Synchronization of ovulation in cultured northern whitefish (Coregonus peled, Gmelin 1788) using [D-Arg6Pro9Net]-sGnRH analogue and its effect on egg quality
Abstract
Female northern whitefish were treated with salmon [D-Arg6Pro9Net]-sGnRHa emulsified in Freund′s incomplete adjuvant as a sustained release treatment (sGnRHa-FIA) or with [D-Arg6Pro9Net]-sGnRHa dissolved in physiological saline as acute release treatment (sGnRHa-PS). Females in four experimental groups (A, B, C, D) and one control group (E) were injected intraperitoneally as follows: A and B – sGnRHa-FIA at doses of 50 and 25 μg kg−1, respectively; C – double injection (DI) of sGnRHa-PS at 25 μg kg−1 administered 3 days apart; D – single injection (SI) of sGnRHa-PS at 25 μg kg−1; and E – control group receiving a 1:1 mixture of FIA and physiological saline. All treatments induced and synchronized ovulation. Mean time to ovulation was significantly reduced (P < 0.05) in GnRHa-treated groups compared with control. Hormone treatments did not affect the relative fecundity. Slight differences were found in ovarian fluid pH between group A and D (P < 0.05). Except for group C, egg size was significantly reduced (P < 0.01) in hormonally synchronized groups compared with the control. Survival to the eyed stage of eggs from females in groups A, B and C was significantly lower (P < 0.01) than in groups D and E. Per cent hatched alevins was lower (P < 0.01) in groups A, B and C than in groups D and E. We conclude that synchronization of ovulation in northern whitefish can be induced by a single acute injection of [D-Arg6Pro9NEt]-sGnRHa at 25 μg kg−1 BW if given near the natural spawning time determined by water temperature below 2°C.
Introduction
Synthetic analogues of gonadotropin-releasing hormone (GnRHa) have proven effective in inducing and synchronizing ovulation in salmonids (Crim & Glebe 1984; Erdahl & McClain 1987; Kouřil, Barth, Štěpán, Fila, Příhoda & Flegel 1987; Breton, Weil, Sambroni & Zohar 1990; Mylonas, Hinshaw & Sullivan 1992; Gillet, Breton & Mikolajczyk 1996; Olito, Loopstra & Hansen 2001; Haffray, Enright, Driancourt, Mikolajczyk, Rault & Breton 2005; Mikolajczyk, Skolowska-Mikolajczyk, Szczerbik, Duc, Goryczko, Dobosz, Glogowski, Epler & Enright 2008; Noori, Amiri, Mirvaghefi & Baker 2010). In contrast to cyprinids, hormone therapy in salmonids mostly does not involve the use of dopamine inhibitors to suppress the inhibitory tone of dopamine on luteinizing hormone (LH) secretion from the pituitary (Podhorec & Kouřil 2009). Salmonids require a more prolonged LH surge, which can be achieved through sustained-release preparations (Breton et al. 1990; Goren, Gustafson & Doering 1995; Zohar & Mylonas 2001) or by multiple acute injections of the peptide (Mylonas et al. 1992). These methods of GnRHa delivery are not always compatible with fish farming practice due to high cost, limited availability and broodstock properties such as small size or fragility.
In immunology, adjuvants are used to initiate and augment the inflammatory reaction necessary for induction of optimal innate and adaptive immune responses to vaccines, as well as to ensure long lasting immunity (Safari, Dekker, Rijkers & Snippe 2011). Adjuvants can also increase the potency of antivenoms, thereby allowing a lower dose (Pratanaphon, Akesowan, Khow, Sriprapat & Ratanabanangkoon 1997) and reducing costs (Singh & O'Hagan 1999). In salmonid artificial reproduction, Freund′s incomplete adjuvant (FIA) has been used as a GnRHa carrier. Such GnRHa-FIA mixtures have shown an effect similar to that of commercial sustained release formulas when administered to rainbow trout (Oncorhynchus mykiss) (Arabaci, Diler & Sari 2004; Vazirzadeh, Hajimoradloo, Esmaeili & Akhlaghi 2008) and chum salmon (Oncorhynchus keta) (Park, Lee, Lee, Kim, Kim, Tamaru & Sohn 2007). Most recently GnRHa-FIA was successfully used for advancement and synchronization of ovulation in small-size and sensitive broodstock of brook char (Salvelinus fontinalis) (Svinger, Policar, Polakova, Steinbach, Jankovych & Kouril 2012).
Coregonid farming has a long history in the Czech Republic. In 1890, European whitefish (Coregonus lavaretus L.) were introduced to be extensively reared in polyculture with common carp (Cyprinus carpio) in deep ponds with cool water conditions. Northern whitefish (Coregonus peled), able to tolerate warmer water than the European whitefish, were introduced from Siberia 80 years later (Hochman 1987). In 1997, market-size whitefish production ranged approximately 140 tons (Annual report 2002, Czech Ministry of Agriculture). In the past decade, however, coregonid farmers have been challenged by expansion in the population of cormorants (Phalacrocorax carbo sinensis), and annual production decreased dramatically to 24 tons (Annual report 2011, Czech Ministry of Agriculture). The harvested survived fish are often severely wounded and exhibit signs of stress (Adamek, Kortan & Flajshans 2007). This is especially seen in the fragile northern whitefish.
Northern whitefish mature at 2 years. Similar to some salmonid species, spontaneous ovulation is extended over several weeks, and broodstock must be frequently checked for ovulatory status, due to early occurring arthretic processes occurring in eggs as early as 2–3 days after ovulation (Hochman 1987). In addition, unpredicted mass ovulation may occur in this species especially following a sharp drop in winter water temperatures to below 2°C (Hochman 1987). The combination of wounded broodstock and frequent handling results in high pre-spawning mortality, making artificial reproduction of this species difficult and unprofitable.
The first use of GnRHa to optimize coregonid broodstock management was reported by Mikolajczyk, Kuźmiński, Dobosz, Goryczko and Enright (2005) using [D-Nal(2)6aza-Gly10]-GnRHa azagly-nafarelin (Gonazon®) in European whitefish. Svinger, Kouril and Pavlista (2010) attempted to synchronize ovulation in northern whitefish using [D-Tle6Pro9NEt]-GnRHa Lecirelin (Supergestran®). While single acute injections of 16 and 32 μg kg−1 body weight (BW) of the Gonazon induced and synchronized ovulation in European whitefish (Mikolajczyk et al. 2005), a single acute injection of 25 μg kg−1 BW was not effective in northern whitefish, and a double injection protocol had to be employed to achieve the desired effect. This suggests that the northern whitefish requires the more prolonged stimulation of continuously elevated LH levels, which are ensured by GnRHa emulsified in FIA.
Good quality eggs are usually defined as those yielding low levels of mortality at fertilization, eying, hatch and first-feeding and those that produce the fastest-growing and healthiest fry and older fish (Bromage, Jones, Randall, Thrush, Davies, Springate, Duston & Barker 1992). Although the effectiveness of GnRHa on synchronizing ovulation in salmonids has been widely reported, thorough investigations of the impact of hormone treatments on egg quality and progeny are lacking, not only in northern whitefish, but in the subfamily Coregoninae in general.
The objectives of the present study were to investigate efficacy of GnRHa treatments on induction and synchronization of ovulation and its effect on egg quality in northern whitefish.
Material and methods
The experiment was carried out at the experimental facility of Faculty of Fisheries and Protection of Waters (FFPW). On November 24, 2010, 160 sexually mature 3-year-old northern whitefish of the same strain were transported to FFPW from Kinsky JSC Fish Farm, Czech Republic, Zdar nad Sazavou (49°35'1”N, 15°56'13”E). After transport, fish were sorted by sex, and 60 females (599 ± 73 g) were randomly divided into five flow-through raceways (0.8 m3) supplied with water from the Blanice River and held 10 days for acclimatization under the natural photoperiod. Sixty spermiating males (550 ± 126 g) were kept in a single raceway (4 m3) under similar water conditions. Only healthy and non-injured size-balanced fish were chosen for the experiment. Water temperature during acclimatization was gradually decreased from 3°C to 0.5°C at hormone administration. Oxygen content was 12.1 ± 0.8 mg L−1 throughout the experiment. Fish were not fed during the experiment.
- Group A: sGnRHa-FIA at 50 μg kg−1 BW
- Group B: sGnRHa-FIA at 25 μg kg−1 BW
- Group C: acute double injection (DI) of sGnRHa at 25 μg kg−1 BW given 3 days apart
- Group D: acute single injection (SI) of sGnRHa at 25 μg kg−1 BW
- Group E: control, injected with 0.9% NaCl mixed with FIA 1:1v/v (0.5 mL per individual)
To prepare GnRHa-FIA, GnRHa was dissolved in 0.9% NaCl and mixed with FIA (Sigma Aldrich, Czech Republic) 1:1 v/v using an Ika T-10 homogenizer. Each female received a total volume of 0.5 mL GnRHa-FIA preparation. For acute treatments, sGnRHa was diluted in 0.9% NaCl to the required concentration (25 μg mL−1). Females treated with acute injections (Group C and D) received 1 mL of solution per 1 kg BW. A quantity of 1 mL insulin in syringes fitted with a 0.7 × 30 mm needle was used for the administration of sGnRHa-FIA treatments. Acute treatments were made using a 1 mL insulin syringe fitted with a 0.33×12 mm needle.
After the second injection in group C, the ovulation status of each female was checked every 3 days by manual stripping. Females were considered to have ovulated if eggs were released with gentle manual pressure on the abdomen. Ovulating females were weighed and eggs were stripped. The total amount of eggs obtained from each female was weighed to calculate relative fecundity (RF = weight of stripped eggs × 100 × g−1 fish weight). Stripped females were tagged by fin cut for group identification and held for a short time in a weak potassium permanganate (KMnO4) bath, after which they were placed into 54 m3 concrete pond to observe post-spawning mortality for a 3 month period. At the end of the observation period (31 March 2011) fish were counted, killed and dissected to evaluate health status.
Immediately after stripping, ovarian fluid was collected and pH was measured using a multifunctional inoLAB 720 pH meter (WTW, 823 62 Weilheim, Germany). Egg diameter was measured to the nearest 0.001 mm using Quick PHOTO CAMERA 2.2 software (Olympus, Hamburg, Germany) from photographs taken with a binocular microscope Olympus BX51 fitted with an Olympus E-510 digital camera. Three samples of 150 ± 10 eggs were taken from each female and fertilized with an equal volume of pooled sperm from three males. Milt was collected at the time of fertilization using a 1 mL syringe for each male. Sperm was mixed in a small dish during the process of fertilization and examined for motility before use. Eggs were incubated in separate small incubators (see Kallert 2009, p. 22) with independently controllable inflow. Incubators were placed in a recirculation system equipped with a thermostatic cooler maintaining the water temperature at 2.5 ± 0.3°C throughout the incubation period. Shortly before hatching, the temperature was raised to 5°C based on information gained from hatchery practices (Hochman 1987). Oxygen content during incubation was maintained at 10.1 mg L−1, and pH level was 7.80 ± 0.02. Other water parameters such as Cl−, Fe, NH3, NH4, N-NO2 and N-NO3 were well below limits that could negatively impact egg development. Survival to the eyed stage and hatching rate was calculated in all samples as the per cent of eyed eggs and hatched alevins in the total number of eggs in the incubator.
All data were analysed by Statistica 9 Cz (StatSoft, Tulsa, USA). Ovulation course and per cent ovulated females were analysed using survival analysis (Z test). Differences in mean time to ovulation were analysed using non-parametric multicomparison Kruskal–Wallis test. One-way anova was used to characterize differences in ovarian fluid pH. Differences in egg diameter, per cent of eyed eggs and hatched alevins were assessed by hierarchical (nested) anova with individual females nested in treatment. Arcsin transformation was applied for all per cent values. If significant differences were found by anova, Tukey′s HSD test was applied for detailed multicomparison assay. The Kolmogorov–Smirnov test was used to confirm normal distribution of the data following homoscedasticity verification by Chochran–Bartlett′s test. Linear regression analysis was applied to correlate per cent of eyed eggs and hatched alevins and latency time. A significance level (α) of 0.05 was applied to all tests except where indicated. Data are presented as mean ± SEM.
Results
Induction and synchronization of ovulation
Compared with control group, all treatments significantly induced synchronized ovulation, whereas no significant differences were found among the treated groups (Fig. 1). First ovulation occurred on day 9 after the first injection in groups C and D, when 8.3% (1 of 12) of females ovulated in both groups. On day 12 after first injection 25% of females ovulated in groups A and B and 58.3%, 16.7%, 8.3% ovulated in groups C, D and E respectively (Fig. 1). On day 15, 66.7% of females had ovulated in groups A and B, and 100%, 75% and 25% in groups C, D and E respectively. On day 18, 91.7%, 100%, 91.7% and 66.7% of females were ovulated in group A, B, D and E respectively (Fig. 1). On day 21, all females in treated groups were ovulated, whereas 25% of females in group E remained unovulated. A 100% ovulation was reached on day 30 in group E. The DI of 25 μg kg−1 was the most effective treatment, reducing the mean time to ovulation to 13±1.9 days. Other treatments significantly reduced the mean time to ovulation to 15.5±2.7, 15.3±2.3 and 15.3±2.9 days in groups A, B, C and D, respectively, compared with 19.5±5 days in the control group, E (P < 0.05).
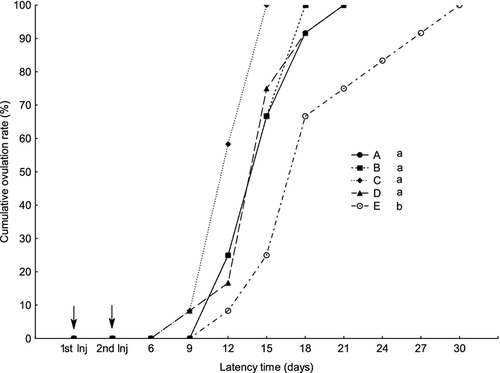
Effect of the treatments on egg quality
Relative fecundity was not influenced by the hormone treatments and no significant differences were found among groups. The relative fecundity reached 13.8 ± 2.9, 15.2 ± 1.6, 14.8 ± 2.6, 13.7 ± 3.2 and 14.8 ± 2.4% in groups A, B, C, D and E respectively (data not shown).
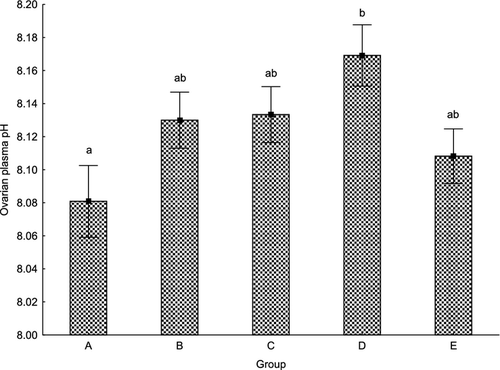
Statistical differences were found in ovarian fluid pH levels between groups A (8.08 ± 0.07) and D (8.17 ± 0.06) (P < 0.05) (Fig. 2). Among other groups, ovarian fluid pH levels were not significantly different. The measured levels were 8.13 ± 0.06 in groups B and C, and 8.11 ± 0.05 in group E. These values were also not different from those in groups A and D (Fig 2). No relationship between ovarian fluid pH level and survival to the eyed stage and hatching rate was found. Ovarian fluid pH remained constant throughout the experiment, and no relation between ovarian fluid pH and latency time was recorded in any of the groups.
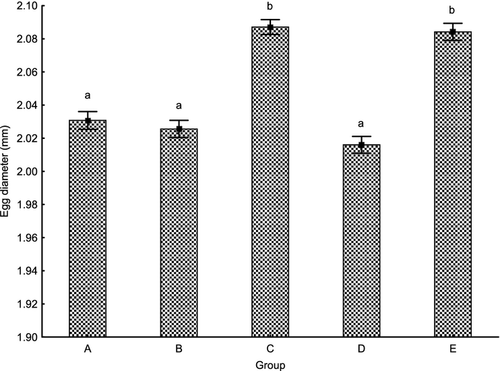
Egg size was significantly reduced in all GnRHa-treated groups compared with the control except group C (P < 0.01). Egg diameter was smaller in groups A, B and D (2.031 ± 0.005, 2.026 ± 0.005 and 2.016 ± 0.005 mm, respectively), compared to 2.087 ± 0.005 and 2.084 ± 0.005 mm in groups C and E respectively (Fig. 3). No relationship between egg size and survival to the eyed stage, hatching rate or latency time was found.
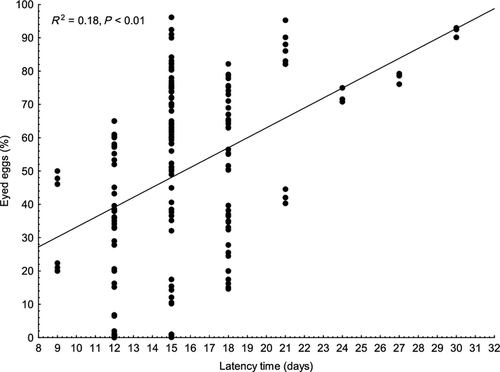
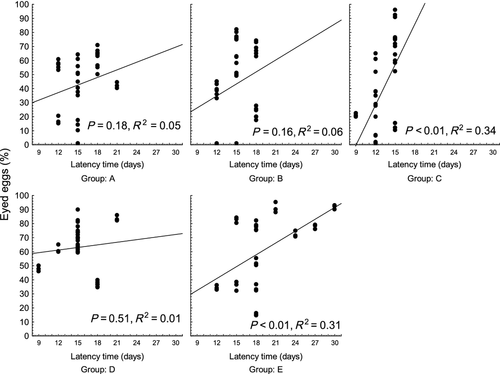
Survival to the eyed stage of eggs from females in groups A, B and C (43.5 ± 21.3, 44.0 ± 27.0 and 38.1 ± 31.1% respectively) was significantly lower (P < 0.01) than in groups D and E (63.2 ± 15.6 and 62.0 ± 25.5% respectively) (Fig. 4). A significant relationship was found if survival to the eyed stage was plotted against the latency time (P < 0.01, R2 = 0.18) (Fig. 5). Females with longer latency time appeared to produce slightly higher numbers of eggs at the eyed stage. The relationship was not strong, thus detailed regression analysis was applied within individual groups. This analysis revealed a significant relationship between eggs at the eyed stage and latency time in groups C and E (P < 0.01, R2 = 0.34 and P < 0.01, R2 = 0.31, respectively), with no relationship found in groups A, B and D (R2 = 0.05, R2 = 0.05 and R2 = 0.01) (Fig. 6).
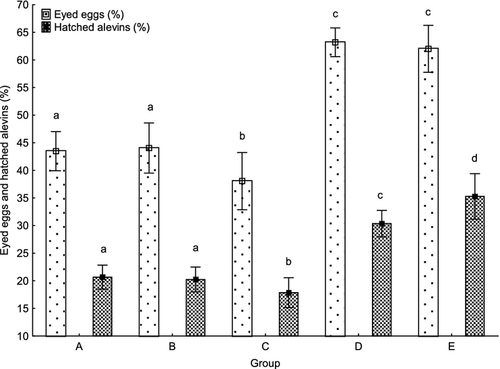
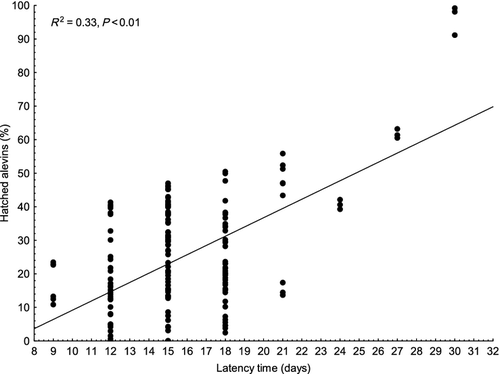
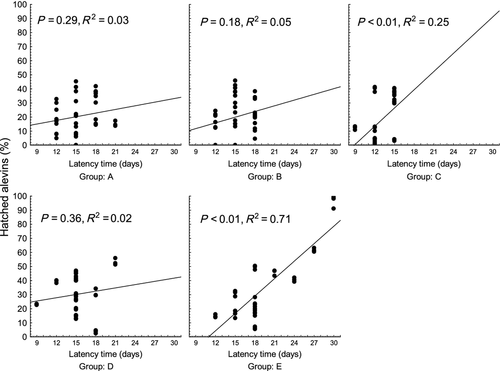
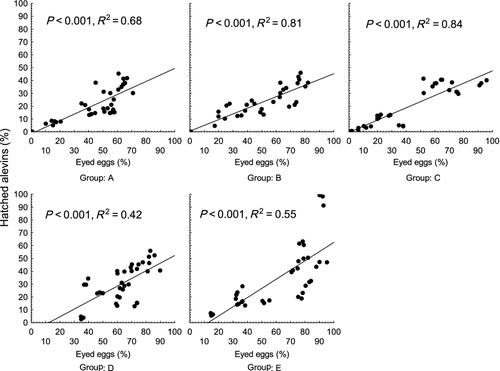
Per cent of hatched alevins was lower (P < 0.01) in groups A, B and C (20.7 ± 13.1, 20.2 ± 13.5 and 17.8 ± 16.2% respectively) than in groups D and E (30.4 ± 14.4 and 35.3 ± 24.7%, respectively), whereas the per cent of hatched alevins was significantly lower in group D compared with group E (P < 0.05) (Fig. 4). A significant relationship was found when per cent of hatched alevins was plotted against the latency time (P < 0.01, R2 = 0.33) (Fig. 7). Those females with longer latency time seemed to have a slightly higher per cent of hatched alevins. As in data on survival to the eyed stage, detailed regression analysis was applied within individual groups. The analysis revealed a significant relationship between per cent of hatched alevins and latency time in groups C and E (P < 0.01, R2 = 0.25 and P < 0.01, R2 = 0.71), with no relationship found in groups A, B and D (R2 = 0.03, R2 = 0.05 and R2 = 0.02) (Fig. 8). In all groups, survival to the eyed stage was highly predictive of the per cent of hatched alevins (R2 = 0.68, R2 = 0.81 R2 = 0.84, R2 = 0.42 and R2 = 0.55 for groups A, B, C, D and E, respectively, P < 0.001 for all) (Fig. 9).
No significant differences in post-spawning broodstock mortality were found in any group. In the 3 month post-spawning period 2, 1, 3, 1 and 2 females died in groups A, B, C, D and E respectively. Normally developing ovaries were found in the dissected fish, and no visible abnormalities (intra-abdominal lesions, bruises, haematomas or granulomas) were observed in either body wall or organ epithelium. Small residues (white flakes 1–1.5 mm diameter) of GnRHa-FIA emulsion were found in body cavity of all females treated with GnRHa-FIA.
Discussion
Synthetic analogues of gonadotropin releasing hormone neuropeptide (GnRHa) are effective in stimulating secretion of luteinizing hormone (LH) from the pituitary (Zohar, Muñoz-Cueto, Elizur & Kah 2010). Luteinizing hormone stimulates ovarian production of maturation-inducing steroids (MIS), e.g. 17α,20β-DP, and maturation-promoting factors, followed by ovulation (Nagahama & Yamashita 2008). The results from this study demonstrate that sGnRHa is effective for induction and synchronization of ovulation in northern whitefish. All utilized hormone treatments in this study significantly induced and synchronized ovulation compared with the control. These results are comparable with those obtained in other salmonid species using sustained GnRHa treatments (Breton et al. 1990; Arabaci et al. 2004; Park et al. 2007; Vazirzadeh et al. 2008; Svinger et al. 2010) or acute GnRHa treatments (Mylonas et al. 1992; Taranger, Stefansson & Hansen 1992; Mikolajczyk et al. 2008; Noori et al. 2010). On the other hand, our results indicate no differences between sustained release and acute treatment in northern whitefish. This is contrary to results of other authors reporting the efficacy of sustained release preparations in salmonids as much higher than that of acute release preparations. Breton et al. (1990) reported 100% ovulation in rainbow trout females treated with GnRHa sustained release form (using commercial microencapsulated form, in a polygylcolic ‒ polylactic biodegradable matrix), despite dosages of 12.5-50.0 μg kg−1, whereas 100% ovulation rate was never achieved in those females treated with acute injections at dose of 20 μg kg−1. Arabaci et al. (2004) and Vazirzadeh et al. (2008) used sustained GnRHa-FIA treatment at dosages of 25–50 μg kg−1 in rainbow trout, and its efficacy was again far above that of acute hormone injections. Furthermore, no significant differences were found between single or double acute injection in northern whitefish in the present study, although the double injection seemed to be slightly more effective. This is in contrast to previous results by Svinger et al. (2010) in northern whitefish using acute injections of mammalian [D-Tle6Pro9NEt]-GnRHa at the same dosages as those of the salmon [D-Arg6Pro9NEt]-GnRHa used in the present study. A single injection of 25 μg kg−1 of [D-Tle6Pro9NEt]-mGnRHa (Lecirelin, Supergestran®) induced ovulation only in a small number of the treated females, and the double injection at 25 μg kg−1 induced ovulation in only 75% of females. Similar to the results of the present study, single acute injections of [D-Nal(2)6Pro9Aza-Gly]-GnRHa at 16 μg kg−1 and 32 μg kg−1 have been reported to effectively induce and synchronize ovulation in European whitefish (Mikolajczyk et al. 2005) and European grayling (Thymallus thymallus) (Mikolajczyk et al. 2008). Distinct GnRHa molecules are known to have different LH release potency. [D-Arg6Pro9NEt]-GnRHa has been shown to be the most effective of GnRHa formulations tested in rainbow trout (Breton et al. 1990) and coho salmon (Oncorhynchus kisutch) (Kraak, Donaldson, Dye, Hunter, Rivier & Vale 1987). In the case of [D-Nal(2)6Pro9Aza-Gly]-GnRHa (Azaglynafarelin, Gonazon™), Haffray et al. (2005) reported a much longer half-life in rainbow trout than other GnRHa molecules. Therefore, potential differences between [D-Tle6Pro9NEt]-mGnRHa and [D-Arg6Pro9NEt]-sGnRHa in LH release potency may have led to the discrepancies in these two experiments in northern whitefish.
Exposure to reduced water temperatures during the weeks prior to ovulation appears to enhance steroidogenic activity (Vikingstad, Andersson, Norberg, Mayer, Stefansson & Taranger 2005) in salmonids and induce or stimulate ovulation, perhaps acting as a late-stage environmental cue (Taranger & Hansen 1993; Taranger, Stefansson, Oppedal, Andersson, Hansen & Norberg 2000; Vikingstad, Andersson, Norberg, Mayer, Klenke, Zohar, Stefansson & Taranger 2008). Results of Pankhurst and Thomas (1998) in rainbow trout and King and Pankhurst (2004) in Atlantic salmon (Salmo salar) suggest that maintenance of salmonids at elevated temperatures may impair pituitary responsiveness to the hormone treatment. The northern whitefish in a trial of Svinger et al. (2010) were injected at temperatures of nearly 5°C prior to the sharp drop in winter water temperature, while the fish in the present study were injected at 0.5°C, 1 week after the temperature drop. Whether this played a role in the high effectiveness of the single acute injection [D-Arg6Pro9NEt]-sGnRHa remains unclear, as we did not measure either the LH or steroid levels.
Relative fecundity is a useful working index for the farmer, as it allows egg production to be directly related to stocking levels, feeding rates, water supply, effluent constraints and the age and number of broodfish (Bromage et al. 1992). In our study, relative fecundity in hormone-treated females remained unchanged compared with control, and its values fell within the normal range for northern whitefish fecundity in the Czech Republic (Hochman 1987, Fish Farm Kinsky JSC).
Ovarian fluid pH level is considered one of the main parameters of ovarian fluid quality. Poor quality ovarian fluid is characterized by pH less than 7.4 in rainbow trout (Wojtczak, Dietrich, Slowinska, Dobosz, Kuzminski & Ciereszko 2007), whereas high quality ovarian fluid pH is 8.44–8.57 in lake trout (Salmo trutta m. lacustris) (Lahnsteiner, Weismann & Patzner 1999). Decreased ovarian fluid pH (to 7.29–7.67) negatively influenced spermatozoa motility, velocity and duration of movement in rainbow trout (Wojtczak et al. 2007). A decline in ovarian fluid pH (pH 6.5–6.55, Krishna 1953; pH 6.47, Dietrich, Wojtczak, Slowinska, Dobosz, Kuzminski & Ciereszko 2007) may occur if egg content escapes into the ovarian fluid, which may be due to over-ripening (Lahnsteiner 2000) or broken eggs (Dietrich et al. 2007). As no measurements of ovarian fluid in northern whitefish have been made before, we can only compare our values to those observed in other salmonid species and state that they are most similar to those observed in rainbow trout (Aegeter & Jalabert 2004, Wojtczak et al. 2007). The pH levels measured in northern whitefish in the present study were 8.00–8.23, and significant differences were found only between groups A and D, with no differences found between hormone-treated groups and control. Only one female of the 60 had ovarian fluid pH less than 8.0 at 7.93. In contrast to results of Lahnsteiner et al. (1999), no relationship between ovarian fluid pH and survival to the eyed stage was found, as narrow distribution of ovarian fluid pH values and high variability of survival to the eyed stage made the detection of any relation impossible. This suggests that optimum ovarian fluid pH in northern whitefish may lie within the range 8.00–8.23.
Egg size is implicated as a likely indicator of egg quality in a number of studies dealing with hormone-induced ovulation in salmonids, regardless of controversy over other egg quality determinants. Some researchers report that hormone treatment did not affect egg size (Hunter, Donaldson, Stone & Dye 1978; Donaldson, Hunter & Dye 1981a; Hunter, Donaldson & Dye 1981; Haraldsson, Sveinsson & Skúlason 1993; Bonnet, Fostier & Bobe 2007), whereas Donaldson, Hunter and Dye (1981b) reported reduced egg size with hormone treatment. In the present study, females subjected to three of four hormone treatments exhibited reduced egg size compared with the control group. Egg size in rainbow trout was reported to be reduced from 3.9 ± 0.1 to 3.7 ± 0.2 mm after ovulation was synchronized using similar GnRHa and GnRHa-FIA treatments (Arabaci et al. 2004), although the authors considered the differences non-significant. However, the measurement method for the large rainbow trout egg diameter might be questionable, as significant differences in our study could be observed on the order of hundredths of millimetre in small northern whitefish eggs. No relationship of egg size to survival at the eyed stage, hatching and latency period was found, suggesting that size does not affect on egg quality in northern whitefish. This is in accordance with other studies in salmonids (Glebe, Appy, T.D. & Saunders 1979; Kato & Kramler 1983; Thorpe, Miles & Keay 1984; Bromage & Cumaranatunga 1988). It has been demonstrated, however, that larger eggs produce larger first feeding fry (Pitman 1979; Springate & Bromage 1985; Springate, Bromage & Cumaranatunga1985), so it is possible that smaller fry might suffer higher mortality due to a smaller mouth gape if they do not receive enough food or are fed on large pellets or particles (Bromage et al. 1992). This might be crucial for northern whitefish fry needing minuscule food particles for first feeding. Thus, further experiments regarding the effect of hormone synchronization of ovulation on egg size are needed, especially as egg size was not reduced in the most synchronized and advanced acute double injection group.
Survival to the eyed stage was reduced in GnRHa-treated groups except for group D, treated with single acute injection of 25 μg kg−1, while hatching rate was reduced in all GnRHa-treated groups compared with controls. Many authors relate decrease in egg quality with the amount of GnRHa administered. Mylonas et al. (1992) recorded lowered eying when a double injection of 10 μg kg−1 was applied. Crim and Glebe (1984), Haraldsson et al. (1993), Taranger et al. (1992) and Olito et al. (2001) report that egg quality was reduced with a dose of 100–150 μg kg−1, and some authors have reported that egg quality following hormone treatment remained unaffected (Billard, Reinaud, Hollebecq & Breton 1984) or was better than in untreated fish (Sower, Iwamoto, Dickhoff & Gorbman 1984; Jansen 1993; Svinger et al. 2010). Results of the present study suggest that factors other than GnRHa dose affected egg quality, as eying and hatching was also reduced in GnRHa-FIA treatment at the 25 μg kg−1 GnRHa dose.
Survival to the eyed stage and hatching rate were positively correlated with latency time. Fish that ovulated later tended to have higher survival to the eyed stage as well as higher hatching rates. This is consistent with results of Mylonas et al. (1992), who obtained similar results in brown trout (Salmo trutta), suggesting time to ovulation as the major factor determining fertility regardless of GnRHa treatment. Crim and Glebe (1984) also recorded lowered viability of Atlantic salmon eggs stripped early in the course of their experiment. Closer analysis of our data in separate groups revealed that latency time influenced eying and hatching only in treated group C and control E, while no relationship was found among the other groups. This compares favourably with results obtained in coho salmon by Fitzpatrick, Suzumoto, Schreck and Oberbillig (1984), where low fertility was higher in GnRHa-treated fish, and egg fertility was lowest in females that ovulated early in the season in both GnRHa-treated and control groups. Mylonas et al. (1992) suggest that hormone treatment may increase natural asynchrony of the two processes, mediated by different reproductive hormones that take place at the completion of oocyte development: final oocyte maturation controlled by MIS and ovulation regulated by prostaglandins (Bradley & Goetz 1994; Goetz & Garczynski 1997), which may lead to ovulation of under-ripe and unfertilizable oocytes. This phenomenon was, however, also observed in rainbow trout stripped without hormone treatment, (Springate, Bromage, Elliot & Hudson 1984) and eggs stripped between 4 and 10 days following ovulation at 10°C consistently achieved high rates of fertilization. Under culture conditions, the eggs of rainbow trout are ovulated but not oviposited; they remain in the body cavity until they are artificially stripped from the fish. During the period of retention in the body cavity, the eggs undergo a ripening process (Bromage et al. 1992), including meiotic maturation, which may not be completed until some time after ovulation (Nagahama 1983). Thus, the reduction in survival to the eyed stage and hatching in other treated groups (A, B, C) and some individuals in group C and E might have resulted from stripping eggs immediately after ovulation and some of slightly immature or under-ripe eggs might have been forced out by the manual stripping (Bromage et al. 1992). In fact, one might ask what the results might be if the individuals with lower eying and hatching would have been stripped a day later, since the physiological and egg developmental stage of individual females may differ at the time of artificial stripping as well as at the time of hormone administration (Billard et al. 1984; Gillet et al. 1996). This may explain high and consistent survival to the eyed stage in group D, suggesting that these females were stripped at the appropriate time. Further experiments are needed to confirm this, since data on fertilization rates are lacking in our study and we are unable to state if egg size played a role in fertilizability of the eggs. On the other hand, Springate et al. (1984) and Bromage et al. (1992) reported that eggs with moderate or low fertilization rates invariably experience similar low rates of survival to eying and hatching. Thus, we assume that fertilization rate in our study may also have been reduced in those groups with low eying and hatching rate. This is supported by a strong relationship between survival to the eyed stage and hatching rate found in all groups in the present study. Increased mortality between the eyed stage and hatching was observed in group D, which resulted in significantly reduced hatching compared with the control group regardless of comparable survival to the eyed stage. This is in accordance with results of Bonnet et al. (2007) who concluded that spawning induction treatment in rainbow trout can produce limited but significant egg quality defects, which may be characterized by embryonic mortalities occurring after eying and possibly after hatching.
GnRHa-FIA treatment has been reported to be associated with high post-spawning mortality in rainbow trout broodstock (Arabaci et al. 2004). Although some females died in the post-spawning period in our experiment, no differences were found among the groups, and loss of fish was related to the intensive handling. This is in accordance with more recent studies where no post-spawning mortality was associated with GnRHa-FIA in rainbow trout and brook char (Vazirzadeh et al. 2008; Svinger et al. 2010). However, experiments focused specially on endocrine and immunological action of GnRHa-FIA treatments should be carried out in the future to exclude the possibility that GnRHa-FIA pose a risk for the broodstock. After 3 months, small residues of white GnRHa-FIA emulsion were still visible in the body cavity, which might call into question the suitability of the GnRHa-FIA-treated northern whitefish for human consumption. No such residue was detected in brook char 6 months post spawning (Svinger et al. 2010).
Conclusion
This study demonstrates that synchronization of ovulation in northern whitefish can be induced by a single acute injection of [D-Arg6Pro9NEt]-sGnRHa at 25 μg kg−1 BW if given near the natural spawning time determined by water temperature below 2°C. Induction and synchronization of ovulation by sGnRHa was associated with reduced egg size, survival to the eyed stage and hatching rate. Thus, further experiments are needed to clarify the cause of these changes and to determine whether reduction in egg size might influence egg fertilizability and survival of the fry.
Acknowledgements
This study was financially supported by projects: CENAKVA CZ.1.05/2.1.00/01.0024, GAJU 025/2011/Z, GAJU 047/2010/Z, NAZV QH 71305, Kontakt ME 10126 and NAZV QH91310. We thank the Lucidus Consultancy for providing English corrections.




