Why is cannibalism less frequent when larvae of sutchi catfish Pangasianodon hypophthalmus are reared under dim light?
Abstract
Survival rates of the larvae of sutchi catfish Pangasianodon hypophthalmus are reported to be three times higher under dim conditions (0.1 lx) than those under 100 lx. In this study, larval behaviour of sutchi catfish was examined under various light intensities (<0.01, 0.1, 1, 10 and 100 lx) using a CCD camera to understand why survival rates vary under different light intensities. Five-day-old larvae showed significantly higher swimming activity under <0.01, 0.1 and 1 lx than those under 10 and 100 lx. On the other hand, the larvae showed significantly higher aggressive behaviour under 10 and 100 lx; swimming larvae attacked resting individuals more frequently under 10 and 100 lx than those under 0 and 0.1 lx. Aggressive behaviour was considered to induce lesions, inflicted by the sharp teeth of attacking larvae, on larval skin surfaces. It is considered that the chemical substances would generate from injured skin surfaces then acted as stimuli, causing the cannibalistic behaviour in other fish around the injured fish. This study provided evidence that the observed higher survival rates depended on lower frequency of aggressive behaviour under dark or dim conditions. It is therefore recommended that larval rearing of sutchi catfish be conducted under dim (less than 1 lx) conditions.
Introduction
Sutchi catfish Pangasianodon hypophthalmus (formerly: Pangasius hypophthalmus) has its origins in the Mekong River basin (Roberts & Vidthayanon 1991; Amin, Bapary, Islam, Shahjahan & Hossain 2005; Morioka, Sano, Phommachan & Vongvichith 2010). This fish is widely cultured in Asian countries, and Vietnam is the largest producing country, with a total production of about 1 250 000 tonnes in 2010 (Phan, Bui, Nguyen, Gooley, Ingram, Nguyen, Nguyen & De Silva 2009; Phuong & Oanh 2009; Halls & Johns 2013).
Sutchi catfish larvae show low survival rates due to their strong propensity towards cannibalism, particularly, at early larval stage (Subagja, Slembrouck, Hung & Legendre 1999; Ali, Hossain & Mazid 2005). Cannibalism is governed by morphological, behavioural and physiological factors; in case of sutchi catfish, the larvae have large mouths, sharp teeth, oral spines and long barbels (Baras, Hafsaridewi, Slembrouck, Priyadi, Moreau, Pouyaud & Legendre 2010; Baras, Slembrouck, Cochet, Caruso & Legendre 2010; Mukai, Tuzan, Sitti Raehanah & Manjaji-Matsumoto 2010).
Sutchi catfish larvae can be reared under dim light or darkness (Mukai 2011a,b). They can also eat food under dark conditions, which implies that their feeding behaviour must be dependent on sensory organs other than eyes. Previous studies demonstrated that sutchi catfish have numerous taste buds that play an important role in their feeding behaviour under dark conditions (Mukai, Tuzan, Lim & Yahaya 2010; Mukai, Tuzan, Sitti Raehanah et al. 2010). Moreover, Mukai (2011a) also reported that sutchi catfish show survival rates three times higher under dim conditions (0.1 lx) than those under brighter conditions (100 lx). Mukai (2011a) postulated that this was a result of the less aggressive behaviour of catfish under dim conditions; however, there is no quantitative data to support this claim.
This study was therefore conducted to detect the differences in larval behaviour under various light intensities. The behaviour of sutchi catfish larvae was observed under <0.01, 0.1, 1, 10 and 100 lx light, using a CCD camera under infrared light; their swimming activity and aggressive behaviour (cannibalistic activity) under these different light conditions were then compared.
Materials and methods
Sutchi catfish larvae for experiments
Fertilized eggs of sutchi catfish, P. hypophthalmus, were obtained from brood fish reared in a hatchery at the Borneo Marine Research Institute, Universiti Malaysia Sabah. The eggs were incubated at 28–29°C and hatched 24 h after artificial fertilization. The larvae were reared in 40-L glass aquaria at a temperature of 28–30°C and fed Artemia nauplii from the second day after hatching. Total lengths (mean ± SD) of the larvae from the same source larvae (n = 10, each age) for this experiment were 5.6 ± 0.20 mm at 3 days old, 6.6 ± 2.6 mm at 5 days old, 12.5 ± 0.7 mm at 12 days old and 14.1 ± 1.1 mm at 15 days old.
Illuminating method and recording of fish behaviour
Experiments were conducted in a blind dark room (<0.01 lx). Various light intensities (<0.01, 0.1, 1, 10 and 100 lx) were created using fluorescent lamps (Power-Glo, 20w, Hagen, Saint-Laurent, Canada) and neutral-density filters (NDx8, HOYA, Tokyo, Japan). Light intensities were measured using a light meter (No. 40103, Extech Instruments, Waltham, MA, USA). A CCD camera [WAT-232/NTSC (colour & IR), Watec, Tsuruoka, Yamagata Prefecture, Japan] was used to record fish behaviour under the different light intensities. An infrared lamp (940 nm; DC12 V, 380 mA) with a 920 nm filter (IR92; Fuji Film, Tokyo, Japan) was used to record the fish behaviour under dark conditions: 0.01, 0.1 and 1 lx. CCD camera, Watec images were recorded by DVD recorder for 30 min. Larvae were examined at 3, 5, 12 and 15 days after hatching,
Ten fish were maintained in a glass basin (18-cm diameter with one litre water). Before the larval behaviour was recorded, they were left for 30 min under each light intensity in the dark room for adaptation. Thereafter, the larval behaviour was observed and recorded for 30 min using a CCD camera system and a DVD recorder (DVD Recorder DR 166H; LG, Seoul, Korea). Water temperature during the behaviour experiments was 28–30°C.
Swimming activity
Ten fish were put in the glass basin. The total number (out of 10) of swimming fish was counted in each minute for 30 min (n = 30, under five different light intensities in each age). The larvae were considered to be swimming when they showed movements for swimming. Any motionless behaviour (defined as fish lying motionless at the bottom of the tank, either on their bellies or on their sides) was not considered as an active behaviour.
Aggressive behaviour
The number of attack activities in every minute was counted for 30 min (n = 30, under five different light intensities in each age). It was expressed as the sum of frequency of attack activities of the ten individuals. The frequency of attack activity was counted as aggressive contact to some part of the head or body of another individual (e.g. colliding with other individuals). Usually, this attacking behaviour occurred when the larvae were resting at the bottom of the tank and were attacked by another individual. The numbers of larvae attacked in every minute were counted for 30 min to obtain the frequency of attack activity.
Feeding behaviour
After the swimming behaviour of the larvae was examined, continuous feeding behaviour experiments were conducted using the same larvae. A total of 300 (0.1 ind mL) Artemia nauplii were put into the glass basin, and the larvae were given 30 min for ingesting Artemia. This density was determined to compare their feeding ability under different light intensities. Thereafter, Artemia were removed from the gut of each larva, and then the number of Artemia was counted. (n = 10, under five different light intensities in each age).
Statistical analysis
Statistical analyses of larval swimming, aggressive and feeding behaviours between groups were performed using the Kruskal–Wallis test with multiple comparisons and the Steel–Dwass test (P < 0.05 was considered statistically significant). (Ekuseru–Toukei 2012, Social Survey Research Information).
Results
Swimming activity
Figure 1 shows the larval swimming activity under different light intensities. Three days after hatching, larvae had inflated their gas bladder. The larvae showed active swimming behaviour during experiments, swimming around the entire glass basin. Three-day-old larvae were not so active under 0.1 lx. By 5, 12 and 15 days, their swimming behaviour became more active, and they also demonstrated more active swimming under dim conditions.
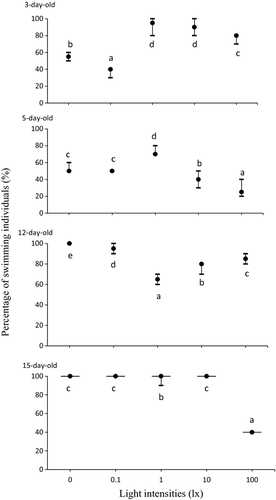
Under low light intensity (<0.01 and 0.1 lx) larvae almost constantly, at about the same speed, neared the surface. By contrast, under 1, 10 and 100 lx they swam throughout the water column and some larvae rested on the bottom.
Aggressive behaviour
Figure 2 shows larval aggressive (collision) behaviour under different light intensities. The larvae demonstrated attack activities (colliding with other individuals) only at 3 and 5 days. This behaviour was absent at 12 and 15 days under any different light intensities. The frequency of attack activities varied significantly between different light intensities; however, the larvae showed more aggressive behaviour under brighter light intensities. The larvae showed the highest level of aggressive behaviour under 100 lx. They swam throughout the water column and some of them stopped to swim at the bottom under 1, 10 and 100 lx, then they accidentally attacked the larvae those were resting on the bottom.
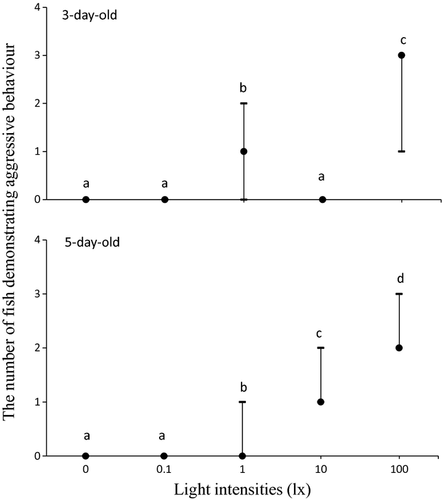
From the observation of larval behaviour in the fish-rearing aquarium, the long barbels of one individual became entwined with other individual's teeth. The larval bodies eventually became entwined in long strings with many other individuals. They could not escape from this chain, and consequently, they died.
Feeding behaviour
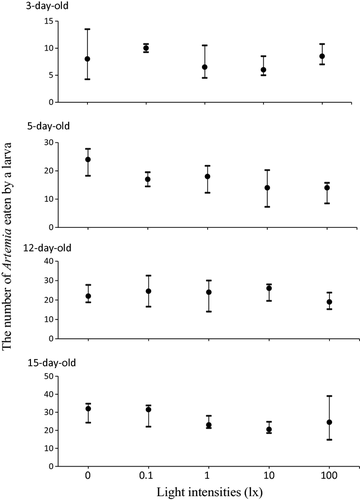
Figure 3 shows feeding behaviour under different light intensities. The larvae ingested almost the same number of Artemia nauplii, with no significant differences, under different light intensities. However, 5-day old larvae showed a tendency to eat a greater number of Artemia under lower light intensities than higher light intensities.
Discussion
Feeding behaviour of fish larvae is closely related to their sensory organs (Blaxter 1986; Mukai & Kobayashi 1991, 1995; Fuiman, Higgs & Poling 2004; Mukai 2006, 2011a; Mukai, Lang, Sitti Raehanah & Senoo 2007; Mukai, Tuzan, Lim et al. 2010).
Previous studies demonstrated that sutchi catfish have numerous taste buds, which may play an important role in their feeding behaviour(Mukai, Tuzan, Lim et al. 2010; Mukai, Tuzan, Sitti Raehanah et al 2010). During growth of sutchi catfish, the larvae showed strong cannibalistic behaviour, biting other individuals and injuring them. Body fluid could have been generated from the injured skin surface, releasing chemical substances into the environment, and triggering or stimulating the cannibalistic behaviours of other individuals.
The morphological characteristics of sutchi catfish larvae are large mouths, sharp oral teeth, oral spines and long barbels (Baras, Slembrouck et al. 2010; Mukai, Tuzan, Sitti Raehanah et al. 2010). Their long barbels become entwined with the sharp teeth or spines of other individual. This unusual behaviour possibly results from attacks (collisions) between individuals in the aquarium. Authors have often observed this phenomenon in seed production tanks (of 5 or 10 tonnes) of sutchi catfish. In the tanks, many larval bodies became entwined with each other, forming a long string from which they could not escape, eventually causing the death of many individuals. Baras, Slembrouck et al. (2010) also reported the similar phenomenon from their cultured larvae. Previous studies (Poulsen, Hortle, Valbo-Jorgensen, Chan, Chhuon, Viravong, Bouakhamvongsa, Yoorong, Nguyen & Tran 2004; Nguyen, Nguyen, Vu & Truong 2005) report that sutchi catfish spawn their eggs on the roots of plants in the river. Thereafter, they hatch out, drift downstream from the spawning ground and are eventually considered to remain in a huge water body. Under these circumstances they would seldom collide with each other in the field. Hence, the cannibalism of sutchi catfish must occur only under artificial conditions. They showed high activity from an early larval stage, and this behaviour would be well adapted to help them move downstream of the spawning grounds to enter the floodplain as their nursery grounds (Poulsen et al. 2004; Nguyen et al. 2005). When the larvae go downstream into the river, they remain in the huge water body, with very little likelihood of a collision. Baras, Ndao, Maxi, Jeandrain, Thome, Vandewalle and Melard (2000) also reported the similar larval behaviour of dorada, Brycom moorei.
Sutchi catfish larvae showed higher survival rates under dim conditions (0.1 lx) than those under light conditions (Mukai 2011a). In this study, we demonstrated that the larvae showed different behaviours under dark or dim and light conditions. Sutchi catfish showed strong cannibalism in early larval stages (less than 10 days). The results of this study indicate that the larvae accidentally attacked other individuals under higher light intensities. Then, in many a case, fish attacked the individuals that were resting on the bottom. We do not yet know the reasons why they showed increased aggressive behaviour (attacked other individuals) under higher light intensities, and further studies are needed to explore this observation; however, our results clearly demonstrated that larvae exhibit lower frequencies of aggressive behaviour under dark or dim conditions.
Mukai (2011a) also demonstrated that the body weights of larvae reared from 3 to 19 days after hatching with equal amounts of food varied between light intensities (<0.01 lx–100 lx). Although survival was about three times as high as under other light intensities, the body weights of larvae at 0.1 lx was not that different from other groups. Therefore, we recommend that larvae of sutchi catfish are reared under dim light during their early larval stage (first 10 days). Our results suggest that there is no aggressive behaviour thereafter, so there is apparently no need to rear under dim conditions beyond 10 days.
Acknowledgements
This study was supported by the Fundamental Research Grant Scheme (FRGS 0002-ST-1/2006) of the Ministry of Higher Education, Malaysia and the e-Science Fund (SCF 0056-AGR-2008) of the Ministry of Science, Technology and Innovation, Malaysia. We wish to thank Mr Ng Kam Foo for his technical assistance.




