Protective potential of CpG 1668 motif-harbouring plasmids against Miamiensis avidus (Ciliophora: Scuticociliatida) infection in olive flounder (Paralichthys olivaceus)
Although several scuticociliate species are known to involve in scuticociliatosis in cultured marine fish (Dragesco, Dragesco, Coste, Gasc, Romestand, Raymond & Bouix 1995; Munday, O'Donoghue, Watts, Rough & Hawkesford 1997; Iglesias, Paramá, Álvarez, Leiro, Fernández & Sanmartín 2001; Jee, Kim & Park 2001; Kim, Cho, Kim, Nam & Kim 2004a; Kim, Cho, Lee, Kwon, Kim, Nam & Kim 2004b), the disease caused by infection of Miamiensis avidus (= Philasterides dicentrarchi) has been the most fatal parasitic disease in farm-reared olive flounder (Paralichthys olivaceus) in Korea, which usually leads to high cumulative mortalities (Kim et al. 2004a; Jung, Kitamura, Song & Oh 2007). As M. avidus is a histophagous ciliate and can invade into all internal organs of fish, eradication of the parasites from systemically infected fish using chemotherapeutics has not been possible. Therefore, immuno-prophylaxis using enhancement of adaptive or/and innate immunity would be a way to control scuticociliatosis.
Synthetic oligodeoxynucleotides (ODN) containing unmethylated CpG dinucleotides (CpG motif) are known as the strong stimulants for enhancing innate immunity of vertebrates (Krieg, Yi, Matson, Waldschmidt, Bishop, Teasdale, Koretzky & Klinman 1995; Krieg, Hartmann & Yi 2000; Krieg 2002), in which CpG motifs are recognized as a pathogen-associated molecular pattern (PAMP) by the Toll-like receptor 9 (TLR9) of immune cells. It has been reported that CpG-ODNs stimulate innate immune responses and enhance disease resistance of fish (Jørgensen, Zou, Johansen & Secombes 2001; Jørgensen, Johansen, Steiro & Johansen 2003; Lee, Jeong, Chung, Lee & Kim 2003; Tassakka & Sakai 2003; Rhodes, Rathbone, Corbett, Harrell & Strom 2004; Carrington & Secombes 2007). However, a little is known about the effects of CpG-ODNs on the resistance of fish against parasitic ciliates infections. Recently, we have demonstrated that CpG-ODN 1668 induced high serum scuticocidal activity and high protection against M. avidus infection in olive flounder (Lee & Kim 2009; Kang & Kim 2012).
Plasmids with stimulatory CpG motifs in their backbone also have potential to be used as immunostimulants and vaccine adjuvants in vertebrates. According to the results of Cornélie, Hoebeke, Schacht, Bertin, Vicogne, Capron and Riveau (2004), murine TLR9 specifically binds plasmids that contain unmethylated CpG motifs. In fish, Chen, Xiang and Shao (2007) had reported that plasmids harbouring tandem arranged 10 CpG motifs sequence (ODN 1681, 1669, 2133, 2102, 2143, 2006, 1826, 1670, 1668 and 1651) enhanced in vitro innate cellular immune responses, such as respiratory burst and bactericidal activities of head kidney macrophages of Crucian carp (Carassius auratus) and Japanese bass (Lateolabrax japonicus). Lately, Liu, Sun, Hu and Sun (2010) also demonstrated that a plasmid (pCN6) with sequences of four CpG motifs (209, 2133, 201 and 203) significantly enhanced respiratory burst, acid phosphatase, bactericidal activities of head kidney macrophages, serum bactericidal activity and resistance against Aeromonas hydrophila and Edwardsiella tarda infections in olive flounder.
Not only different CpG motifs, but also different copy number of a CpG motif can influence on the potency of immune responses in a species (Krieg 2002; Pontarollo, Babiuk, Hecker & Van Drunen Littel-Van Den Hurk 2002). In this study, to know whether the plasmid-based CpG 1668 motif and the copy number of CpG 1668 motif in plasmids can exert a beneficial effect on the resistance of olive flounder against M. avidus infection, fish were injected with plasmids containing various copies of the motif, and then the protective effect was analysed by investigating serum scuticocidal activity and survival rate of fish against M. avidus challenge.
M. avidus were isolated from diseased olive flounders obtained from local fish farms in South Korea. Identification of ciliates species was carried out using PCR using species-specific oligonucleotide primer pairs (Kim et al. 2004a). M. avidus were grown using Chinook salmon epithelia (CHSE)-214 cells incubated at 20°C in Eagle's minimum essential medium (MEM, Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco).
Fifteen copies of CpG 1668 motif were artificially synthesized and inserted into pUC57 vector (Cosmo Genetech, Korea). The fragment containing 15 copies of CpG 1668 motif was isolated by digestion of the vector with BglII and BamH1, and then ligated into LITMUS 28i vector (NEB) or pUC18 vector that were pre-digested with the same restriction enzymes, then designated them as pL-CpG15 and pUC-CpG15 respectively. The CpG15 fragment in the pL-CpG15 vector was isolated by digestion with BglII and BamHI, and ligated into pUC-CpG15 vector that was pre-digested with BamHI, resulting in a vector with 30 copies of the CpG 1668 motif (pUC-CpG30). After digestion of the vector with XbaI and KpnI, the CpG30 fragment was inserted into pLITMUS28i that was pre-digested with the same enzymes, resulting in pL-CpG30. Another CpG30 fragment isolated by digestion of the pUC-CpG30 with HindIII and EcoRI was ligated into the pL-CpG30, resulting in a vector with 60 copies of CpG 1668 motif, pL-CpG60.
Olive flounder fingerlings weighing approximately 6–7 g were obtained from a local fish farm, divided into ten 50 L tanks (20 fish tank−1), and were acclimated at least for 2 weeks before the experiment. Seawater temperature was maintained between 21 and 22°C during whole experimental period. Fish in the 10 tanks were divided into two replicates, and each replicate consisted of five groups. Fish in group I were intraperitoneally injected (i.p.) with 50 μL of phosphate buffered saline (PBS); fish in group II were i.p. injected with 100 μg of LITMUS 28i vector in 50 μL PBS; fish in group III, IV and V were i.p. injected with 100 μg of pL-CpG15, pL-CpG30 and pL-CpG60 in 50 μL PBS respectively. At 3 days post injection, five fish in each tank were randomly sampled and bled to isolate serum. The rest of fish (15 fish tank−1) in one replicate were i.p. challenged with 1 × 104 ciliates in 50 μL MEM, and fish in the other replicate (15 fish tank−1) were challenged with 2.5 × 104 ciliates in 50 μL MEM. The mortality was monitored for 20 days, and dead fish were necropsied to confirm the presence of ciliates. The analysis of scuticocidal activity of serum was performed according to the method described in Kang and Kim (2012). Briefly, all the sera were serially diluted ranging from 1/4 to 1/1024 in Hank's balanced salt solution (HBSS, Sigma) using 96-well flat-bottomed micro- titration plates. The ciliates were added to the wells (4 × 102 ciliates well−1) of the plate, incubated at 20°C and observed every 1 h for 24 h to analysis scuticocidal activity of the sera. The titre of each serum was the last dilution at which 100% of the ciliates were lysed or non-motile, which was observed under an inverted microscope at 40–100 × magnification. In all assays, control wells containing heat-inactivated pooled sera (at 50°C for 30 min; 1/4 dilution) of each experimental group and wells containing no serum were included.
The data were analysed using the Student's t-test, and significant differences were determined at P < 0.05.
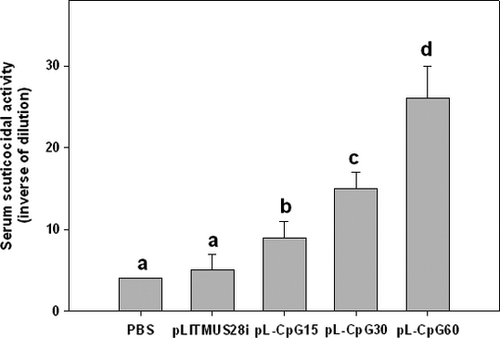
As a result, fish injected with plasmids harbouring CpG 1668 motif showed significantly higher serum scuticocidal activity than fish injected with PBS alone or LITMUS 28i vector alone (Fig. 1). Furthermore, the serum scuticocidal activity was significantly increased in proportion to the number of CpG 1668 motif in the plasmids. No scuticocidal activity was observed in the wells containing heat-inactivated sera or HBSS alone.
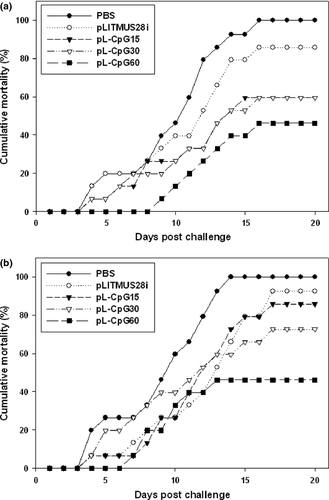
In the replicate that was challenged with 1 × 104 ciliates, the cumulative mortality rates of fish injected with plasmids harbouring CpG 1668 motif were clearly lower than fish injected with PBS alone or LITMUS 28i vector alone (Fig. 2a). The group injected with pL-CpG60 showed the highest survival rate, and there were no differences in survival rate between groups of fish injected with pL-CpG15 and pL-CpG30. In another replicate that was challenged with 2.5 × 104 ciliates, the group of fish injected with pL-CpG60 showed consistently high survival rate (Fig. 2b). Although fish injected with pL-CpG30 showed increased mortality compared with the fish challenged with lower number of ciliates, the survival rate was still clearly higher than fish injected with PBS alone or LITMUS 28i vector alone.
It has been reported that the immunostimulatory effect of CpG motifs are sequence specific (Hartmann & Krieg 2000; Verthelyi, Ishii, Gursel, Takeshita & Klinman 2001; Krieg 2002; Vollmer, Weeratna, Payette, Jurk, Schetter, Laucht, Wader, Tluk, Liu, Davis & Krieg 2004). According to the recent structure-based classification of CpG-ODNs (Krieg 2002), CpG-ODN 1668 is belonging to the B-class ODN that is characterized by possessing at least one CpG motif and phosphorothioate backbone. Lately, we have compared immunoprophylactic potential of CpG-ODNs belonging to three different classes (A, ODN 2216; B, ODN 1668; C, ODN 2395) against M. avidus infection, and found that CpG-ODN 1668 has the highest capability to enhance resistance of olive flounder against the ciliates challenge (Kang & Kim 2012). However, as the cost for synthesis of CpG-ODNs is too high to use them in aquaculture, the use of plasmids containing multiple CpG motifs instead of artificially synthesized CpG-ODNs would be a way to low the cost. In this study, we performed experiments to know whether CpG motif-containing plasmids can be used to increase resistance of fish against infectious agents, and have firstly demonstrated that plasmids harbouring multiple CpG 1668 motif enhance the resistance of olive flounder against M. avidus infection. This result suggests that plasmids harbouring CpG motifs might be used as immunostimulants in fish instead of costly CpG-ODNs.
In the results of this study, the plasmids containing 15–60 copies of CpG 1668 motif induced higher scuticocidal activity in serum and higher survival rates against M. avidus challenges than the control PBS alone or the plasmid without CpG 1668 motif, suggesting that the plasmid with multiple copies of CpG 1668 motif can be recognized as a danger signal and can activate anti-parasitic immune responses in olive flounder as in CpG-ODN 1668 (Lee & Kim 2009; Kang & Kim 2012). Furthermore, results of this study showed that the scuticocidal activity and the survival rates were increased in proportion to the copy number of CpG 1668 motif in the plasmids, and fish injected with the plasmids harbouring the largest copy number of CpG 1668 motif (pL-CpG60) showed the highest serum scuticocidal activity and survival rate. These results suggest that the copy numbers of CpG motif in the plasmid exert a positive effect on the induction of immune responses that play critical roles in defence against M. avidus infection. It has been demonstrated that recognition of CpG-ODNs by TLR9 activates complement signalling in mammals (Henry, Giclas, Leeds, Pangburn, Auletta, Levin & Kornbrust 1997; Zhang, Kimura, Fang, Zhou, Sfyroera, Lambris, Wetsel, Miwa & Song 2007; Mangsbo, Sanchez, Anger, Lambris, Ekdahl, Loskog, Nilsson & Tötterman 2009). Therefore, the present loss of serum scuticocidal activity by heat-inactivation indicates that the present scuticocidal activity might be mediated through activation of alternative complement pathway.
Selection of exact CpG motifs and optimizing the copy number in CpG-enriched plasmids could influence on the induction of favourable immune responses. The present results indicate that at least 60 copies of CpG 1668 motif are needed to effectively defence against even a high challenge dose of M. avidus. However, it remains unclear whether additional copies of CpG 1668 motif can more strongly enhance the anti-scuticociliate responses in olive flounder. Furthermore, which host factors are involved in the recognition of different copies of CpG motif in the plasmid remains unknown. Investigations to uncover this mechanism must be conducted further. In summary, we have demonstrated that CpG 1668 motif-enriched plasmids can be used to enhance resistance of olive flounder against M. avidus infection.
Acknowledgments
This study was supported by the research fund (Project no. #20088033-1) from the Ministry of Land, Transport and Maritime Affairs, Republic of Korea.




