Effects of dietary magnesium on the growth, carapace strength and tissue magnesium concentrations of soft-shelled turtle, Pelodiscus sinensis (Wiegmann)
Abstract
A feeding trial was conducted to evaluate the effects of dietary magnesium on the growth, carapace strength, tissue and serum Mg concentration of soft-shelled turtles, Pelodiscus sinensis (Wiegmann). Juvenile soft-shelled turtles of approximate 5.4 g body weight were fed diets with seven levels of Mg (48, 206, 369, 670, 955, 1195 and 1500 mg Mg kg−1) for eight weeks. No significant difference (P ≥ 0.05) was found in weight gain (WG), feed conversion ratio or protein efficiency ratio among treatments. However, the WG of turtles continued to increase with increasing dietary Mg levels up to 670 mg kg−1, beyond which the WG levelled off. The plasma alkaline phosphatase activity and the muscle, bone Mg concentrations of the turtles increased with the increasing dietary Mg levels between 48 and 955 mg kg−1, beyond which the tissue Mg concentrations remained relatively constant. Furthermore, the carapace strengths of turtles fed with the control diet of 48 mg Mg kg−1 were significantly weaker (P < 0.05) than that of turtles fed with diets containing higher Mg levels. Based on a broken-line modelling analysis, the required dietary Mg level for the optimal WG of juvenile soft-shelled turtles was estimated to be approximately 650 mg kg−1. By contrast, the required dietary Mg levels for turtles to reach the optimal muscle and bone Mg concentrations were 1050 and 1000 mg kg−1 respectively. The required dietary Mg level for maximal alkaline phosphatase activity was approximately 980 mg kg−1.
Introduction
Soft-shelled turtles, Pelodiscus sinensis (Wiegmann), have long been a delicacy and have also been used neutraceutically in China and other Asian countries to address human health issues such as hypertension, menopause, diabetes, anaemia etc. The consumption of ground dehydrated turtle bodies or spray-dried soft-shelled turtle eggs has been shown to enhance the immune system of mice, inhibiting the growth of certain types of tumours (Feng, Yamazaki, Matsuki & Saito 1996; Yu, Li, Wang, Zheng & Yan 2005). Due to its high nutritional value and high economic profits, the soft-shelled turtle has been an important aquaculture species in Asia for decades. The nutrient requirements for this species, including protein (Nuangsaeng & Boonyartapalin 2001), methionine (Huang & Lin 2002), calcium (Huang, Lin & Wu 2003),vitamin E (Huang & Lin 2004), lipid (Huang, Lin & Chu 2005), iron (Chu, Chen & Huang 2007), copper (Wu & Huang 2008) and zinc (Huang, Chen & Huang 2010) have been well established.
Magnesium (Mg) is another major element in animal bodies, essential for the growth of bone tissues, the metabolism of nucleic acids, carbohydrates, lipids and proteins, as well as the balance and ion-exchange of the biological systems (Davis & Gatlin 1996; Lall 2002; Vormann 2003). Dietary Mg supplement is not critical for marine species (Davis & Gatlin 1996; Ye, Tian, Mai, Yang, Niu & Liu 2009) due to an abundant supply of this element in the seawater. For freshwater species, however, dietary Mg supplementation is often necessary due to its scarcity in the freshwater (Gatlin, Robinson, Poe & Wilson 1982; Shearer 1989; Dabrowska, Meyer-Burgdorff & Günther 1991; Lin, Ku & Shiau 2013). The Mg requirements of fresh water soft-shelled turtle must therefore come from its diets. This study evaluates the effects of dietary Mg on the growth, tissue Mg status and carapace strength of soft-shelled turtles and determines the dietary Mg requirement of soft-shelled turtles.
Materials and methods
Juvenile Chinese soft-shelled turtles were fed a basal diet (Table 1) that meets the dietary requirements for soft-shelled turtles (Chu et al. 2007; Wu & Huang 2008; Huang et al. 2010; Chen & Huang 2011; Huang & Huang 2012) for eight weeks. Mg sulphate (MgSO4) obtained from Mallinckrodt Baker (Phillipsburg, NJ, USA) was premixed with cellulose and then mixed with regular food ingredients to create a control and six treatment levels with supplementary Mg of 0, 150, 300, 600, 900, 1200 or 1500 mg Mg kg−1. Atomic absorption spectral (AAS) analysis revealed that the Mg contents were 48, 206, 369, 670, 955, 1195 and 1500 mg kg−1 for feeds supplemented with 0, 150, 300, 600, 900, 1200 or 1500 mg Mg kg−1 respectively. Clean rearing water in which animals would be kept was sampled and analyzed using AAS for Mg concentration weekly. The Mg concentration of the rearing (pond) water was 13.7–14.7 mg L−1. Feed ingredients were mixed in a Kitchen Aid multi-function mixer (Whirlpool, St. Joseph, Michigan, USA). Water was added to the feed in a 1:2 (v/w) ratio to produce feed dough, which was then pressed to form spaghetti-like strings. The finished strings were broken, freeze-dried, and then stored in a −20°C freezer until the time of feeding. The mean proximate composition of the diet was 11% moisture, 40% crude protein, 5.3% crude lipids and 3.8% ash.
| Ingredient | (g kg−1) |
|---|---|
| Casein | 420 |
| α-starch | 200 |
| Dextrin | 150 |
| Lipidb | 60 |
| Vitamin premixc | 20 |
| Mineral premix (Mg-free)d | 50 |
| Attractante | 60 |
| Cellulose + Mgf | 40 |
- a Casein, α-starch, dextrin, fish oil are products of Sigma Chemicals (St. Louis, MO, USA).
- b Lipid source is a 2:1 blend of soybean oil (Uni-President, Tainan, Taiwan) and fish oil (Sigma Chemicals).
- c One kg vitamin premix contains 500 000 IU vitamin D3, 1 000 000 IU vitamin A, 7.8 g vitamin K3, 10.0 g tocopheryl acetate, 4.0 g thiamine-HCl, 6.25 g riboflavin, 10.2 g calcium pantothenate, 20.1 g niacin, 30.0 g biotin, 4.0 g pyridoxine-HCl, 1.9 g folic acid, 1.0 g B12, 5.1 g B1, 204 g inositol, 71.4 g ascorbyl-monophosphate-Mg and 628 g choline chloride. All vitamin ingredients were obtained from Nice Garden (Taipei, Taiwan).
- d One kg mineral premix contains 134 g calcium phosphate, 476 g calcium lactate, 13.2 g ferric citrate, 240 g potassium phosphate, 87.2 g sodium phosphate, 43.5 g sodium chloride, 0.15 g aluminium chloride hexahydrate, 0.15 g potassium iodine, 0.37 g cupric sulphate pentahydrate, 0.8 g manganese sulphate monohydrate, 1.0 g cobalt chloride hexahydrate, 4.41 g Zinc sulphate heptahydrate, 0.023 g sodium selenite.
- e Attractant contained 7.4 g L-arginine, 6.7 g glycine, 1.7 g L-histidine-HCl, 4.4 g L-isoleucine, 3.6 g L-leucine, 2.2 g L-methionine, 4.7 g cysteine, 4.1 g L-phenylalanine, 0.5 g L-tyrosine, 1.3 g tryptophan, 3.4 g L-valine and 20 g betaine. All components were products of Sigma Chemicals.
- f Magnesium sulphate (MgSO4) was mixed with cellulose before adding to the diet at Mg levels of 0, 150, 300, 600, 900, 1200 and 1500 mg kg−1 diet at the expense of cellulose.
Fertilized soft-shelled turtle eggs were incubated in our laboratory at 30°C for approximately 45 days until hatch. Juvenile turtles were acclimated to laboratory conditions for 1 week and fed the basal diet with no supplemented Mg. Animal care conditions were similar to those described elsewhere (Chu et al. 2007). Following the acclimation period, healthy turtles, with a mean initial weight of 5.4 g, were selected and randomly assigned to 140 plastic cylindrical containers (18 cm deep × 20 cm high). Individual turtles were kept in separate containers to prevent injuries from fighting. A total of 140 individuals were randomly assigned to the seven treatment levels, resulting in 20 turtles for each treatment level. To standardize the activity level required by turtles to respire, the depth of the water in each container was adjusted according to the height of each individual turtle. All animal containers were kept indoors at 30 ± 1°C for the entire trial period.
Turtles were fed 3% of their body weight once daily at 17:00 for eight weeks. This feed ratio was determined based on observations made during the acclimation period and the amount of feed given could be consumed within 1 h. The water in each container was replaced and the containers cleaned each morning. The body weights of individual turtles were measured every two weeks, and the daily feed allowance of experimental animals was adjusted according to their measured body weights.
To measure the strength of the turtle carapace, we ran a simple physical procedure using a Rheo Tex rheometer (Sun Scientific, Tokyo, Japan) equipped with a spherical probe (Chou & Huang 2013). The turtles were placed on the platform of the instrument. The spherical shaped probe was directed at 1/3 the length of the carapace measured from the front edge. A force of 200 g was then applied onto the carapace through the probe. Distance (mm) travelled by the probe pressed into the carapace was measured automatically by the rheometer. The datum was then converted to g-force mm−1 – the force required to push down the shell per mm. A greater force required by the probe to travel one mm distance indicates a stronger carapace.
At the end of the feeding trial, all turtles were sacrificed by decapitation. The growth performance of the turtles was determined by the relative weight gain (WG), feed conversion ratio (FCR), protein efficiency ratio (PER) and survival; the calculation of each is shown as follows: WG (%) = 100 (Final body weight − Initial body weight)/Initial body weight; FCR = Total feed fed (g)/weight gain (g); PER = weight gain (g)/total protein fed (g); Survival = 100 (final turtle number/initial turtle number).
Turtles from each treatment level were randomly assigned to one of the four composite groups of 4–5 turtles each. Four composite samples were used for each dietary treatment. Data and samples from individuals within the same composite group were lumped together. Blood samples were drawn from the neck and collected in BD Vacutainers (Brand, Franklin Lakes, NJ, USA) containing 68 USP of sodium heparin. Plasma, muscles and livers from within each composite group were separately taken, blended and stored at −80°C for further analysis. Moisture, crude protein and ash levels were determined by following standard methods (AOAC 1984). Crude protein was determined by the Kjeldahl procedure using a Tecator Kjeltec System (Foss, Hoganas, Sweden). Crude lipids were extracted using the method of Folch, Lees and Sloane-Stanley (1957). Alkaline phosphatase (ALP) activity of plasma was determined using a commercial kit (Merck, Darmstadt, Germany). ALP catalyzes the hydrolysis of excess monoester phosphate in the kit. The residue monoester phosphate was measured at 405 nm in a spectral photometer to obtain the activity of ALP.
To measure the tissue and dietary Mg, samples were digested in a microwave sample preparation system (Anton Parr, Graz, Austria) and Mg contents were analyzed using atomic absorption spectroscopy (AAS) equipped with a graphite furnace (AA-240Z, Varian, Australia).
Statistical analyses and graphics were performed with Sigma Stat and Sigma plot software ( SPSS, Chicago, IL, USA). One-way analysis of variance (Steele, Torrie & Dickey 1997) was used to determine differences among treatment means at the 5% significance level followed by Duncan's new multiple range comparison for difference between treatment means. The required dietary Mg levels derived from WG, plasma ALP activity, muscle and bone Mg were estimated by a broken-line regression model.
Results
No visible signs of abnormality were observed for turtles reared on different treatment diets. No mortality was observed in turtles fed diets supplemented with Mg. However, a 15% mortality was observed in turtles fed control diet containing 48 mg kg−1 endogenous Mg from feed ingredients (Table 2). The weight gain of the soft-shelled turtles generally increased with the increasing dietary Mg level up to approximately 670 mg Mg kg−1 diet and then levelled off beyond this level.
| Dietary Mg (mg kg−1) | IW (g) | FW (g) | WG (%) | FCR | PER | Survival (%) |
|---|---|---|---|---|---|---|
| 48 | 5.4 ± 0.1 | 13.3 ± 0.6 | 144 ± 12 | 2.02 ± 0.12 | 1.44 ± 0.09 | 85 |
| 206 | 5.5 ± 0.1 | 13.1 ± 0.5 | 140 ± 8 | 2.02 ± 0.09 | 1.45 ± 0.06 | 100 |
| 369 | 5.4 ± 0.1 | 14.7 ± 0.7 | 170 ± 12 | 1.84 ± 0.14 | 1.67 ± 0.12 | 100 |
| 670 | 5.4 ± 0.1 | 15.1 ± 0.7 | 179 ± 12 | 1.70 ± 0.13 | 1.81 ± 0.13 | 100 |
| 955 | 5.4 ± 0.1 | 15.3 ± 0.8 | 181 ± 15 | 1.67 ± 0.11 | 1.75 ± 0.10 | 100 |
| 1195 | 5.4 ± 0.1 | 15.1 ± 0.7 | 182 ± 14 | 1.68 ± 0.11 | 1.76 ± 0.10 | 100 |
| 1500 | 5.4 ± 0.1 | 15.0 ± 1.0 | 177 ± 16 | 1.81 ± 0.11 | 1.63 ± 0.10 | 100 |
- There was no significant difference among means ± SE of treatments (n = 17 or 20, P ≥ 0.05).
Moisture, crude protein and lipid of turtle muscles showed no difference among different dietary Mg levels (Table 3). Muscle ash contents were significantly higher for turtles fed diets containing 48, 1195 and 1500 mg Mg kg−1.
| Dietary Mg (mg kg−1) | Moisture (%) | Crude protein (%) | Lipid (%) | Ash (%) |
|---|---|---|---|---|
| 48 | 78.4 ± 0.9 | 15.9 ± 0.3 | 9.4 ± 1.0 | 0.54 ± 0.00ab |
| 206 | 76.0 ± 1.9 | 16.3 ± 0.5 | 8.9 ± 0.9 | 0.48 ± 0.02b |
| 369 | 77.4 ± 0.3 | 15.2 ± 0.3 | 9.9 ± 0.3 | 0.47 ± 0.04b |
| 670 | 75.1 ± 0.3 | 16.1 ± 0.5 | 8.2 ± 0.3 | 0.49 ± 0.03b |
| 955 | 75.3 ± 0.5 | 15.7 ± 0.6 | 8.0 ± 0.3 | 0.48 ± 0.06b |
| 1195 | 75.1 ± 0.5 | 16.3 ± 0.6 | 10.1 ± 1.1 | 0.56 ± 0.00ab |
| 1500 | 76.4 ± 0.3 | 16.2 ± 0.3 | 10.1 ± 0.6 | 0.61 ± 0.02a |
- Within a column, means ± SE sharing different superscript are significantly different (n = 4, P < 0.05).
The concentration of Mg in muscle, bone and plasma of turtles increased with increasing dietary Mg between 48 and 670–955 mg kg−1, beyond which tissue Mg level stabilized (Table 4). Both the carapace strength and the plasma ALP activity were the lowest for turtles fed 48 mg Mg kg−1 (control) diet (Table 5). The carapace strength and plasma ALP activity of the turtles reached the highest level when fed dietary Mg level of 1195 and 955 mg kg−1 diet respectively.
| Dietary Mg (mg kg−1) | Muscle (μg g−1) | Bone (μg g−1) | Plasma (μg mL−1) |
|---|---|---|---|
| 48 | 139.5 ± 3.6b | 2070 ± 36c | 36.55 ± 3.49b |
| 206 | 147.1 ± 5.3b | 2059 ± 37c | 33.72 ± 0.97b |
| 369 | 147.5 ± 5.0b | 2142 ± 26c | 35.67 ± 5.11b |
| 670 | 162.5 ± 3.1a | 2372 ± 35b | 47.20 ± 0.92a |
| 955 | 174.3 ± 5.4a | 2572 ± 54a | 49.79 ± 2.17a |
| 1195 | 173.3 ± 3.5a | 2576 ± 122a | 42.66 ± 2.43ab |
| 1500 | 176.2 ± 4.2a | 2570 ± 25a | 35.19 ± 1.43b |
- Within a column, means ± SE sharing different superscript are significantly different (n = 4, P < 0.05).
| Dietary Mg (mg kg−1) | Carapace strength (g-force mm−1) | ALP (unit L−1) |
|---|---|---|
| 48 | 134 ± 7c | 590 ± 65b |
| 206 | 171 ± 13b | 618 ± 34b |
| 369 | 174 ± 14b | 696 ± 26b |
| 670 | 187 ± 10ab | 716 ± 54ab |
| 955 | 177 ± 14b | 837 ± 44a |
| 1195 | 227 ± 18a | 754 ± 36ab |
| 1500 | 157 ± 10b | 627 ± 20b |
- Within a column, means ± SE sharing different superscript are significantly different (n = 17 or 20, P < 0.05).
The dietary Mg requirements of juvenile soft-shelled turtles for maximal WG estimated by a broken-line modelling were approximately 650 mg kg−1 (Fig. 1). Meanwhile, the dietary Mg levels for turtles to reach the maximal bone and muscle Mg concentration were 1000 and 1050 mg kg−1 respectively. The dietary Mg level for the maximal ALP activity was approximately 980 mg kg−1.
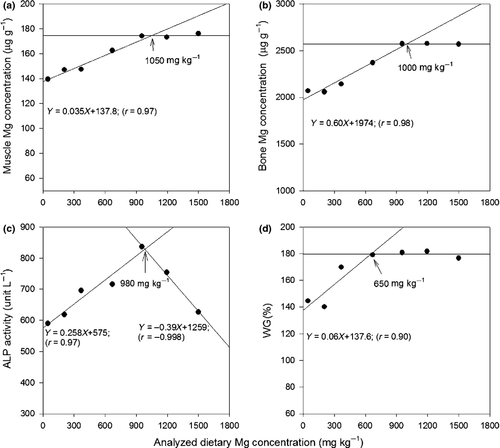
Discussion
Estimating dietary requirements based on the dose-response growth is normally performed with a regression modelling (Shearer 2000). Therefore, the WG in soft-shelled turtles did not always show significant differences among treatment levels (Wu & Huang 2008; Chen & Huang 2011). Moreover, a feeding period of eight weeks may not have been sufficiently long enough to show a significant difference in the WG. It is not clear how long the feeding period would be required to show a significant difference in the growth of soft-shelled turtles.
The regression model was more suitable as a tool to identify the dietary requirement based on the WG values provided the sample size is adequately large. Therefore, a maximal sample size that could be handled in our lab was used in the trial (n = 20). Although the WG among treatments were not significantly different, a broken-line analysis was able to identify the dietary Mg requirement for the growth of turtles based on the WG. For this, a dietary Mg level of 650 mg kg−1 was established as the requirement. Yet, this value is somehow lower than the dietary Mg level required for optimal bone and muscle Mg concentrations (1000 and 1050 mg kg−1 diet respectively). It has been noted that the WG is often a poor indicator for the dietary mineral requirement as the negative impact of mineral deficiency on growth may take a long time to appear. Other indicators such as tissue and serum mineral contents are preferred for estimating the dietary requirement of different nutrients (Shearer & Åsgård 1992; Wilson & El Naggar 1992; Maage & Julshamn 1993; National Research Council (NRC) 2011). Therefore, the required dietary Mg level based on the bone and muscle Mg concentration may be a better estimate in the present study.
Dietary requirements reported for terrestrial and aquatic animals vary greatly. Terrestrial animals obtain Mg mainly through their dietary intakes, particularly from plant sources. Feed stuffs derived from plant source normally contain good amounts of Mg, as Mg is a constituent of chlorophyll. For marine aquatic animals, water-borne Mg is a major source. Thus, fish reared in sea water are not likely to require dietary Mg supplementation (Ye et al. 2009; Lin et al. 2013). In contrast, fish reared in freshwater in general require dietary Mg supplementation, unless the water contains high amount of Mg. For example, a rearing water containing 46 mg Mg L−1 meets the requirement of rainbow trout that was fed a diet containing 78 mg kg−1 endogenous Mg (Shearer & Åsgård 1992). Soft-shelled turtles acquire minerals through their diet and the surrounding water. The semi-purified basal diet used in the present study (for the control group) contained 48 mg of Mg kg−1. The rearing water from the tap water system contained about 14 mg Mg L−1. Our results indicated that this level of Mg failed to meet the Mg requirement of soft-shelled turtles.
Magnesium deficiency causes imbalance in ion ratios such as calcium and sodium, resulting in calcium sedimentation in soft tissues. This probably explains why the high muscle ash content of turtles fed 48 mg Mg kg−1 diet (Table 3). These results coincide with reports for both terrestrial and aquatic animals in that the deficiency of dietary Mg will cause calcium sedimentation in organs, muscle, bone or kidney (Morris & O'Dell 1961; Bunce, Sauberlich, Reeves & Oba 1965; Dabrowska et al. 1991; Lim & Klesius 2003).
Magnesium plays important roles in enzyme stimulation, and is involved in more than 300 enzymatic reactions in biological systems. The deficiency of Mg will result in reduced ALP activity as Mg is involved in the active site of ALP (Stec, Holtz & Kantrowitz 2000). This is well supported in the present study, shown through the enhanced plasma ALP activity as the dietary Mg level increased up to 955 mg kg−1. As a result of increasing ALP activity with increasing dietary Mg level, the carapace strengthened. An important physiological function of ALP is to catalyze the bone generation and mineralization. ALP activity increases during the development of bone formation (Coleman 1992; Nakamura, Noda, Shimpo, Oikawa, Kawasaki & Hirashita 2004). In this study, the plasma ALP activity of the soft-shelled turtles showed the exact patterns as the plasma ALP activity reported in tilapia (Lin et al. 2013) where the enzyme activity increased when dietary Mg increased and then decreased when dietary Mg exceeded the requirement. As the carapace of soft-shelled turtle is fused from bone that contained significant proportions of body Mg, a dietary Mg level may be estimated by measuring carapace strength. Magnesium deficiency may have well resulted in the weakness of the carapace in this study. A similar observation has been reported in a study where the leg bones were weak in baby pigs fed with Mg deficiency diets (Miller, Ullrey, Zutaut & Baltzer 1965). In conclusion, based on a broken-line analysis, a dietary Mg level of 650 mg kg−1 satisfies the growth requirement for the soft-shelled turtles. However, the dietary level of 1000 mg Mg kg−1 is recommended furthering enhancing the carapace strength in soft-shelled turtles.
Acknowledgments
The funding of this study was provided by the National Science Council of the Republic of China under grant NSC-99-2313-B-415-004-MY3. We appreciate Dr Chin Sun and Ms Angel Huang of the University of British Columbia for their assistances during the preparation of the manuscript.




