an indicator represented by the ratio between each registered EMG and the Ucrit EMG of each tagged fish was used to avoid the influence of size on the swimming performance (Carbonara et al. 2006) and to have an index of the level of activity of red muscle (aerobic activity) (Fig. 2).

Blood sampling and analytical determination
Thirty fish were randomly selected at the beginning and at the end of the experiment in both the two experimental groups (10 for each tank in the density L and 15 per tank in density H) for the determination of blood parameters. Fish implanted with the EMG-tag were not sampled for blood. Therefore, data are representative of mean population changes. Fish were individually caught from the tanks with a landing net and suddenly anaesthetized, in separate sedation tanks (50 L) using a 30 mg L−1 clove-oil solution. Blood was collected with a heparinized syringe (needle 23G) from the first branchial arch. The sampling procedure was the same for each fish during all experimentation to avoid the influence of methodological bias on the data analysis (comparison between two densities). The amount of blood drawn up was of about 0.5 mL. Every sample was divided into three aliquots of 5 μL for the haemoglobin determination and stored at −20°C. Five microliters were diluted in one millilitre of Hendrik's solution (Houston 1990) for the erythrocyte counts (RBCC) and stored at +4°C. Three microhaematocrit tubes for each sample were filled with blood. The remaining part of the sample was centrifuged at 2000 g for 5 min in order to obtain the plasma, stored at −80°C until the analysis of cortisol, lysozyme, glucose and lactate were performed.
Lysozyme concentration, an indicator of the functionality of the aspecific immune system, was measured using turbidimetric assay modified for microplate reader (Carbonara, Corsi, Focardi, Lembo, Rochira, Scolamacchia, Spedicato & McKinley 2010). The results were expressed as μg mL−1 HEWL (hen egg white lysozyme). Plasma cortisol concentration was expressed as ng mL−1 and measured by HPLC according to Carbonara et al. (2010). Haemoglobin (Hb) concentration was determined through cyanmethemoglobin method using a commercial kit (Sigma, St. Louis, MO, USA) and expressed as g dL−1. Haematocrit (per cent packed cell volume Hct) was determined using a heparinized microhaematocrit tube filled directly from the syringe needle and centrifuged (15000 g for 3 min) and read immediately after. Haematocrit value was expressed as percentage of red blood cells on whole blood volume. The red blood cells (RBCC) were counted in a Bürker counting chamber under a light microscope (Nikon 400E; Chiyoda, Tokyo, Japan) at 40× magnification and final concentration was expressed as number of cells 106 mm−3. Plasma glucose and lactate concentrations were determined using a commercial kit (Sentinel, Milan, Italy) based on the enzymatic–colorimetric Trinder reaction (GOD/PAP for glucose and PAP for lactate) and both expressed as mmol L−1.
Growth monitoring
In order to evaluate the growth performances of the specimens, the following parameters were calculated:
1 – SGR (specific growth rate) was calculated on replicates at population levels as follows:
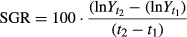
where 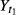 and
and 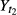 are, respectively mean total weights of fish at time t1 (time of fish in the tanks) and t2 (end of the experiment) expressed in days for each experimental group.
are, respectively mean total weights of fish at time t1 (time of fish in the tanks) and t2 (end of the experiment) expressed in days for each experimental group.
2 – FCR (food conversion ratio), calculated as:
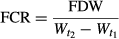
where, FDW is food dry weight expressed in grams, Wt is the fish mean weight at t times. Thus, the lower was the value the more efficiently the food was turned into fish flesh;
3 – PER (protein efficiency rate), calculated as:
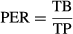
where, TB is the total biomass for each tank and TP the total proteins assumed. It was expressed as percentage;
4 – SR (survival rate), calculated as:

where, fisht2 is the final number of fish in the tank and fisht1 the initial number of fish per tank.
5 – CF (condition factor), calculated as:
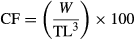
where, W is the mean weight of fish in a tank (g), and TL is the total length (cm).
Statistical analysis
The goodness of fit of a sigmoid model to the observed calibration data was tested using the Kolmogorov–Smirnov test. The ratio between each registered EMG and the Ucrit EMG, derived from the calibration, was calculated and averaged on a daily basis for each fish. To avoid possible bias, data from the last days of tag functioning were excluded from the analysis. All the ratios of individuals for each tank were pooled and used to calculate the cumulative ratio frequencies, grouped into 0.1 classes. A logistic model was then fitted to cumulative frequency curves and model parameters were estimated using regression analysis followed by a maximum likelihood estimation. The differences among the ogives obtained by tanks of the same density were compared through the Chen test (Chen & Paloheimo 1994). If differences were not significant, all the ratios of individuals from the same density were pooled and a logistic model was fitted for the fish reared at L and H densities. Also the two ogives (density L and H) were compared by Chen test. The ogive parameters representing the muscle activity at 25%, 50% and 75% of the Ucrit were thus estimated.
The comparison of the results of haematological and serological analyses within and between the experimental groups was accomplished using non-parametric tests, given the sample sizes: the Wilcoxon–Mann–Whitney (WMW test) and the Kruskal–Wallis test (R software) were applied respectively for L and H density.
Specific growth rate at population level was expressed as percentage and is reported as mean ± SE (standard error of the mean). SGRs, FCRs and PERs from the two densities were statistically compared with the Wilcoxon–Mann—Whitney non-parametric test (R software), introducing for the high density a third simulated sample. CFs from the two densities were compared with t-test.
Ethical considerations
All the fish manipulations (morphometric measurements, blood samples, surgical implantations) were performed on fish anaesthetized with a 30 mg L−1 clove-oil solution to minimize pains and discomfort on experimental animals and to prevent stress rise (Iversen et al. 2003). Survival after manipulations (morphometric measurements, blood sample, surgical implantations) was 100%.
Care and handling of fish were accomplished in accordance with the European Commission recommendations 2007/526/EU C2007 2525.
Results
Muscle activity associated with swimming
The level of the relative Ucrit EMG was estimated by the EMG-tag calibration (Fig. 1). The EMG calibration results were significantly fitted with a sigmoid model (Kolmogorov–Smirnov test, P > 0.05).
The cumulative frequency curves of EMG ratios from the same density were compared and did not show any significant difference (Chen test, Fobs < Fcrit; α = 0.05) (Fig. 3). Thus, data were pooled to compare curves from the two densities.
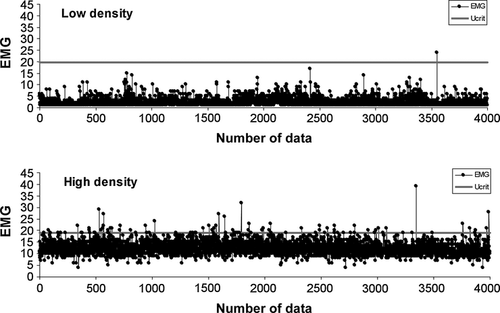
Parameters of the cumulative frequency curves of EMG ratios, estimated according to a logistic model (Table 2) and represent the muscle activity at 25%, 50% and 75% of the Ucrit. The comparison of the ogive parameters obtained from the two density trials, shows that in the density H the activity level at 50% of the Ucrit (Table 2 and Fig. 3) is about twofold higher than that in the density L. The analysis between the two density groups showed a significant difference between the two curves (Chen test, Fobs > Fcrit; α = 0.05), with H group exhibiting a decrease in the metabolic energy useful to cope with a further stress event. Fish from the highest density (50 kg m−3) swam generally closer to their Ucrit than fish reared at 10 kg m−3 (Fig. 2).
| Density | Activity 25% | Activity 50% | Activity 75% |
|---|---|---|---|
| L | 0.187 | 0.269 | 0.351 |
| H | 0.421 | 0.522 | 0.623 |
Haematological and serological parameters
The results of the haematological and serological analyses for both densities are reported in Fig. 4. No statistical differences were found among the replicates (tanks) of each density group (P > 0.05). All the measured biochemical parameters showed significant differences between the experimental groups at the first blood sampling except for glucose. In particular, all the oxygen transport descriptors (haematocrit, haemoglobin and erythrocytes concentrations) were significantly more elevated in the higher density group both at the beginning (Hb, P = 3.439E-5; Hct, P = 0.00105; RBCC, P = 0.0023) and at the end (Hb, P = 0.00010; Hct, P = 0.001891; RBCC, P = 0.00032) of the experiment, but the level resulted unchanged between the beginning and the end of the experiment within each densities (Hb-density L, P = 0.4464; Hb-density H, P = 0.5900; Hct-density L, P = 0.5145; Hct-density H, P = 0.4376; RBCC-density L, P = 0.3525; RBCC-density H, P = 0.9518). Lactate showed significant lower levels both at the beginning and at the end of the experiment in density H than in density L (lactate-begin, P = 0.0172; lactate-end, P = 0.0086), but the levels remained unchanged between the beginning and the end in each density (lactate-density L, P = 0.4203; lactate-density H, P = 0.8072).
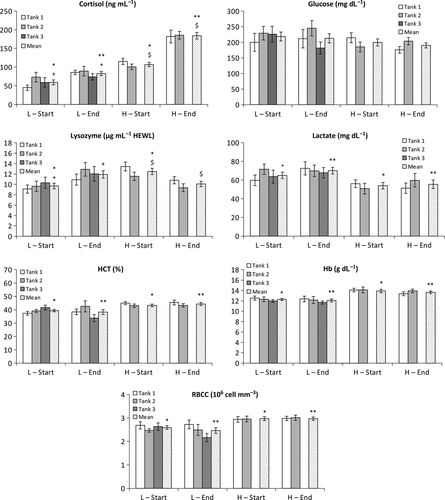
Regarding glucose levels, fish from the two densities showed the same levels both at the beginning (glucose-begin, P = 0.3695) and the end (glucose-end, P = 0.2399) of the monitoring period. Within each density, glucose levels were not significantly changing (glucose-density L, P = 0.82; glucose-density H, P = 0.229).
In contrast, cortisol levels were significantly different between densities (cortisol-begin, P = 1.662E-5; cortisol-end, P = 6.315E-13) and within each density (cortisol-density L, P = 0.0049; cortisol-density H, P = 3.223E-7; A significant increment of cortisol levels in both the densities was evident at the end of the experiment, moreover cortisol resulted significantly higher in the density H than in density L.
Lysozyme levels were significantly different at beginning between densities (lysozyme-begin, P = 0.0033) and within densities (lysozyme-density L, P = 0.0361; lysozyme-density H, P = 0.00067); in particular lysozyme concentration showed an increasing trend in the density L and a decreasing trend in the density H.
Growth performances
The main fish growth parameters used in the experiment are showed in Tables 3. The survival rate (SR) was higher in the low density experimental group (97.4% vs. 85.8%). Although in both the densities the condition factor (CF) was close to 1.1, in the high density group the mean CF value is significantly higher than the other (P < 0.05). The growth rates (SGR) of sea bass held at lower density (0.30 ± 0.007) were significantly higher (P < 0.05) than those of fish held at high density (0.17 ± 0.011). While the PER is lower in the high density group (P < 0.05), the FCR was greater for fish reared at the higher density (P < 0.05).
| Tanks | SGR | FCR | PER | SR (%) | CF |
|---|---|---|---|---|---|
| Density L | |||||
| 1 | 0.285 | 1.03 | 2.22 | 97.7 | 1.14 |
| 2 | 0.306 | 0.97 | 2.33 | 98.6 | 1.14 |
| 3 | 0.302 | 0.98 | 2.33 | 95.9 | 1.09 |
| Density H | |||||
| 4 | 0.191 | 3.21 | 0.70 | 91.6 | 1.21 |
| 5 | 0.151 | 3.18 | 0.71 | 80.1 | 1.17 |
| 6 | 0.166* | 3.20* | 0.70* | 86.7* | 1.19* |
- SGR, specific growth rate; FCR, food conversion ratio; PER, protein efficiency rate;SR, survival rate; CF, condition factor.
Discussion
Stocking density is one of the most important factors affecting fish welfare at aquaculture sites. A great inter-specific variability could be associated with the responses to stocking density. However, for many teleosts high density induces the increase in the energetic expenditure for basal life functions, that in turn could become detrimental for growth, immune-resistance, and could also affect the social interaction between fish (Huntingford 2004; Martins et al. 2012).
Literature lacks information about the growth performances on adult of sea bass as, in this stage. Moreover, it resulted pretty difficult to compare growth performances of this study with the most present in literature, as most of them were conducted in cages and therefore subjected to very different environmental conditions such as temperature and photoperiod. These two variables in particular, are known to influence strongly growth as food intake generally decreases in winter time and increase in spring/summer time (Kavadias et al. 2003).
Although the FCR and the PER values may indicate a worse ability of the animals held at the higher density to convert food (and in particular proteins, that account as a major component in fish muscle), the condition factor, a bit lower in the high density, on the contrary, is not indicative of a particular compromised situation. Fish growing rate decreases progressively in time with decreasing PER and SGR and increasing FCR values. Because of the link between FCR, PER and SGR with the growth scale factor, these zoo-technical parameters, in our experimental conditions, are probably not completely reliable to describe the effects of the density on fish growth. In any case, these parameters contribute all together, with the others parameters studied in this experiment, to give a more comprehensive description of physiological response to density.
From a physiological point of view, the experiment shows that high density condition increases red muscle activity leading to a rise of the global scope for activity (Lembo et al. 2007), defined as the increase in oxygen uptake in a swimming fish (Fry 1971). At density H fish muscle activity was on average twofold higher than one in density L (lower density). The value of EMG at maximum critical swimming, derived from the Ucrit calibration, represents a threshold limit of red muscle energy expenditure, therefore the maximum sustainable aerobic activity for a fish (Plaut 2001; Carbonara et al. 2006). This threshold limit allows the measurement of the energy fraction used versus the total amount of the available energy. The higher red muscle activity, close to the Ucrit, recorded in fish reared at 50 kg m−3 is linked also to a higher use of the anaerobic substrates, which represent the reserve energies (Lembo et al. 2007). Less availability of anaerobic energetic reserves has consequences on the reactivity of stress systems rather than on the level of the stress parameters, reflecting on a reduced ability for the fish to compensate a further stress event (Korte, Olivier & Koolhaas 2007). In this sense, the measure of muscle activity could be considered an index of well-being condition. The lower SGR observed at 50 kg m−3 might be the consequence of a higher energetic expenditure (Lupatsch, Kissil, Sklan & Pfeffer 1997; Montero, Izquierdo, Tort, Robaina & Vergara 1999; Attia, Millot, Di-Poi, Bégout, Noble, Sanchez-Vazquez, Terova, Saroglia & Damsgard 2012) and of the higher cortisol level (Barton, Schreck & Barton 1987).
The most significant result is that for the first time EMG (activity levels) has been used to predict the activity based energy consumption (Cooke, Hinch et al. 2004), expressed as the fraction of the maximum aerobic activity (Ucrit), and so to estimate stress-related effects of stocking density in European sea bass. Significantly, different activity levels were found in the two tested densities, providing insights into relative energetic strategies and their metabolic consequences, such as the increase in the cortisol levels.
Haematological parameters are indicators of fish oxygen demand to maintain the basal metabolism. Hct, Hb and RBCC were significantly different between the two densities (both at the beginning and the end of the trials), generally showing the higher levels in the density H. Haemoconcentration is reported as a strategy for increasing oxygen carrying capacity of blood during periods of high energy demand (Houston 1990), such as a stress event or an important swimming activity. In the European sea bass, an acute stress does not seem to influence the haematological parameters (Carbonara et al. 2010), while significant haematological changes are reported to be affected by long term changes in environmental parameters. Indeed, the haematological parameters are reported to be very sensitive to intrinsic and extrinsic factors such as sexual maturity, feeding regime, exposure to organic and inorganic compounds, temperature, salinity, dissolved oxygen and photoperiod (Messanger, Spéphan, Quentel & Laurencin 1992; Roche & Bogè 1996; Kavadias et al. 2003; Lupi et al. 2005). Basically, the modulation of haematological parameters is strictly connected to the ecthotermic condition of fish, which reflects the wide range of values reported in the literature (Houston 1990). In any case the values obtained are comparable with the higher values reported in the literature on Hct (Kavadias et al. 2003; Caruso, Genovese, Maricchiolo & Modica 2005), RBCC (Hadj Kacem, Aldrin & Romestand 1986; Messanger et al. 1992) and Hb (Caruso et al. 2005; Carbonara et al. 2010).
Cortisol levels reported in literature as ‘control’ or ‘unstressed’ show a high variability (from 10 to 600 ng mL−1; reviewed by Ellis et al. 2012). This wide range of ‘basal’ values could be due to external factors as differences in experimental conditions, sampling methods, anaesthetic used, manipulation procedures, analytical techniques (Ellis et al. 2012) and internal factors (circadian and seasonal rhythms) (Planas, Gutierrez, Fernandez, Carrillo & Canals 1990). The values found in this study appear closer to the smaller value present in the literature (Ellis et al. 2012). Moreover, a significant increase in cortisol levels between the beginning and end of the trial was also detected in each density. The differences in cortisol concentration between the two experimental groups seem to be directly related with the increased rearing density. Indeed, there is evidence that, between certain limits, the cortisol concentration increases proportionally with the stress levels, just before down-regulation control and saturation of the cortisol receptors occur (Mommsen, Vijayan & Moon 1999; Carbonara et al. 2010; Ellis et al. 2012). These trends are comparable with those reported by Vazzana et al. (2002) for adult sea bass after a 48 h confinement, although Di Marco et al. (2008) did not found significant differences in cortisol levels for sea bass reared at different densities for 6 weeks, while a longer exposure (3 months) to high stocking density (80–100 kg m−3) induced a significant increase in plasma cortisol in juvenile sea bass (Terova, Gornati, Rimoldi, Bernardini & Saroglia 2005). So the response of the cortisol concentration seems be influenced by the duration of the stress stimuli and by the life stage as well. In any case, the cortisol plays a pivotal role in many physiological process in fish (i.e. glucose metabolism, energetic metabolism, osmoregulation function), but its elevation may be an adaptive response for positive and negative feeling in fish (Ellis et al. 2012). In itself, the elevation cortisol is an indicator of the physiological stress and affected function, but cannot give indication on the welfare state.
Glucose release in blood is generally associated with the secondary stress response being modulated by the action of cortisol which influences glucidic metabolism in fish (Mommsen et al. 1999). Also, environmental factors modulate plasmatic glucose levels such as photoperiod (Kavadias et al. 2003), dissolved oxygen and temperature (Lupi et al. 2005). Mommsen et al. (1999) reported that plasmatic, hepatic and muscular glucose levels in teleosts and in particular in carnivorous species, such as sea bass, may not be univocally correlated with the stress condition (cortisol level). The glucose levels measured in this study were comparable with some results of literature (Caruso et al. 2005; Lupi et al. 2005; Roncarati, Melotti, Dees, Mordenti & Angelotti 2006). Glucose did not show any significant variation between the two densities and within each density tested in this experiment. This finding is in accordance with the results by Di Marco et al. (2008) for juvenile sea bass while an increase in glucose is reported by Vazzana et al. (2002) in adult sea bass.
Increments of blood lactate have been generally reported as a secondary effect of the stress (Montero et al. 1999; Caruso et al. 2005), whereas in the present study a decrease in lactate was found. Such suppression of the acute-like response to the stress in chronic exposition are probably caused by a down-regulation of adrenocorticotrophic hormone (ACTH) or cortisol receptors as reported in several studies (Wendelaar Bonga 1997; Barton, Ribas, Acerete & Tort 2005).
Plasmatic lysozyme is considered one of the principal indicators of the non-specific immune system (Balfry, Maule & Iwama 2001). Cortisol seems to have an active role in the modulation of the non-specific immune system of teleosts. Acute stress stimuli seem to enhance the non-specific response, while chronic stressors seem to depress this kind of immunological reaction (Weyts, Cohen, Flik & Verburg-Van Kemenade 1999), increasing the susceptibility of fish to disease, depressing the phagocytic activity of the head kidney macrophages and decreasing the spleen-mass index (Wang, Li, Cai & Wang 2005). Little fluctuations in cortisol levels do not induce significant variations in the immunitary response, while wider plasmatic cortisol elevation could cause chronic depressive effect on the immunitary system. The observed pattern of lysozyme changes seems consistent with the depressive effect of high and prolonged exposure (chronic) to circulating cortisol in density H. The same depressive effects on lysozyme concentration was reported in specimens of grass carp injected with cortisol in coconut oil (chronic elevation effect), while the cortisol in physiological water (acute elevation effect) injected fish didn't influenced the lysozyme level (Wang et al. 2005). However, the role of cortisol as a regulator of fish immune system has not been completely clarified and in this way it seems that the type, intensity and duration of the stressor applied may play an important role (Mommsen et al. 1999).
Rotllant, Ruane, Caballero, Montero and Tort (2003) showed, in D. labrax, how the raise of the plasmatic cortisol levels suddenly occurs after a stress event. This happens because the hormone is stored into the inter-renal cells of the head kidney. Anaesthesia could also have affected these responses, indeed clove oil as anaesthetic is reported to be a strong blocker of the cortisol increase in some species (Small 2003; Holloway, Keene, Noakes & Moccia 2004), but in some others did not act as such (Martìnez-Porchas, Martìnez-Còrdoba & Ramos-Enriquez 2009). The sampling procedures or the anaesthetic adopted has probably influenced the absolute values of both serological and haematological parameters in each group. However, the differences caused by the diverse density conditions can be detectable in the two groups observed.
Nine different parameters were used to describe fish physiological state during these trials. The higher muscular activity of fish kept at higher density was associated with the cortisol elevation, the rise of the RBCC and, in turn, of the haematocrit and haemoglobin concentrations, the decrease in lysozyme levels and growth performances, all giving a consistent description of the lower welfare condition of fish maintained at ~50 kg m−3.
However, haematological and serological parameters in fish lack of both normal and threshold reference levels useful to univocally diagnose the impaired welfare (Houston 1990). According to this, single measures of blood parameters have a high level of uncertainty and can be only used in a comparative way.
In a perturbed condition, a more metabolic energy mobilization occurs to cope with the stress event, allowing fish to escape from a predator or a polluted or overcrowded environment. The synergic use of the stress (cortisol, glucose, lactate) and functional (EMG) indicators gives a more comprehensive metabolic description of fish condition and behaviour. Assessments of individual and group behaviour can be used as an operational on-farm welfare indicator and it is what most farmers use daily to evaluate the hunger, stress level and health status of fish (Martins et al. 2012).
The complementary use of functional (e.g. growth) and physiological (haematological and biochemical) profiles could give a more comprehensive view, validating the diagnosis of fish stress induced by culture practices. In addition, an indicator as EMG, could better represent the integrated response of the whole fish organism to stress conditions. Furthermore, the possibility of estimating, by means of EMG, the amount of energy reserves (anaerobic metabolism) that fish could use to cope with stress (Lembo et al. 2007) may help to give insights into the boundary between eustress and distress in fish, highlighting that to reach a new equilibrium state (allostasis) is crucial to a good animal welfare (Korte et al. 2007; Lembo & Zupa 2010).
Acknowledgment
This study was funded by the Regional Government of Apulia (Italy).




