Collagen proportionate area predicts clinical outcomes in patients with alcohol-related liver disease
Mads Israelsen and Marta Guerrero Misas shared co-first authorship. These authors contributed equally to the work.
The Handling Editor for this article was Professor Gideon Hirschfield, and it was accepted for publication after full peer-review.
Funding information
Mads Israelsen is supported by Odense University Hospital and The University of Southern Denmark. Alberto Quaglia and Massimo Pinzani are supported by the National Institute for Health Research (NIHR) UCLH/UCL Biomedical Research Centre (BRC). Anastasios Koutsoumourakis was supported by an educational scholarship from the Hellenic Society of Gastroenterology. The Danish cohort of 110 patients were acquired from a Galaxy sub-study that has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement number 668031 and Challenge Grant “MicrobLiver” grant number NNF15OC0016692 from the Novo Nordisk Foundation. The data from the Australian population was acquired from a NHMRC-funded study – APP 1128889.
Summary
Background
No prognostic tools are established for alcohol-related liver disease (ALD). Collagen proportionate area (CPA) measurement is a technique that quantifies fibrous tissue in liver biopsies using digital image analysis.
Aim
To assess the predictive value of CPA on hepatic decompensation and liver-related mortality in ALD
Methods
In a multicentre cohort study, we included 386 patients with biopsy-verified ALD and with long-term follow-up. In the development cohort of 276 patients, we assessed the predictors of hepatic decompensation and liver-related death in standard and competing risk multivariable Cox regression analyses. The results were validated in an independent prospective cohort of 110 patients, where CPA was also correlated with liver stiffness measurement (LSM).
Results
In the development cohort, 231 (84%) patients had early/compensated ALD (non-cirrhotic or compensated cirrhosis) and 45 (16%) had decompensated cirrhosis. In the validation cohort, all patients had early/compensated ALD. Independent predictors of liver-related mortality were higher CPA values (HR = 1.04, 95% CI 1.02-1.04) and advanced fibrosis (HR = 2.80, 95% CI 1.29-6.05) with similar results in standard and competing risk multivariable Cox regression analysis. In early/compensated ALD, CPA was the only independent predictor of hepatic decompensation and liver-related death (HR = 1.08, 95% CI 1.06-1.11). In the prospective cohort, we validated that CPA independently predicts hepatic decompensation in early/compensated ALD. The predictive power of CPA and LSM was equally strong.
Conclusions
CPA predicts liver-related mortality in ALD and hepatic decompensation and/or liver-related death in early/compensated ALD. Traditional histological assessment may benefit from the addition of CPA to the evaluation of ALD.
1 INTRODUCTION
Globally 2.4 billion people consume alcohol on a regular basis with an average intake of 32.8 g of pure alcohol per day.1, 2 In the UK, 22% of the population drink alcohol above the recommended limits and 4% above the high-risk limits.3 Alcohol is attributable to almost half of all cirrhosis deaths worldwide and the numbers are rising.4-6 From 1999 to 2016, the United States have experienced a 2%-10%5 annual increase in mortality due to alcohol-related liver disease (ALD) and in the UK, alcohol-related deaths have tripled from 1980 to 2008 mainly due to liver disease.7 The pathogenesis of ALD is complex and less than 20% of those who drink in excess will progress to cirrhosis.8 Several non-invasive methods are available for ruling out advanced fibrosis in ALD; however, a liver biopsy is still required to establish the diagnosis and assess the exact stage of disease in selected patients.9, 10 There is no histological scoring system developed specifically for ALD; however, liver fibrosis is routinely reported using the semiquantitative Brunt histological scoring system.11 A recent study confirmed that advanced fibrosis is associated with liver-related mortality in patients with early/compensated ALD.12 However, there are large variations in mortality within patients with similar histological lesions. In patients with biopsy-proven alcohol-related cirrhosis, who survive their index hospitalisation, 15% die within 1 year, while 36% survive beyond 10 years. Similarly, 9% of patients with fibrosis (but not cirrhosis) die within the first year of their diagnosis, while 49% survive beyond 10 years.13 Consequently, quantitative measurement of liver fibrosis in such patients might enhance the prognostic ability of semiquantitative liver biopsy fibrosis staging.
Collagen proportionate area (CPA) is a method that accurately quantifies the amount of collagen in liver biopsies by digital-assisted analysis with very high reproducibility.14 Our group has demonstrated that applying CPA to the histological assessment of liver biopsies improves the prediction of hepatic decompensation and mortality in chronic viral hepatitis, non-alcoholic fatty liver disease (NAFLD) and cirrhosis of mixed aetiologies over and above traditional semiquantitative staging of fibrosis.15-19 However, the prognostic utility of CPA has never been evaluated in ALD.
Our primary aim was to assess the association of CPA with liver-related events and overall mortality in two independent cohorts of patients with available biopsies and longitudinal follow-up. Our secondary aim was to assess the relationship between CPA with other diagnostic modalities for alcohol-related liver fibrosis including liver stiffness measurements (LSMs) and semiquantitative fibrosis staging.
2 PATIENTS AND METHODS
This was a multicentre, retrospective study comprising two retrospective cohorts and one prospective research cohort in patients with biopsy-proven ALD. The study received approvals from the institutional review board of each participating institution.
2.1 Patients
Consecutive patients with available histology and longitudinal follow-up were identified from a structured search in the pathology archives at the Royal Free Hospital in London, United Kingdom and the Sir Charles Gairdner Hospital in Perth, Australia. Inclusion criteria were a history of alcohol misuse, histological features in keeping with ALD and known survival status at follow-up. Both patients with compensated and decompensated cirrhosis were included. Patients with all fibrosis stages (F0-F4) and no previous episode of hepatic decompensation were classified as early/compensated ALD. Patients with competing liver diseases including alcoholic hepatitis were excluded. Demographic, biochemical and data on daily alcohol intake were recorded at the time of liver biopsy.
Follow-up data were obtained until last clinical follow-up or at the time of liver transplantation or death. Data collection was censored in May 2017. The development of hepatic decompensation (defined as the development of either ascites, variceal bleeding, hepatic encephalopathy or clinical non-obstructive jaundice) during the follow-up period, as well as the survival status of each patient were recorded at the end of the data collection, through the clinical notes, general practitioner enquiries or the national health system-integrated hospital register. Patients were classified as abstinent during the follow-up period, only if abstinence was consistently stated in medical records from the time of the liver biopsy onwards.
In an independent prospective research cohort of patients with early/compensated ALD from Odense University Hospital in Denmark, the association between CPA and LSM by transient elastography (FibroScan®) and 2D shear wave elastography (Supersonic Aixplorer®) was assessed.20 Inclusion criteria were a history of chronic excess alcohol drinking for at least 1 year and consenting to a liver biopsy and no prior hepatic decompensation. Liver biopsy and LSM were performed on the same day after an overnight fast.20 Long-term outcome data were obtained through medical records until last clinical follow-up or at the time of liver transplantation or death. Hepatic decompensation (defined as the development of either ascites, variceal bleeding, hepatic encephalopathy or clinical non-obstructive jaundice) during the follow-up period, as well as the survival status of each patient were recorded at the end of the data collection.
2.2 Histological assessment
Liver biopsy samples were assessed centrally by a single liver pathologist (AQ) blinded to all clinical data. Liver fibrosis was assessed according to the Brunt scoring system for fibrosis; F0, no fibrosis; F1, zone 3 perisinusoidal fibrosis; F2, perisinusoidal and portal fibrosis; F3, bridging fibrosis; F4, cirrhosis.11 Cirrhosis was subclassified according to the Laennec scoring system; 4A, Marked septation with rounded contours or visible nodules, most thin fibrotic septa (one broad septum allowed); 4B, two or more broad fibrotic septae, but no very broad septa and less than half of biopsy length composed of minute nodules; 4C, at least one very broad septum or more than half of biopsy length composed of minute nodules (micronodular cirrhosis).21
2.3 Measurement of collagen proportionate area
Paraffin-embedded liver biopsies were stained centrally in one batch with picroSirius red and whole section digital images were obtained using a digital camera. To obtain the standard magnification (SM) CPA, the whole section digital image was hereby measured by image analysis software, with a step of manual elimination of structural collagen and artefacts. CPA was measured with Zeiss KS300 image analysis software. A RGB (Red, Green, Blue) threshold was then used to detect areas of stained collagen and the collagen mask was calculated as an area in pixels. A ratio (CPA) is expressed as a percentage.14, 17 The same procedure was used to obtain high magnification (HM) CPA, but high power (x4) image capture was performed using a microscope and Zeiss Axiocam ICc5 as previously described.19
The difference between SM and HM CPA was markedly lower (<30%) across all fibrosis stages when compared with patients with NAFLD.19 Due to the strong concordance between SM and HM CPA, we chose to continue using SM CPA for the statistical analyses, because SM is easier to measure and has been used for the majority of previous studies of CPA in liver disease. For simplicity, we use the term CPA in the manuscript referring to SM CPA.
CPA measurement is associated with significantly less interobserver variability compared to semiquantitative histological staging as it is automated with only one manual step of removal of structural collagen. Previously, we demonstrated that the interobserver agreement was excellent (k = 0.912) with median CPA difference between observers of 2%.19 Furthermore, for this study, CPA measurements were performed centrally by a single operator (MGM) who was blinded to the clinical data of the patients.
2.4 Statistics
Descriptive data are reported as counts with frequencies, means with standard deviations and medians with interquartile ranges (IQR) according to data distribution. Categorical data were compared using Fisher's exact test. All comparisons between two groups with regard to continuous and categorical data were assessed with Wilcoxon rank-sum test. Spearman's rank-order correlation was used to test the association between CPA and LSM. Univariable, standard multivariable and competing risk Cox regression analyses were performed to determine the predictors of clinical outcomes (liver-related mortality and all-cause mortality in the overall cohort and hepatic decompensation and/or liver-related mortality in patients with early/compensated ALD). Except for all-cause mortality, liver transplantation and death unrelated to liver disease were classified as competing risks. In a univariable analysis, we assessed the predictors as follows: recruitment centre, sex, age at time of biopsy, abstinence from time of biopsy, volume of alcohol intake at time of biopsy, CPA, Brunt fibrosis stage, advanced fibrosis (≥F3) and the Model for End-Stage Liver Disease (MELD) score. Parameters with P-value < 0.10 from the univariable analysis were included in the multivariable analysis, where stepwise backward elimination was applied. As there were only minor variation between the result from the standard and competing risk analyses, we reported results from the competing risk analysis. The analyses were repeated in a subgroup of patients (81%) where FIB4 and APRI values were available. Area under the receiver operating characteristics (AUROC) curve was used to compare predictors of liver-related outcomes at 2 years and maximum follow-up. The Youden's index was used to determine CPA cut-offs with optimal sensitivity and specificity for predicting such events. Subsequently, the cohort was split according to these cut-offs and the outcome distribution was compared using log rank test and illustrated by Kaplan-Meier plots. Harrell's C index was used to compare predictive power between prognostic parameters in the validation cohort. All calculations were performed using STATA 16 (College Station, TX, US).
3 RESULTS
3.1 Patients
We included 386 patients with biopsy-proven ALD, 276 in the development cohort and 110 in the validation cohort. Baseline characteristics of the patients in the development cohort are summarised in Table 1 and of the validation cohort in Table S1. In the development cohort, 177 (64%) patients were non-cirrhotic, while 99 (36%) had cirrhosis, of which 231 (84%) had early/compensated ALD and 45 (16%) had decompensated cirrhosis. There was a significantly lower prevalence of cirrhosis and previous decompensation in the UK compared to the Australian cohort (AU).
| Variable | UK, n = 207 | AU, n = 69 | P-value | Overall, n = 276 |
|---|---|---|---|---|
| Age, years | 51 ± 11 | 53 ± 13 | 0.403 | 52 ± 12 |
| Ethnicity, Caucasian n (%) | 149 (72) | NA | NA | |
| Males, n (%) | 154 (74) | 40 (58) | 0.014 | 194 (70) |
| BMIb, kg/m2 | 27.5 ± 5.5 | NA | ||
| Alcohol intake, g/day | 126 (157) | 50 (100) | 0.003 | 120 (167) |
| Hypertension, n (%) | 53 (26) | NA | ||
| Dyslipidaemia, n (%) | 40 (19) | NA | ||
| Diabetes, n (%) | 18 (9) | NA | ||
| CVD, n (%) | 15 (7) | NA | ||
| Brunt fibrosis staging, n (%)a | ||||
| F0, n | 5 (2) | 0 (0) | 0.339 | 5 (2) |
| F1, n | 49 (24) | 8 (12) | 0.039 | 57 (21) |
| F2, n | 57 (28) | 5 (7) | <0.001 | 62 (23) |
| F3, n | 41 (20) | 10 (15) | 0.471 | 51 (19) |
| F4, n | 55 (27) | 44 (66) | <0.001 | 99 (36) |
| Early/compensated ALD, n (%) | 184 (89) | 47 (78) | <0.001 | 231 (84) |
| Decompensated ALD, n (%) | 23 (11) | 22 (32) | <0.001 | 45 (16) |
| CPA, (%) | 4.1 (8.9) | 16.2 (20.9) | <0.001 | 5.1 (15.8) |
| MELD score | 6 (2) | 9.5 (12) | <0.001 | 6 (3) |
| Platelets, 109/L | 224 (44) | 171 (38) | 0.023 | 214 (39) |
| ALT, U/L | 44 (44) | 36.5 (38) | 0.127 | 43 (39) |
| Bilirubin, μmol/L | 10 (9) | 16 (76) | <0.001 | 11 (12) |
| Albumin, g/L | 43 (8) | 36 (13) | <0.001 | 42 (9) |
| INR | 1 (0.2) | 1.1 (0.4) | <0.001 | 1 (0.2) |
Note
- Values are reported as mean ± standard deviation, counts (proportion) and median (IQR). P-values compare centres and are obtained by unpaired t-test, Fisher's exact test and Wilcoxon rank-sum test according to the distribution.
- Abbreviations: ALD, alcohol-related liver disease; ALT, alanine aminotransferase; AU, Australian cohort; BMI, body mass index; CPA, collagen proportionate area; CVD, cardiovascular disease; HDL, high-density lipoprotein; INR, international normalised ratio; LDL, low-density lipoprotein; MELD, the Model for End-Stage Liver Disease; NA, not available; TG, triglycerides; UK, the United Kingdom cohort.
- a Biopsies adequate for Brunt fibrosis staging 274/276.
- b BMI was available in 127 patients. Alcohol intake was recorded at the time of biopsy. Abstinence was recorded from time of biopsy.
The mean age was 52 ± 12 years, 194 (70%) were males and 86 (31%) reported that they were abstaining from alcohol from the time of the biopsy onwards. In the validation cohort, all patients had early/compensated ALD of which 43 (39%) had advanced liver fibrosis (≥F3) and 27 (25%) had compensated cirrhosis. Mean age was 56 ± 9 years and 82 (75%) were males.
3.2 Correlation of CPA with fibrosis stage and LSM
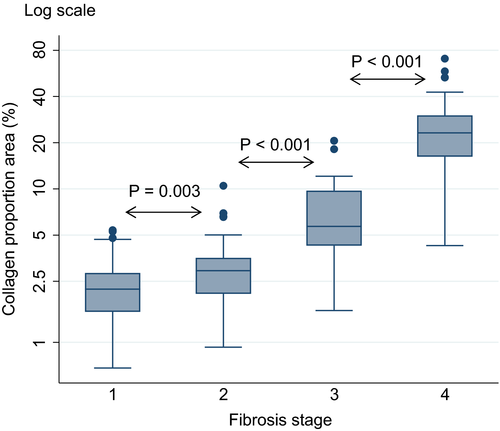
CPA increased gradually with increasing fibrosis stage and the Laennec subclassification of cirrhosis, with significant differences of the CPA values between each stage (Figure 1).
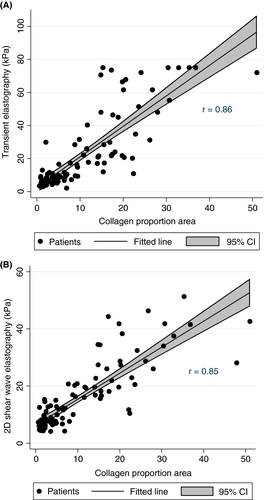
In the validation cohort of 110 patients with liver biopsy and LSM performed on the same day, we found a strong correlation between CPA and LSM by transient elastography (r = 0.86) and 2D shear wave elastography (r = 0.85) (Figure 2 and Table S2).
3.3 Long-term outcomes in the development cohort
The clinical outcomes are summarised in Table S3.
3.4 All patients
In total, 79 (29%) patients died after a median follow-up of 81 (IQR 103; range 1-317) months. The cause of death was liver-related in 58 cases (73%) and 21 patients (27%) died from non-liver-related disease causes including non-primary liver cancer or cardiovascular disease. Four patients had liver transplantation during follow-up, of which three had decompensated cirrhosis at the time of biopsy. Twenty-two deaths happened within the first 24 months after the liver biopsy, of which 16 deaths were related to liver disease. From the time of biopsy to the end of follow-up, 75 (35%) patients reported that they were consistently abstinent from alcohol.
3.5 Early/compensated ALD
Seventy-eight (34%) hepatic decompensations or deaths were recorded in patients with early/compensated ALD in a follow-up of 90 (IQR 100, range 1-317) months. Fifty-three patients developed hepatic decompensation, five died of liver disease without a preceding episode of hepatic decompensation and 20 deaths were from non-liver-related disease causes. Hepatic decompensation was in most cases associated with multiple manifestations (n = 21, 40%), whereas the most frequent single manifestation was ascites (n = 15, 28%) followed by non-obstructive jaundice (n = 8, 15%), variceal bleeding (n = 6, 12%) and hepatic encephalopathy (n = 3, 6%). Hepatic decompensations or death occurred in 28 patients within the first 24 months after liver biopsy of which 23 events were related to liver disease.
3.6 Long-term outcomes in the validation cohort
Thirty-two (29%) patients experienced hepatic decompensation and 19 (17%) died in a follow-up of 45 (IQR 28, range 1-75) months (Table S3).
3.7 CPA predicts liver-related death in ALD
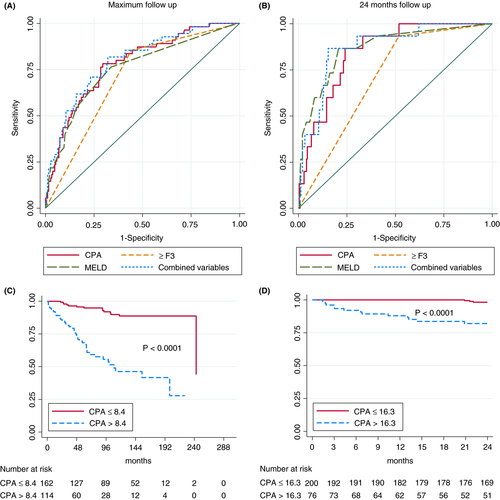
We tested the predictors of liver-related death in univariable analysis in the overall development cohort including also patients with decompensated liver disease at baseline. Predictors with P-value < 0.1 were entered in multivariable Cox regression analysis (Table 2). In the competing risk multivariable Cox regression analysis, higher CPA values (HR = 1.04, 95% CI 1.02-1.07) and advanced fibrosis (≥F3) (HR = 2.77 95% CI 1.26-6.09) predicted liver-related death at maximum time follow-up. When follow-up was restricted to 24 months after biopsy, predictors of liver-related mortality were higher CPA values (HR = 1.03, 95% CI 1.002-1.061), higher MELD score (HR = 1.07, 95% CI 1.03-1.011) and being from the AU (HR = 5.44, 95% CI 1.58-18.77). Figure 3A,B show the AUROCs of CPA, MELD and the presence of advanced fibrosis. Using cirrhosis instead of advanced fibrosis did not result in a higher AUROC value (0.69). The optimal cut-off for CPA was 8.4% for predicting liver-related death at maximum follow-up (AUROC = 0.72, sensitivity 76% and specificity 69%). The best CPA cut-off for prediction of liver-related death within 24 months was 16.3% (AUROC = 0.79, sensitivity 81% and specificity 76%). Figure 3C,D illustrate the liver-related survival probability according to the optimal CPA cut-offs. Figure S1 illustrates liver-related survival probability according to fibrosis stage and abstinence. Abstinence (compared to non-abstinence) had a more pronounced beneficial effect on liver-related events in patients with F0-F2 than F3-F4 fibrosis (Figure S2). When follow-up was restricted to 48 months after biopsy, results were similar to maximum follow-up (data not shown).
| Variable | Univariable | Multivariable | Competing multivariable | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| (A) At maximal follow-up | ||||||
| Cohort (AU) | 3.07 (1.74-5.42) | <0.001 | NS | NS | ||
| Sex (female) | 1.70 (1.00-2.90) | 0.051 | NS | NS | ||
| Age at biopsy | 1.01 (0.99-1.03) | 0.363 | ||||
| Abstinence | 0.71 (0.40-1.25) | 0.238 | ||||
| Alcohol quantity | 1.00 (1.00-1.00) | 0.149 | ||||
| CPA | 1.07 (1.05-1.08) | <0.001 | 1.05 (1.03-1.08) | <0.001 | 1.05 (1.03-1.07) | <0.001 |
| Brunt fibrosis stagea | 2.21 (1.67-2.93) | <0.001 | ||||
| ≥F3 | 5.95 (2.99-11.84) | <0.001 | 2.57 (1.15-5.70) | 0.021 | 2.81 (1.29-6.13) | 0.009 |
| MELD | 1.06 (1.04-1.07) | <0.001 | NS | |||
| (B) At 24-months follow-up | ||||||
| Cohort (AU) | 7.22 (2.51-20.80) | <0.001 | 5.47 (1.75-17.13) | 0.004 | 5.43 (1.58-18.73) | 0.007 |
| Sex (female) | 1.12 (0.39-3.21) | 0.839 | ||||
| Age at biopsy | 0.98 (0.94-1.02) | 0.241 | ||||
| Abstinence | 0.69 (0.22-2.14) | 0.522 | ||||
| Alcohol quantity | 1.00 (0.99-1.00) | 0.155 | ||||
| CPA | 1.06 (1.04-1.09) | <0.001 | 1.04 (1.01-1.06) | 0.018 | 1.03 (1.00-1.06) | 0.036 |
| Brunt fibrosis stagea | 3.40 (1.49-7.75) | 0.004 | ||||
| ≥F3 | 12.66 (1.66-96.28) | 0.014 | NS | NS | ||
| MELD | 1.08 (1.05-1.10) | <0.001 | 1.07 (1.03-1.11) | <0.001 | 1.07 (1.03-1.11) | 0.001 |
Note
- Univariable, standard and competing risk Cox regression analysis on liver-related mortality. Alcohol intake was recorded at the time of biopsy. Abstinence was recorded from the time of biopsy.
- Abbreviations: AU, Australia; CPA, collagen proportionate area; MELD, the Model for End-Stage Liver Disease.
- a Variables not included in the multivariable analysis. Competing risk at maximum follow-up (n = 25), Competing risk at 24 months follow-up (n = 6). Variables marked non-significant (NS) in multivariable analyses did not pass the stepwise backward elimination.
3.8 CPA predicts clinical outcomes in early/compensated ALD
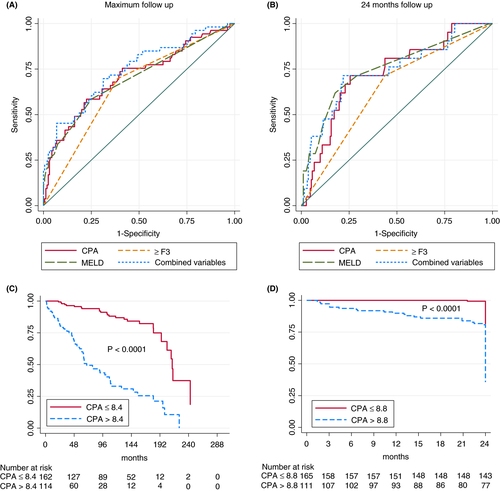
We established the predictors of hepatic decompensation and liver-related death in patients with early/compensated ALD (Table 3). In the competing risk multivariable Cox regression analysis, CPA was the only independent predictor of hepatic decompensation and liver-related death at maximum (HR = 1.08, 95% CI 1.06-1.11) and 24 months follow-up (HR = 1.08, 95% CI 1.04-1.12). Figure 4A,B show the AUROCs of CPA, MELD and the presence of advanced fibrosis. The optimal CPA cut-offs for prediction of hepatic decompensation or liver-related death were 8.4% (AUROC = 0.72, sensitivity 71% and specificity 73%) at maximum follow-up and 8.8% (AUROC = 0.78, sensitivity 87% and specificity 69%) at 24 months follow-up. Figure 4C,D illustrates the liver-related survival probability according to the optimal CPA cut-offs. CPA was the only independent predictor of hepatic decompensation in the subgroup of patients with compensated cirrhosis (n = 60) at maximum follow-up, whereas there were no significant predictors at 24 months follow-up (Table S4). When follow-up was restricted to 48 months after biopsy, results were similar to maximum follow-up (data not shown).
| Variable | Univariable | Multivariable | Competing multivariable | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| (A) At maximal follow-up | ||||||
| Cohort (AU) | 1.71 (0.89-3.28) | 0.108 | ||||
| Sex (female) | 1.99 (1.17-3.38) | 0.011 | NS | NS | ||
| Age at biopsy | 1.00 (0.98-1.02) | 0.982 | ||||
| Abstinence | 1.02 (0.59-1.74) | 0.955 | ||||
| Alcohol quantity | 1.00 (1.00-1.00) | 0.852 | ||||
| CPA | 1.08 (1.06-1.11) | <0.001 | 1.08 (1.06-1.11) | <0.001 | 1.09 (1.06-1.11) | <0.001 |
| Brunt fibrosis stagea | 1.84 (1.43-2.37) | <0.001 | ||||
| ≥F3 | 3.23 (1.86-5.62) | <0.001 | NS | NS | ||
| MELD | 1.06 (1.03-1.08) | <0.001 | NS | NS | ||
| (B) At 24 months follow-up | ||||||
| Cohort (AU) | 1.86 (0.77-4.53) | 0.170 | ||||
| Sex (female) | 1.98 (0.87-4.51) | 0.105 | ||||
| Age at biopsy | 0.98 (0.94-1.02) | 0.277 | ||||
| Abstinence | 0.72 (0.28-1.83) | 0.493 | ||||
| Alcohol quantity | 1.00 (1.00-1.00) | 0.907 | ||||
| CPA | 1.08 (1.04-1.12) | <0.001 | 1.08 (1.04-1.12) | <0.001 | 1.08 (1.04-1.12) | <0.001 |
| Brunt fibrosis stagea | 2.00 (1.31-3.03) | 0.001 | ||||
| ≥F3 | 3.53 (1.39-8.96) | 0.008 | NS | NS | ||
| MELD | 1.05 (1.02-1.08) | 0.003 | NS | NS | ||
Note
- Univariable, standard and competing risk Cox regression analysis on hepatic decompensation and liver-related mortality in early/compensated ALD. Alcohol intake was recorded at the time of biopsy. Abstinence was recorded from the time of biopsy.
- Abbreviations: AU, Australia; CPA, collagen proportionate area; MELD, the Model for End-Stage Liver Disease.
- a Variables not included in the multivariable analysis. Competing risk at maximum follow-up (n = 20), Competing risk at 24 months follow-up (n = 5). Variables marked non-significant (NS) in multivariable analyses did not pass the stepwise backward elimination.
To validate our findings in an independent cohort, we established the predictors of hepatic decompensation in the prospective validation cohort of patients with early/compensated ALD (Table S5). In the multivariable Cox regression analysis, CPA (HR = 1.06, 95% CI 1.01-1.11) and transient elastography (HR = 1.03, 95% CI 1.00-1.05) were both independent predictors of hepatic decompensation. The MELD score and the presence of advanced fibrosis (≥F3) did not independently predict hepatic decompensation. We found no significant differences in the predictive power of CPA (Harrell's C = 0.80, 95% CI 0.70-0.88) and transient elastography (Harrell's C = 0.82, 95% CI 0.74-0.89) as single predictors of hepatic decompensation (P-value = 0.358). The combination of CPA and transient elastography did not improve their predictive power (Harrell's C = 0.81, 95% CI 0.73-0.90). Finally, when we applied the optimal CPA cut-off (8.4%) of the development cohort in the validation cohort, the AUROC for prediction of hepatic decompensation was comparable (AUROC = 0.74, sensitivity 78% and specificity 71%) (Figure S3).
3.9 CPA predicts all-cause mortality in ALD
We tested the predictors of all-cause mortality in the overall development cohort also including patients with decompensated cirrhosis (Table S6). Independent predictors of all-cause mortality were CPA (HR = 1.05, 95% CI 1.03-1.07) and alcohol abstinence from the time of biopsy (HR = 0.52, 95% CI 0.32-0.87). Figure S4 illustrates all-cause survival probability according to CPA and abstinence. Independent predictors of 24 months all-cause mortality were MELD (HR = 1.06, 95% CI 1.03-1.10) and recruitment centre (HR = 3.96, 95% CI 1.71-9.18) (Table S6).
In the validation cohort, CPA (HR = 1.05, 95% CI 1.02-1.09) was the only independent predictor of all-cause mortality in the multivariable analysis (Table S5). The MELD score, presence advanced fibrosis (≥F3) and liver stiffness were not independent predictors.
3.10 Subgroup analyses including FIB4 and APRI
FIB4 and APRI were available in 81% of the patients. When these scores were included in the multivariable models, predictors of liver-related mortality were CPA, advanced fibrosis (≥F3) and being from the AU at maximum follow-up (Table S7). Predictors of hepatic decompensation and/or liver-related death in patients with early/compensated ALD were CPA, FIB4 and female gender at maximum follow-up (Table S8). All-cause mortality was independently predicted by CPA, FIB4 and being abstinent after time of biopsy at maximum follow-up (Table S9).
3.11 Effect of smoking and metabolic comorbidities
Potential confounders, such as BMI, smoking, type 2 diabetes, cardiovascular disease, hypertension and dyslipidaemia, were explored with data available from the UK cohort (Table S10). BMI, smoking status and metabolic comorbidities at the time of biopsy did not predict hepatic decompensation or liver-related mortality in patients with early/compensated ALD or liver-related mortality in the overall cohort.
4 DISCUSSION
In this large study of patients with biopsy-proven ALD, we demonstrated for the first time that CPA independently predicts liver-related mortality in such patients. CPA predicted hepatic decompensation and liver-related death with greater accuracy than a semiquantitative fibrosis score in patients with early/compensated ALD and also liver-related mortality in the whole cohort that also included patients with hepatic decompensation. We further showed that CPA values strongly correlate with LSM values and that CPA and LSM are independent predictors of liver-related outcome and mortality. We also provide optimal CPA cut-offs for the prediction of liver-related events that were validated in the prospective cohort. Despite the increasing use of non-invasive fibrosis tests, liver biopsy will remain essential for a subset of patients in the foreseeable future. CPA provides important additional prognostic information and can be performed in tandem with the traditional semiquantitative histological scoring, thus maximising the information that can be derived from a liver biopsy.
Liver fibrosis quantified using CPA has been mainly assessed in viral hepatitis.14, 16, 17, 22, 23 Histologically, ALD resembles NAFLD in terms of fibrosis formation and its perisinusoidal/pericellular distribution.24 The presence of advanced fibrosis and CPA were independent predictors of liver-related death in our ALD cohort. CPA quantifies fibrosis, whereas semiquantitative histological scoring systems describe both fibrosis and architecture. This implies that CPA and a semiquantitative histological score may have synergistic value on prognosis in liver diseases in general and also in ALD. Despite the high specificity of non-invasive fibrosis tests to rule out fibrosis in ALD,25 performance of liver biopsy is recommended in clinical trials also to rule out coexisting liver disease.9 In such clinical trials, CPA could be considered as one of the effect measurements due to the close relation to clinical outcomes demonstrated in the present study.
We previously investigated the correlation between CPA and LSM in NAFLD,19 showing a correlation (r = 0.73) that was weaker than in the present study (r = 0.86). A potential explanation could be that the LSM prediction of CPA is less reliable in NAFLD compared to ALD, particularly for earlier fibrotic stages, where the diagnostic accuracy of transient elastography is moderate in NAFLD.26 However, LSM was performed within 6 months of the liver biopsy in the NAFLD study, whereas the investigation was performed on the same day in the present study. It is therefore possible that the weaker correlation could be in part due to the changing dynamics of liver inflammation and oedema in addition to fibrosis. This hypothesis is supported by another study comparing CPA and LSM in viral hepatitis and post-transplant patients.23 Similarly to the present study, the biopsy and LSM were performed on the same day and the correlation of CPA and LSM was comparable to the present study (r = 0.77-0.80). CPA and LSM were both independent predictors of hepatic decompensation in patients with early/compensated ALD and can therefore provide complementary information in such patients. While it is well established that LSM is clinically useful to assess the severity of liver fibrosis at the time of diagnosis,20 our findings confirm that LSM is also clinically useful for the prognosis of ALD. Interestingly, CPA but not LSM was an independent predictor of all-cause mortality. Prior studies have also shown that the extent of fibrosis at baseline predicts both further progression of fibrosis 27 and the clinical outcome in ALD.12, 28, 29 Similar to our study, Lackner et al divided their cohort of 192 patients with ALD into 60 (31%) patients with early/compensated and 132 (69%) with decompensated ALD. The authors demonstrated that fibrosis (pericellular), female gender, INR and bilirubin independently predicted outcomes in decompensated ALD, whereas the presence of advanced fibrosis was the only independent predictor of clinical outcomes in early/compensated ALD. In recent years, the focus has shifted on early detection of chronic liver disease.30 Consequently, the number of identified patients with early/compensated ALD is rapidly increasing, which generates an emerging need of better understanding of the prognosis and the risk factors for disease progression. This emphasises the importance of our findings in patients with early/compensated ALD, which made up 84% of our cohort.
Alcohol abstinence or significant alcohol reduction is currently the only way to lower the risk of progression in patients with ALD8, 9; around 50% of patients with evidence of liver fibrosis manage to reduce harmful alcohol consumption,31 and alcohol abstainers have lower mortality and prevalence of hepatic decompensation when diagnosed with alcohol-related cirrhosis.32, 33 However, the findings in the present study were that the daily alcohol consumption at the time of biopsy and abstinence after biopsy did not predict liver-related outcomes. A potential explanation could be due to underreporting, which is commonly seen in relation to alcohol use34, 35 and to the retrospective collection of data. Moreover, the lifetime exposure to alcohol is likely of greater significance rather than alcohol use at a single time point. Interestingly, studies that included ALD patients with all stages of liver fibrosis were unable to show an independent association between alcohol intake and disease progression,12, 27 whereas others showed that abstinence had a beneficial albeit small effect compared to the stage of fibrosis.29 A likely confounder in this type of studies is that the non-abstainers may have significantly reduced their alcohol intake and/or changed the pattern of drinking and this was not measured. Most importantly, the present study found that alcohol abstinence was significantly associated with lower all-cause mortality emphasising that harmful drinking has detrimental consequences that goes beyond liver disease and should be addressed regardless of the severity of liver fibrosis.36 Overall, since data on alcohol abstinence are based on retrospective clinical records, they are probably not entirely accurate. This significantly reduces the reliability of results regarding abstinence from alcohol in this paper.
Limitations of this study are related to the retrospective data collection for the development cohort, lack of predefined criteria for performing liver biopsy and reliance on medical records for critical information such as abstinence from alcohol. It is not recommended to perform a diagnostic biopsy in ALD,9 and therefore the development cohort may have been selected due to clinical suspicion for coexisting liver disease and consequently differs from the general population of patients suspected to have ALD. For instance, the proportion of patients with decompensated cirrhosis was significantly higher in the AU cohort. This probably explains why being from the AU cohort were associated with higher mortality in the development cohort when looking at all patients, but not when looking at patients with early/compensated ALD. However, by replicating the results in an independent prospective cohort, we significantly reduced the risk of selection bias. Although we were unable to establish the exact cause of death in all patients, we were able to identify the survival status of the whole cohort during follow-up. Moreover, CPA was a predictor not only of liver-related mortality but also of all-cause mortality with equal hazard ratios, thus minimising the risk of detection bias. It should be emphasised that the continuous scale is one of the major advantages of CPA, which makes CPA suitable for measuring efficacy of intervention in clinical trials.
In conclusion, CPA is an independent predictor of clinical outcomes in patients with ALD. Adding CPA to traditional histology improves the prognostication in ALD and could be considered an additional effect measurement in clinical trials.
ACKNOWLEDGEMENT
Declaration of personal interest: None.
AUTHORSHIP
Guarantor of the article: None.
Authors contributions: MM and ET study concept and design; MM, AK, YH, MT, AH, DRa, CC, EB, LP, DRo, SD and TVL data collection; MI, MM and ET data analyses; MI and ET drafted the manuscript; all authors contributed to the manuscript with important intellectual content and approved the final version.
Open Research
DATA AVAILABILITY STATEMENT
Anonymised data available on request.




