Hot water immersion lowers survival, shell growth rate and lysosomal membrane stability of oysters Crassostrea virginica (Gmelin)
Abstract
This study compares the effect of two anti-fouling treatments, hot water immersion (15 s at 60°C) and air drying (72 h) on the physiological status of the Eastern oyster Crassostrea virginica. The negative impact of hot water immersion was greater than that of air drying, but varied depending on the initial size of the oysters (40 vs. 60 mm shell height) and the time of the year (June vs. August). Groups treated with hot water exhibited a higher proportion of haemocytes with destabilized lysosomal membranes (HDLM; 47.5 ± 3.1%) than those exposed to air drying (37.5 ± 2.9%). This suggests that the oyster immunocompetency may be lowered by hot water immersion. Overall, the large oysters had lower HDLM values (32.9 ± 3.5%) than the small individuals in June (45.7 ± 2.8%) but similar values in August (46.6 ± 3.5%). Small oysters subjected to hot water immersion in June exhibited a 50% reduction in shell growth and a 50% mortality rate after one month. Our results indicate that air drying is more suitable than hot water immersion as an anti-fouling treatment for <45 mm oysters.
Introduction
Eastern Oyster (Crassostrea virginica) aquaculture is of major economic and social significance in New Brunswick as it provides employment in rural areas that have been heavily affected by the fisheries decline. Eighty eight small enterprises employ more than 250 people (Department of Agriculture Aquaculture and Fisheries, New Brunswick, unpublished data) and the industry is poised for major expansion. Improving production by reducing the negative impact of biofouling is an important step towards insuring the profitability and longterm survival of these aquaculture businesses.
The development of biofouling communities on oyster bags, cages and Oystergro® systems leads to a progressive reduction in water flow through the structures (Ross, Thorpe, Norton & Brand 2002), thereby restricting the input of food and oxygen and the output of faeces and metabolic by-products. Most studies have shown that the accumulation of fouling organisms on the rearing structures impairs bivalve growth (Enright, Krailo, Staples, Smith, Vaughan, Ward, Gaul & Borgese 1983; Quayle & Newkirk 1989; Claereboudt, Bureau, Côté & Himmelman 1994; Lodeiros & Himmelman 1996; Willemsen 2005), although there are some contradictory findings (Ross et al. 2002; Mallet, Carver & Hardy 2009). Certain biofouling species may further reduce the available food supply to the cultured oysters by competing directly for suspended particles (Lodeiros & Himmelman 1996; Cigarría, Fernández & Magadán 1998; Ross et al. 2002; Willemsen 2005). It has also been suggested that the added weight of biofouling may interfere with the bivalves ability to open and feed effectively (Lodeiros & Himmelman 1996; Ross et al. 2002).
Air drying is the technique traditionally used in eastern Canada to control biofouling on oyster culture structures. Floating oyster bags or OysterGro cages are either flipped over to expose the fouled surface at the growing site, or brought ashore to fully air dry for up to 72 h. This treatment is very effective in eliminating soft-bodied species as long as they are not protected from desiccation by a thick mass of seaweed (Quayle & Newkirk 1989). Although oysters are adapted to respond to air drying by tightly closing their shells, eventually the internal environment will become hypoxic, especially in warm weather (Burnett & Milardo 2000). The more similar the air and the water temperature, the lesser is the physiological stress imposed on the oysters (Moore, Lowe & Moore 1979; Harding, Couturier, Parsons & Ross 2004; Zhang, Li, Vandepeer & Zhao 2006 and Song, Li, Clarke, Wang & Bott 2007a). For example, in Crassostrea gigas (Thunberg) acclimated to 15°C, Song et al. (2007a) observed mortality rates of 6.7–12% at an air temperature of 25°C compared with no mortality at air temperatures of 5°C and 15°C.
Hot water immersion is an anti-fouling technique that consists of submerging bivalves and their rearing structures in a hot water bath for a specified duration. Originally developed in Japan, Korea and France, it has proved to be very effective in eliminating the biofouling community. Trials conducted in Asia (Arakawa 1980; Park, Park, Kim, Hur & Kim 1988) found that 15 s in 60°C water caused 100% mortality of 10–20 mm Mytilus edulis (L.) but only 8% mortality of 10–25 mm Pacific oysters (C. gigas). In France, Pacific oysters are treated at higher temperatures (80-85°C), but only for 2–3 s depending on their size (Centre régional d'expérimentation et d'application aquacole: http://creaa.pagesperso-orange.fr/doc/05_fiche_echaudage_huitres.pdf, consulted 18 February, 2013).
The optimal temperature and exposure duration for effectively eliminating biofouling while minimizing bivalve mortality need to be established for eastern Canada, where both the cultivated species and the biofouling community are different from previous studies. Preliminary tests on the impact of hot water immersion on C. virginica conducted in Prince Edward Island (Smith & MacNair 2000) and New Brunswick (L. Lanteigne, pers. comm., 2008) suggested that 60°C for 10–15 s was effective against biofouling without leading to increased mortality. However, these experiments did not test for any sub-lethal treatment impacts on the physiological status of the cultivated oysters. Sub-lethal damage could lower the oysters' physiological resilience in the long term and eventually lead to lower production and higher mortality rates than those estimated in the short term. We are thus interested in comparing the impact of hot water immersion versus air drying on stress indicators in C. virginica. Given that this is an intertidal species, well adapted to air exposure, we hypothesize that it is likely more vulnerable to the effects of hot water immersion than air drying.
In bivalves, shell and soft-tissue growth are often asynchronous (Kautsky 1982; Hilbish 1986; Lewis & Cerrato 1997; Borrero & Hilbish 1988). According to Lewis and Cerrato (1997), shell growth in Mya arenaria (L.) was directly related to metabolic rate and water temperature, and inversely related to emersion period. In contrast, soft tissue growth was inversely related to metabolic rate and not dependent on temperature or emersion period. Given this variability, shell growth and flesh production may well respond differently to air drying versus hot water immersion. It should be noted that any reduction in shell growth and/or decrease in flesh content associated with the anti-fouling treatment could have serious economic ramifications, as market size is determined by shell height, and lean oysters are less valued by consumers.
Stressed animals usually exhibit cellular responses before the effect becomes detectable at the individual level of organization (Bayne, Moore, Widdows, Livingstone, Salkeld, Crisp, Morris, Gray, Holden, Newell & McIntyre 1979). Haemocyte responses are a useful index of physiological status as these cells participate in the homoeostasis of bivalves in various ways. Haemocytes are involved in intracellular digestion, excretion, nutrient transport, calcium and protein transport for shell building, wound repair, immune defence, and sequestration of toxic molecules (Cheng 1981, 1984, 1996; Auffret 1988, 1989; Nakayama, Nomoto, Nishijima & Maruyama 1997; Lowe & Fossato 2000; Wootton & Pipe 2003). The lysosomes of the granulocytes are a key element in these various functions, and as such their integrity is crucial to homoeostasis. An array of stressors including temperature changes and air exposure may lead to the destabilization of lysosomal membranes. Hauton, Hawkins and Hutchinson (1998), Zhang et al. (2006) and Song et al. (2007a) showed that variations of 5–10°C in water temperature affected the stability of lysosomal membranes, whether the change was rapid or gradual, or directed towards an increase or a decrease in temperature. Changes in the capacity of lysosomes to retain neutral red could be detected in various oyster species after the bivalves had been exposed to the new temperature for as few as 30 min. To our knowledge, no study has yet documented the impact of a short and intense thermal shock such as that used in anti-biofouling treatments. A few studies have reported that air exposure may affect the capacity of oyster lysosomes to retain neutral red (Zhang et al. 2006; Song et al. 2007a). In both cases, oysters were held out of water for 72 h which is similar to the time frame prescribed for treating oyster bags for biofouling. On a shorter time scale, Tremblay and Pellerin-Massicotte (1997) observed cyclic destabilization of lysosomal membranes at low tide when the bivalves were exposed to the air.
The impact of anti-fouling treatments on oyster survival rate and physiological status likely varies with size; smaller individuals have a higher metabolic rate and tend to be more sensitive to stress than larger ones (e.g. Withers 1992; Wells & Baldwin 1995; Sukhotin, Lajus & Lesin 2003). Another factor which may influence the oysters' response is the time of the year at which the treatments are conducted. Not only does the oyster's physiological status vary seasonally, but also the temperature differential between the oyster's environment and the treatment conditions.
The objective of this study was to compare the physiological response of two size classes of oysters subjected to hot water immersion or air exposure treatment in late spring and mid-summer. Response variables were mortality, growth rate, condition index and % haemocytes with destabilized lysosomal membranes (HDLM).
Materials and methods
Experimental set-up
The experiment was conducted at a commercial oyster farm (46° 43′ 13′′ N, 64° 52′ 47′′ W) located in the Richibucto Estuary, NB, Canada (Fig. 1). The oysters were brushed clean before being transferred to clean Vexar bags (85 × 40 × 10 cm) with either a 1.9-cm mesh size for large animals or a 0.7-cm mesh size for small ones. Oyster densities in the experimental floating bags were similar to those used by aquaculturists, i.e. 700 small individuals (35–45 mm shell height) and 275 large individuals (55–65 mm) per bag. A total of 72 bags, 36 of each size group were prepared on May 15, 2009 and randomly tied to a main line at the surface of the water.
Two experimental trials were conducted in 2009, the first from June 2 to 14 and the second from August 5 to 17. These dates were chosen to be representative of the periods at which aquaculturists typically apply anti-fouling treatments. They also reflect changes in the physiological status of the oysters; June is a period of high growth, whereas August is the post-spawning phase when water temperatures are at their highest. It should be noted that because of time constraints associated with the HDLM assessment technique, the number of individuals that could be analysed on a single day was limited to 12. Therefore, the treatments had to be carried out successively over 12 days, taking care to treat one bag of large oysters and one bag of small oysters each time, and to sample oysters treated by hot water immersion (along with the control group) and oysters exposed to air in alternation.
Over the 12-day period, a total of six bags of large oysters and 6 bags of small oysters were treated in a commercial-scale hot water bath located at the farm site. The system had a conveyor belt moving at a constant speed that transported the oyster bags down into a reservoir tank of 60°C heated water; the bags remained immersed in the tank for a period of 15 s and then emerged at the other end. They were returned to the water approximately 30 min later. During the same period, another 6 bags from each size group were brought in and exposed to 72 h of air drying. This was achieved by storing the bags for three days in a building located on the wharf to avoid variations in sun/wind exposure and rain conditions. Another 6 bags from each size group served as the control for both the hot water immersion and the air-drying treatment. These were brought to the wharf and then returned to the water along with the heat-treated bags.
Water salinity was measured manually at the experimental site using a refractometer (Sybon Opticon Series FG100sa, Bethesda, MD, USA). Water temperature was registered with a Vemco Minilog placed by the Department of Agriculture, Aquaculture and Fisheries of New Brunswick (DAAF/MAAP) in a floating oyster bag at a nearby (<1 km) oyster aquaculture site. DAAF/MAAP followed oyster spawning in Bedec Bay, at the mouth of the Richibucto River, less than 10 km east of our experimental site. Daily air temperature, precipitation and wind speed registered at the Bouctouche meteorological station (30 km south of our experimental site) were obtained from Environment Canada. Observations on cloud and sun conditions were recorded daily during the experiments.
Sampling for % haemocytes with destabilized lysosomal membranes
Oysters sampled for % HDLM measurements were retrieved from the floating bags 48 h posttreatment. This time delay was chosen on the basis of the results reported for C. gigas by Zhang et al. (2006) and Song et al. (2007a). These studies showed that the stress associated with temperature changes and air exposure had their full effect on lysosomal membrane stability after approximately 24 h and that these effects lasted for at least 72 h. At the start of each sampling day, we retrieved two large oysters from one of the six replicate bags from each of the three treatments, and two small oysters from the corresponding bags, for a total of 12 individuals (2 oysters × 2 size classes × 3 treatments). These oysters were immediately transported to the laboratory located approximately 100 km from the experimental site. They were packed in a cooler filled with ambient sea water to minimize changes in water temperature and surrounded by eelgrass (Zostera marina) to reduce the risk of movement and collisions inside the cooler. At the laboratory, shell height was measured with an electronic calliper. The oysters were prised open and their stage of gonad development was visually assessed and ranked 0 (not developed), 1 (slightly thickened), 2 (thick enough to hide the underlying digestive gland), or 3 (very thick and enlarged gonads), following the description by Baqueiro, Aldana, Sevilla and Rodríguez (2007). A piece of gonad was examined under the microscope to determine the sex of each individual. This was only successful for large oysters as the gonads of the smaller ones were undeveloped throughout the experiment.
Determination of % haemocytes with destabilized lysosomal membranes
Per cent HDLM was estimated using the neutral red retention technique, adapted from Hauton, Hawkins and Hutchinson (2001), Keppler, Hoguet, Smith, Ringwood and Lewitus (2005) and Song et al. (2007a). A 1000-ml saline solution was prepared with deionized water, 26.5 g sodium chloride, 0.75 g potassium chloride, 1.11 g calcium chloride, 3.33 g magnesium chloride and 2.05 g magnesium sulphate, and the pH was adjusted to 7.3 (Schlieper 1972). The salinity was adjusted with deionized water to match that measured in seawater at the time of sampling. The solution was filtered through a 0.22-μm filter before being used. A stock solution of neutral red (Product No. 72210; Sigma, St. Louis, MO, USA) was prepared by dissolving 2.28 mg of neutral red in 1 ml of dimethylsulphoxide (Sigma D8418). This solution was kept frozen in an amber bottle for no longer than 3 weeks. When the bioassays were performed, a fresh working solution was made up every 3 h by diluting 17 μL of stock solution in 1 ml of saline.
For the bioassays, a 0.2-ml sample of haemolymph was taken from the adductor muscle sinus and transferred to a siliconized microcentrifuge tube containing 0.2 ml of saline. Forty μL of the haemolymph-saline mixture was transferred to an adhesive Colorfrost® Plus slide (Product No. 48512-02; Cole-Parmer, Montr®al, QC, Canada) and incubated at 20°C for 15 min in a humid chamber to allow the adherence of the haemocytes, after which the excess haemolymph was absorbed with the corner of a Kim Wipe cleansing tissue. Twenty μL of working neutral red solution were added to the slide, which was returned to the humid chamber for 15 min, after which time a cover slide was added and the incubation was resumed for another 45 min. This incubation time was consistent with that used by Keppler et al. (2005) on C. virginica digestive gland cells, and was validated for haemocytes by preliminary tests in which we compared % HDLM in unstressed oysters after 15, 30, 45 and 60 min of incubation. The slides were then quickly observed at 630× with a bright-field microscope; at least 50 haemocytes were counted and classified as having either ‘stable lysosomal membranes’ (dark red granules and clear cytosol) or ‘unstable lysosomal membranes’ (bright red granules and pink or bright red cytosol at least in part). Aggregated cells and lysed cells were not counted. The results are expressed as % HDLM or the percentage of haemocytes presenting signs of destabilized lysosomal membranes.
Per cent mortality, condition index and shell growth
The stress indicators (condition index, shell growth, % mortality) were assessed one month after the treatments had been applied, i.e. from July 2 to 14 and from September 5 to 17. Estimates of % mortality were obtained for each of the treated and control bags. To determine the condition, 10 individuals from each bag were dissected and the tissues were dried at 70°C for 48 h. The internal volume of each oyster was obtained by subtracting the volume of water displaced by the two valves alone from the volume displaced by the whole animal. The condition or filling index was calculated as the mass of total dry flesh/internal shell volume (g mL−1).
To estimate shell growth during the month posttreatment, three oysters per bag were individually marked with permanent ink and photographed before the experimental trials in June and August. The shell surface area of each individual was measured using the UTHSCSA Image Tool version 3.0 (San Antonio, TX, USA). Marked oysters were placed in small bags with a mesh size of 2.5 cm, which were then placed inside the main oyster bags. A second picture of each marked oyster was taken one month posttreatment. Shell growth was calculated for each individual by subtracting the first shell surface value from the second value (cm2 month−1). The measurement of changes in shell surface area allows a more precise estimation of shell growth than measuring changes in shell height, as oyster shape is quite variable.
Statistics
Statistical analyses were performed with the software sas (GLM procedure), version 9.1 (sas Institute 2002–2003, Cary, NC, USA). Normality of residues was verified with the Shapiro–Wilk test, and homoscedasticity was visually assessed from the distribution of the residues. Data on condition index and % mortality were log-transformed to meet these requirements. Significance level was set at 0.05.
A preliminary analysis showed that sex did not influence values of % HDLM (Student's t test, P > 0.05). As Soletchnik, Razet, Geairon, Faury and Goulletquer (1997) also showed that sex does not affect the scope for growth of C. gigas (Thunberg), we pooled the data for the subsequent analyses. The dependent variables % HDLM, condition index and shell growth were analysed with a mixed model anova, with anti-fouling treatment (hot water immersion, air drying or control), oyster initial size at the beginning of the growing season (large or small) and timing (late spring or mid-summer) considered as fixed factors. The oyster bags (experimental units) with replicate measurements (2 for % HDLM, 10 for condition index, 3 for shell growth) were set as a random factor in the model. As the calculation of % mortality was based on all the oysters within each bag, there was no replicate measurement for this variable, which was thus analysed with a 3 fixed factor anova. Whenever a significant relationship including a factor with more than two levels was detected, multiple comparisons were carried out with a Tukey–Kramer test.
Results
Water temperature oscillated between 13.5 and 16.5°C during the first period of anti-fouling treatments (June 2–14) and between 19.5 and 24.5°C during the second period (August 5–17). Salinity varied between 25 and 27 g L−1 during the first period, and between 20 and 23 g L−1 during the second.
The small oysters treated in June averaged 40.8 ± 1.1 mm (mean ± standard error) in shell height, and the large ones 61.9 ± 0.7 mm. At the time of the August treatment, the initially small individuals had reached a mean shell height of 56.1 ± 1.2 mm, whereas the large ones measured 67.0 ± 1.0 mm. The initially small oysters had undeveloped gonads in both June and August. In contrast, most of the large oysters had ripe gonads in June; 69% were classified as stage 2 or 3 and the remaining individuals were stage 1. In August, after spawning had occurred, 72% of the large oysters displayed very little gonadic tissue or none at all, whereas the remaining 28% were classified as stage 2.
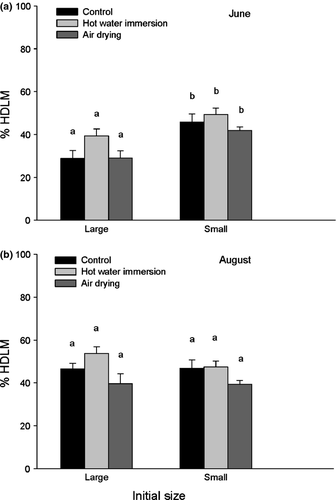
Overall, the oysters treated with hot water showed significantly higher mean values of HDLM (47.5 ± 3.1%) than the oysters exposed to air (37.5 ± 2.9%) as shown by Table 1 and Fig. 2. There was also a significant ‘initial size × timing’ interaction. Large individuals collected in June had the lowest % of HDLM (32.4% ± 3.5), whereas the mean proportion of HDLM was around 45% in small individuals collected in June and August and also in large oysters collected in August.
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Treatment | 2 | 1203.36 | 9.53 | <0.01 |
| Initial size | 1 | 1133.44 | 8.97 | <0.01 |
| Timing | 1 | 1547.11 | 12.25 | <0.01 |
| Treatment × Initial size | 2 | 140.36 | 1.11 | 0.34 |
| Treatment × Timing | 2 | 83.53 | 0.66 | 0.52 |
| Initial size × Timing | 1 | 2116.00 | 16.75 | <0.01 |
| Treatment × Initial size × Timing | 2 | 10.75 | 0.09 | 0.92 |
| Oyster bag (Treatment × Initial size × Timing) | 60 | 126.30 | 0.79 | 0.82 |
| Error | 72 | 159.22 |
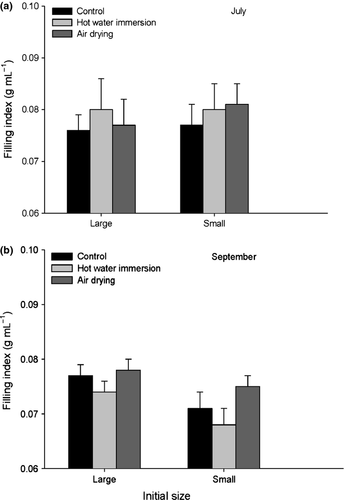
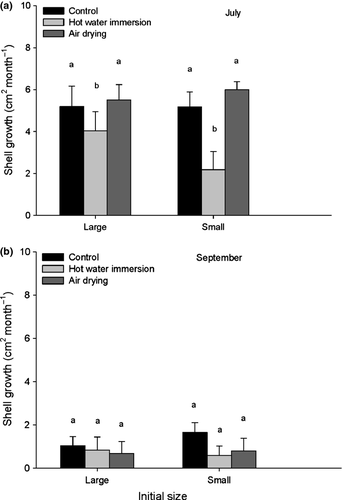
According to the anova results, the timing of treatment was the only factor which had a significant effect on the filling index of the oysters one month posttreatment. Overall estimates from July were significantly higher than those from September (Table 2, Fig. 3). Shell growth rate during the month posttreatment was apparently a more sensitive indicator of sub-lethal stress than the condition index as there was a significant ‘treatment × timing’ interaction. Shell growth rate was higher from June to July (4.7 ± 0.8 cm2 month−1) than from August to September (0.9 ± 0.5 cm2 month−1) for the three treatments. Averaged over both size groups, oysters treated in June with hot water grew more slowly (3.1 ± 0.9 cm2 month−1) than those exposed to air (5.8 ± 0.6) or those used as controls (5.2 ± 0.8), whereas estimates for the August to September period did not differ among treatments (Table 3 and Fig. 4).
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Treatment | 2 | 0.36 | 2.59 | 0.08 |
| Initial size | 1 | 0.32 | 2.28 | 0.14 |
| Timing | 1 | 0.69 | 4.99 | <0.05 |
| Treatment × Initial size | 2 | 0.26 | 1.85 | 0.16 |
| Treatment × Timing | 2 | 0.07 | 0.48 | 0.62 |
| Initial size × Timing | 1 | 0.38 | 2.74 | 0.10 |
| Treatment × Initial size × Timing | 2 | 0.01 | 0.03 | 0.98 |
| Oyster bag (Treatment × Initial size × Timing) | 60 | 0.14 | 1.54 | <0.01 |
| Error | 646 | 0.09 |
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Treatment | 2 | 34.15 | 5.29 | <0.01 |
| Initial size | 1 | 0.61 | 0.09 | 0.76 |
| Timing | 1 | 628.00 | 97.45 | <0.01 |
| Treatment × Initial size | 2 | 7.05 | 1.09 | 0.34 |
| Treatment × Timing | 2 | 27.83 | 4.30 | <0.05 |
| Initial size × Timing | 1 | 4.56 | 0.71 | 0.40 |
| Treatment × Initial size × Timing | 2 | 3.40 | 0.53 | 0.59 |
| Oyster bag (Treatment × Initial size × Timing) | 59 | 6.67 | 2.37 | <0.01 |
| Error | 124 | 2.81 |
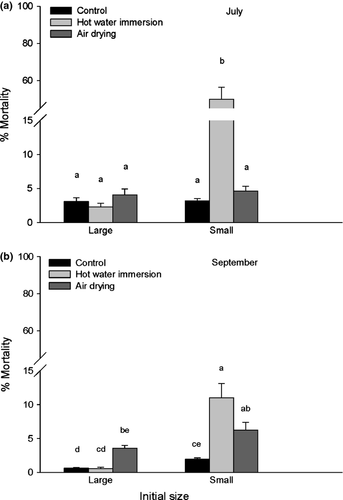
The interaction of the factors ‘treatment × timing × initial size’ had a significant effect on oyster mortality rate one month posttreatment (Table 4 and Fig. 5). The initially small oysters treated with hot water in June had a substantially higher mortality rate (50%) than either the control (2.3%) or the air-dried groups (4.6%), whereas those treated in August (11%) only differed significantly from the control group (1.9%). In contrast, the mortality rate of the large oysters did not vary much among the three treatments and remained consistently below 5%.
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Treatment | 2 | 8.07 | 38.45 | <0.01 |
| Initial size | 1 | 28.81 | 137.29 | <0.01 |
| Timing | 1 | 9.52 | 45.35 | <0.01 |
| Treatment × Initial size | 2 | 10.52 | 50.11 | <0.01 |
| Treatment × Timing | 2 | 3.16 | 15.04 | <0.01 |
| Initial size × Timing | 1 | 0.35 | 1.67 | 0.20 |
| Treatment × Initial size × Timing | 2 | 0.88 | 4.19 | <0.05 |
| Error | 58 | 0.21 |
Discussion
Our results indicate that hot water immersion is generally more harmful to oysters than air drying, and that the magnitude of the physiological impact varies depending on the size of the oysters and the time of year at which the treatment is applied. In particular, the small (35–45 mm) oysters treated with hot water in late spring showed the greatest sub-lethal damage in terms of loss of haemocyte integrity and reduced growth, as well as the highest subsequent mortality rates one month later.
The marked sensitivity of small oysters to hot water immersion is consistent with the results of Arakawa (1980), and may be attributed to the weak protection offered by their thin shells. A size effect was particularly evident in the oysters treated in June when the difference in shell height between the two size classes was the most pronounced. Shell growth and survival were more noticeably depressed in the small individuals treated by hot water immersion than either the control or the air-dried groups, on one hand, and the large oysters immersed in hot water on the other hand. This suggests that shell thickness may be a determining factor in protecting the subjacent tissues against an acute thermal stress. To validate this interpretation, we measured the thickness of the left valve of 12 small oysters (41.3 ± 0.4 mm shell height) and 12 large ones (62.0 ± 0.4 mm). A significant difference of 0.50 mm in shell thickness was indeed noted (small: 1.22 ± 0.09 mm, large: 1.72 ± 0.11 mm, Student's t = −3.606, P = 0.002).
We can suppose that the thinner the shell, the higher the temperature will rise in the extrapallial compartment during hot water immersion. According to the review on the effect of temperature on protein functions by Fields (2001), for species whose maximal environmental temperature is 35–40°C, the protein denaturation temperature is slightly below 60°C. Adult C. virginica are found in waters from −2 to 36°C, and water temperature above 35°C for a complete tidal cycle has been shown to cause mortality (Stanley & Sellers 1986). These data suggest that hot water immersion could have caused the denaturation of the functional proteins and/or the circulating haemocytes involved in shell building (Wilbur & Saleuddin 1983; Mount, Wheeler, Paradkar & Snider 2004), especially in small oysters with thin shells. It may be argued that because the small oysters used in the second set of treatments in August had grown substantially since June and hence presumably thickened their shells, the impact of hot water immersion on shell growth was no longer detectable, and the mortality rate in this size group dropped by a factor of approximately five.
An allometric effect might also explain the vulnerability of the small oysters, as depicted in June by their higher % HDLM values relative to the large oysters. Shell growth represents a considerable investment of energy in heavy-shelled bivalves such as oysters (Wilbur & Saleuddin 1983), and even though both the small and the large individuals belonging to the control and the air-dried groups had comparable shell growth rates from June to July, the small oysters invested in fact a proportionally higher amount of energy. Indeed, a shell increment of 5.5 cm2 month−1 represents an augmentation of their shell surface of 87% compared with their initial size, while the large oysters augmented their surface by only 30%. Small oysters would thus be more likely to experience an imbalance in their energy budget when dealing with stressful conditions during this growth phase. An imbalance between energy expenditure and gains has also been proposed by Song, Li, Clarke, Wang and Bott (2007b) as a plausible causal factor of impaired lysosomal membrane stability in Pacific oysters.
Even though large oysters were less impacted by hot water immersion than small ones, their growth rate and the integrity of their haemocytes were nonetheless impaired. From the aquaculturists' perspective, a slight decrease of 1 cm2 month−1 in shell growth rate from June to July should be acceptable, considering that heavy fouling would likely interfere with shell growth (Willemsen 2005) and the effectiveness of hot water treatment, unlike air drying, does not depend on weather conditions. However, a higher degree of lysosomal membrane destabilization, as compared with the air-dried oysters, might indicate potentially deleterious effects on the immunocompetency of both small and large oysters. The period required by the lysosomal membrane to recover after a stress varies from 5 to at least 12 days in C. gigas (Zhang et al. 2006; Song et al. 2007a). Although a transitory high value of % HDLM might not have significant repercussions on the health of the oyster stock, a prolonged weakening of the oysters' immune defences would increase sensitivity to pollutants and the risk of developing diseases. Further studies on the temporal pattern of lysosomal membrane stability in response to hot water immersion are needed to assess this risk.
Another concern is the impact of hot water immersion on the reproductive success of the oysters. Ringwood, Hoguet, Keppler and Gielazyn (2004) have shown that embryos derived from oysters with lysosomal destabilization rates >35% had very low rates of normal development. It should be noted that the stress to which the oysters were subjected in that study was a prolonged chemical insult as opposed to an acute thermal shock as in our study. Also, the 35% threshold established by these authors as indicative of stress was based on the response of hepatopancreatic cells and might not apply to haemocytes. However, as a majority of the large oysters used in our study had ripe gonads at the time of the first anti-fouling treatments in June, and those that were treated with hot water had 40% HDLM, we cannot dismiss the possibility that hot water immersion causes damage to the gametes. Further investigations on this subject would be relevant as most aquaculturists in New Brunswick depend on wild spat collection to replenish their stocks, and it is not known to what extent this spat originates from cultivated or wild oyster broodstock.
Observations of temporal changes in the oysters' condition index were probably related to different factors in the small and large individuals. The decline in the condition index of the large oysters between August and September was most probably related to spawning. We can safely assume that the large oysters spawned sometime between the end of the first treatment period (June 14), at which time the majority were in stage 2 or 3 of gonad development, and before the onset of the second treatment period (August 5), when most oysters showed spent gonads. This is supported by field data from DAAF-MAAP, New Brunswick, according to which the first signs of oyster spawning occurred on June 20, 2009 in Bedec Bay, 10 km from our study site. Our results also suggest that the reproductive cycle modulates the vulnerability level of the oysters, as the large individuals had a higher level of % HDLM in August, after they had spawned, regardless of the treatment to which they had been subjected. This is in agreement with Cho and Jeong (2005) who observed a reduction in the lysosomal membrane stability of C. gigas which had recently spawned. Bocchetti and Regoli (2006) and Harding (2003) also measured the lowest level of lysosomal membrane stability at the end of the gonadic development period in Mytilus galloprovincialis (Lamarck) and M. edulis respectively. Obviously, spawning cannot be invoked to explain the reduction in the condition index in the small oysters as the latter had undeveloped gonads throughout the experiment. Rather, a time lag between shell and flesh growth may have been a factor, as the shell size and thus internal volume of the small oysters markedly increased between treatment periods. As flesh production often takes place sometime after a bout of shell growth (Hilbish 1986; Borrero & Hilbish 1988), the ratio of flesh mass to internal shell volume would decrease under such circumstances.
It is not clear why the shell growth rate of all the experimental groups was so much lower from August to September than from June to July. In C. virginica, shell growth follows a seasonal rhythm: in Chesapeake Bay, it is most pronounced from June to October and stops when water temperature decreases to 10°C (Paynter & Dimichele 1990). As no adaptation of the valve opening behaviour to cold conditions was found in a population of C. virginica from New Brunswick (Comeau, Mayrand & Mallet 2012), we can assume that shell growth of the Richibucto oysters responds to temperature in a manner similar to that of the Chesapeake population. In this study, water temperature fluctuated between approximately 15 and 25°C during both the late spring and the mid-summer periods and cannot explain seasonal variations in shell growth. Even though salinity was lower in mid- summer than in late spring, it was still well within the optimal range of 14–28 g L−1 that has been delimited for C. virginica (Shumway 1996). Fluctuations in food availability and quality might account for the temporal variations in shell growth, as food level has been related to shell growth although not linearly (Lewis & Cerrato 1997). However, as we measured neither chlorophyll a nor seston concentrations, we cannot draw conclusions on this subject. Finally, strong storm events, which can reduce the apparent shell growth rate by shattering the fragile new shell edge, occurred at a similar frequency in late spring and mid-summer. In fact, wind speeds >50 km h−1 were registered on only one occasion during each period at the nearby Bouctouche meteorological station (Environment Canada).
In conclusion, we recommend that aquaculturists should not use hot water immersion at 60°C for 15 s on eastern oysters <45 mm as this treatment increases % HDLM, impairs subsequent shell growth and may lead to >50% mortality. Based on the results of this study, air drying would appear to be the preferred treatment option for small oysters. However, other combinations of temperature and duration should be tested as different conditions may prove to be more successful with small individuals. Hot water immersion treatment can be safely applied to oysters >55 mm, although the long term effects on immune defence capacity and reproductive success need to be investigated.
Acknowledgments
The authors express gratitude and thanks to Gaétan Moreau, André Mallet, Tobie Surettte and Angéline Leblanc for invaluable assistance with statistical analyses. Maurice Daigle of Maison Beausoleil kindly provided lease space and field resources to carry out the experiments. The authors also acknowledge the useful contributions made by Rémi Sonier and Carla Hicks during data collection, and the collaboration of Marie-Josée Maillet (DAAF/MAAP) who provided the data on oyster spawning and on bivalve larvae abundance. Claire Carver kindly agreed to read a draft of this article and provided very helpful comments. This study was funded by the Professional Shellfish Growers Association of New Brunswick in partnership with the Department of Fisheries and Oceans of Canada (Aquaculture Collaborative Research and Development Program, project MG-08-01-003).




