The gut microbiota can orchestrate the signaling pathways in colorectal cancer
Abstract
Current evidence suggests that bacteria contribute to the development of certain cancers, such as colorectal cancer (CRC), partly by stimulating chronic inflammation. However, little is known about the bacterial impact on molecular pathways in CRC. Recent studies have demonstrated how specific bacteria can influence the major CRC-related pathways, i.e., Wnt, PI3K-Akt, MAPK, TGF-β, EGFR, mTOR, and p53. In order to advance the current understanding and facilitate the choice of pathways to investigate, we have systematically collected and summarized the current knowledge within bacterial altered major pathways in CRC. Several pro-tumorigenic and anti-tumorigenic bacterial species and their respective metabolites interfere with the major signaling pathways addressed in this review. Not surprisingly, some of these studies investigated known CRC drivers, such as Escherichia coli, Fusobacterium nucleatum, and Bacteroides fragilis. Interestingly, some metabolites produced by bacterial species typically considered pathogenic, e.g., Vibrio cholera, displayed anti-tumorigenic activities, emphasizing the caution needed when classifying healthy and unhealthy microorganisms. The results collectively emphasize the complexity of the relationship between the microbiota and the tumorigenesis of CRC, and future studies should verify these findings in more realistic models, such as organoids, which constitute a promising platform. Moreover, future trials should investigate the clinical potential of preventive modulation of the gut microbiota regarding CRC development.
Abbreviations
-
- ACS
-
- adenoma carcinoma sequence
-
- AKT
-
- serine/threonine protein kinase B
-
- AOM
-
- azoxymethane
-
- APC
-
- adenomatous polyposis coli
-
- AvrA
-
- anti-virulence factor A
-
- CB
-
- Clostridium butyricum
-
- CDT
-
- cytolethal distending toxin
-
- CEACAM
-
- carcinoembryonic antigen-related cell adhesion molecules
-
- c-Myc
-
- cellular myelocytomatosis oncogene
-
- CRC
-
- colorectal cancer
-
- CTNNB1
-
- gene-encoding β-catenin
-
- DMH
-
- 1,2-dimethylhydrazine
-
- DSS
-
- dextran sulfate sodium
-
- EGFR
-
- epidermal growth factor receptors
-
- EPS
-
- exopolysaccharides
-
- ETBf
-
- enterotoxigenic Bacteroides fragilis
-
- FadA
-
- Fusobacterium nucleatum adhesin A
-
- GTPase
-
- guanosine triphosphatase
-
- HFD
-
- high-fat diet
-
- HIF-1α
-
- hypoxia inducible factor-1 α
-
- KRAS
-
- Kirsten rat sarcoma virus gene, part of RAS/MAPK pathway
-
- lncRNA
-
- long non-coding RNA
-
- LPS
-
- lipopolysaccharide
-
- MAPK
-
- mitogen-activated protein kinase
-
- Mdm2
-
- murine double minute 2
-
- mTOR
-
- mammalian target of rapamycin
-
- p53
-
- tumor protein p53
-
- Pd
-
- Parabacteroides distasonis
-
- PI3K-Akt
-
- phosphoinositide 3-kinase-protein kinase B
-
- pks+
-
- colibactin-producing
-
- SA
-
- Salternamide A
-
- TDH
-
- thermostable direct hemolysin
-
- TGF-β
-
- transforming growth factor-β
-
- TME
-
- tumor microenvironment
-
- Wnt
-
- Wingless-related integration site
Colorectal cancer (CRC) is the fourth most common cancer, accounting for 10 percent of all new cancer cases in 2020, and the second leading cause of cancer mortality worldwide [1]. Despite decades of intense research into this disease, it still proves challenging to unravel the molecular mechanisms underlying it. However, a common consensus is that CRC is a genetic disease resulting from accumulated mutations in tumor suppressor genes and oncogenes, referred to as genomic instability [2]. The pathogenesis of sporadic CRC is an ordered series of events, also referred to as the adenoma-carcinoma sequence (ACS), denoting the transformation of the normal colonic epithelium to an adenomatous intermediate, ultimately resulting in adenocarcinoma [3]. The ACS hypothesis has been supported in animal and 3D cell models [4-6]. This oncogenic process is mainly driven by mutations in the TP53, APC, and KRAS genes, resulting in deregulation of the signal pathways that maintain cell homeostasis [7]. Other essential genes and pathways (Wnt, PI3K, MAPK, TGF-β, EGFR, and mTOR) have also been reported to be mutated in individual CRC tumors, though less frequently [5, 8].
CRC is divided into sporadic and familial/hereditary cases, emphasizing an etiology with shared environmental factors and genes. Environmental factors that increase CRC risk include smoking, alcohol intake, abdominal obesity, and a western diet [1, 9]. The recognition of the genetic connection between diet and CRC, and thus the involvement of gut microbiota, was explored decades ago [10, 11]. It is now well accepted that an interplay exists between dietary lifestyles, the gut microbiota, and secondary pro-tumorigenic metabolites [12-14]. In addition, the gut microbiota has been linked to CRC carcinogenesis, where known bacterial drivers play a significant role in inducing genomic mutations in human cells or exacerbating tumor-promoting inflammation [15-17].
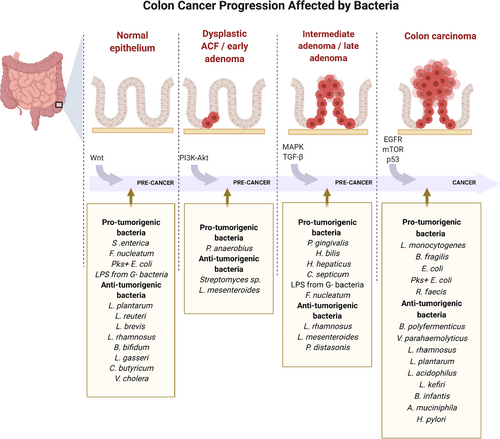
Increased access to genomic analysis, such as next-generation sequencing (NGS), has identified pathways and networks underlying many diseases, including CRC, creating the opportunity to explore and understand the conditions on a molecular basis [7, 18]. Over the last decade, an increasing amount of studies has been published describing individual bacterial strains and altered RNA or gene expression profiles concerning CRC. In this narrative review, we present literature that investigated the bacterial influence on the major signal pathways outlined in the ACS, from the normal epithelium (Wnt), through dysplastic aberrant crypt foci and early adenoma (PI3K-Akt) to intermediate adenoma (MAPK), late adenoma (TGF-β), and finally carcinoma development (EGFR, mTOR, and p53). The findings are divided into pro-tumorigenic (pathogenic and oncogenic bacteria) and anti-tumorigenic bacteria (beneficial bacteria and probiotics). An illustrative figure of the bacterially altered pathways identified in the literature is presented in Fig. 1.
Methodology
All studies included were collected according to the following search criteria: studies on CRC that included bacteria (any species) and one of the major pathways in the ACS. Semi-systematic searches were conducted in the Medline database through Pubmed, and only English-written studies published before 01/10/2021 were included. Table 1 summarizes the studies included in this review, their findings (pro- or anti-tumorigenic), and the in vitro or in vivo model(s) used to investigate the relationship between bacteria, CRC, and the major signal pathways in the ACS model. Table S1 shows the search strings and MESH terms and provides an overview of excluded studies.
| References | Bacteria | Pathway affected (Pro- or anti-tumorigenic) | Cell response1 | Model |
|---|---|---|---|---|
|
Wang et al. 2018 [24] |
Salmonella enterica sv. Typhimurium |
Wnt (Pro) |
↑ IL-8, IL-6, and granulocyte-macrophage colony-stimulating factor |
Pathogen-free female C57BL/6 mice Human colon cancer cell lines HCT116 and Caco-2 |
|
Liu et al. 2010 [25] |
Salmonella enterica sv. Typhimurium |
Wnt (Pro) |
↑ Proliferation, T-cell reporter activity, Wnt2, Wnt3, Wnt6, Wnt9a, and Wnt11 mRNA ↓ IL-8 |
Pathogen-free female C57BL/6 mice Human colonic epithelial HCT116 cells |
|
Rubinstein et al. 2013 [30] |
Fusobacterium nucleatum |
Wnt (Pro) |
↑ Proliferation, tumor growth, transcription factors lymphoid enhancer factor (LEF), T-cell factor (TCF), and NF kappaB, and Wnt oncogenes c-Myc and Cyclin D1 |
HCT116 xenografts in female nude mice Human colon cancer cell lines HCT116, DLD1, SW480, HT29, and RKO |
|
Li et al. 2020 [32] |
Fusobacterium nucleatum |
Wnt (Pro) |
↑ Proliferation, migration, IL- 6, IL-8, COX2, and TNF-α and Wnt oncogenes c-Myc and Cyclin D1 |
DLD1 xenografts in BALB/c nude mice Human colon cancer cell lines DLD1 and SW480 |
|
Lin et al. 2020 [33] |
Fusobacterium nucleatum |
Wnt (Pro) |
↑ Tumor growth, tumor size, migration, Wnt pathway genes, β-catenin, Axin1, and TGIF nucleoproteins |
C57BL/6J- ApcMin/+ mice Human colon cancer cell lines SW480, HT29, and Caco-2, CT26, and bone mesenchymal stem cells (BMSCs) |
|
Wu et al. 2019 [34] |
Fusobacterium nucleatum |
Wnt (Pro) |
↑ TLR4, P-PAK1, phosphorylated β-catenin S675, and Wnt oncogenes c-Myc and Cyclin D1 | C57BL/6-ApcMin/+ mice |
|
Chen et al. 2017 [35] |
Fusobacterium nucleatum |
Wnt (Pro) |
↑ TLR4, P-PAK1, phosphorylated β-catenin S675, and Wnt oncogenes c-Myc and Cyclin D1 | CRC tissue and human colon cancer cell lines SW480 and Caco-2 |
|
Ghanavati et al. 2020 [41] |
Lactobacillus spp. (L. plantarum, L. reuteri, L. brevis, and L. rhamnosus) |
Wnt (Anti) |
↑ Apoptosis, APC, CSNK1ε, gsk3β ↓ Proliferation, CTNNB1, CCND1, pygo2, axin2, and id2, inflammation, and tumor growth |
AOM/DSS colon cancer model (Female BALB/c mice) Human colon cancer cell line HT29 and murine colon cancer cell line CT26 |
|
Benito et al. 2021 [42] |
Bifidobacterium bifidum and Lactobacillus gasseri in combination with quercetin |
Wnt (Anti) |
↓ CTNNB1 | C57BL/6J ApcMin/+ mice |
|
Chen et al. 2019 [43] |
Clostridium butyricum |
Wnt (Anti) |
↑ Apoptosis ↓ Proliferation, β-catenin, cyclin D1, and c-Myc |
Pathogen-free ApcMin/+ mice Human colon cancer cell lines HCT116, Caco-2, and HCT8 |
|
Nadeem et al. 2021 [44] |
MakA from Vibrio cholera |
Wnt (Anti) |
↑ Apoptosis ↓ Proliferation, migration, tumor size, β-catenin, Axin-2, CyclinD1, and VEGF |
A colon cancer murine solid tumor model with CT26 cell line injected to BALB/c mice Human colon cancer cell lines HCT8 and DLD1, normal human colon cell lines CCD-18Co and CCD 841 CoN |
|
Long et al. 2019 [48] |
Peptostreptococcus anaerobius |
PI3K, AKT (Pro) |
↑ Proliferation |
ApcMin/+ mice Human colon cancer cell lines Caco-2, HT29, and SW48 |
|
Bach et al. 2015 [49] |
Salternamide A from Streptomyces sp. |
PI3K, AKT (Anti) |
↑ Apoptosis ↓ HIF-1α, proliferation |
Human colon cancer cell line HCT116 |
|
Mu et al. 2021 [52] |
Porphyromonas gingivalis |
MAPK, PI3K, AKT (Pro) |
↑ Proliferation, MAPK signaling pathways, PI3K/Akt signaling pathways | Human colon cancer cell line S1 and murine colon cancer cell line MC38 |
|
Zununi et al. 2017 [50] |
Leuconostoc mesenteroides |
MAPK, AKT (Anti) |
↑ Apoptosis | Human colon cancer cell line HT29 |
|
Zahran et al. 2017 [53] |
Lactobacillus rhamnosus |
MAPK (Anti) |
↓ Proliferation | DMH colon cancer rat model |
|
Maggio-Price et al. 2006 [58] |
Helicobacter bilis, Helicobacter hepaticus and Helicobacter sp. |
TGF-β (Pro) |
↑ Proliferation and alterations in inflammatory cytokines and pro-oncogene | Helicobacter-infected SMAD3 deficient mice (referred to as SMAD3−/−) and isolated cells from the model |
|
Maggio-Price et al. 2009 [59] |
Helicobacter bilis and Helicobacter hepaticus |
TGF-β (Pro) |
↑ Proliferation/survival of epithelial cells and alterations in inflammatory cytokines, pro-oncogenes, and pro-apoptotic proteins |
Helicobacter-infected SMAD3 deficient mice, which were also deficient in both B- and T-lymphocytes (referred to as Smad3/Rag2-double knockout (DKO) mice) and isolated cells from the model |
|
Engle et al. 2002 [60] |
Helicobacter hepaticus |
TGF-β (Pro) |
N/A | Helicobacter-infected mice deficient in TGF-β1 and B- and T-lymphocytes (referred to as Tgfb1−/− Rag2−/− mice) |
|
Gu et al. 2020 [61] |
Clostridium septicum 2 |
TGF-β (Pro) |
N/A | SMAD4- and β2-spectrin-deficient mice (Sptbn1+/- and Smad4+/-/Sptbn1+/-) |
|
Yoshioka et al. 2001 [64] |
Lipopolysaccharide (LPS) from Gram-negative bacteria |
TGF-β (Pro) |
↑ TGF-β and TLR-2 | Human colon cancer cell lines DLD1 and LoVo |
|
Saito et al. 2016 [65] |
Fusobacterium nucleatum 2 |
TGF-β (Pro) |
N/A | Tumor tissue, adjacent normal colonic tissue, and fecal samples |
|
Koh et al. 2019 [67] |
Parabacteroides distasonis |
TGF-β (Anti) |
↑ IL-10/TGF-β, tight junction protein ZO-1 ↓TLR4 activation and transcription of related cytokines |
A/J mice |
|
Oliveira et al. 2004 [69] |
Listeria monocytogenes |
EGFR (Pro) |
↑ Cancer cell invasion, ErbB2, and ErbB3 | Human colon cancer cell lines HCT8/E11 and HCT8/E11 DNEGFR (dominant-negative for EGFR) |
|
MA et al. 2009 [70] |
Bacillus polyfermenticus |
EGFR (Anti) |
↓ Proliferation, tumor size, angiogenesis, E2F-1, cyclin D1, ErbB2, and ErbB3 |
CD-1 nude mice Human colon cancer cell lines HT29, DLD1, and Caco-2 |
|
Karmakar et al. 2012 [71] |
Vibrio parahaemolyticus |
EGFR (Anti) |
↓ Proliferation, EGFR | Human colon cancer cell line HCT116 and HT29 cells |
|
Bao et al. 2019 [73] |
Bacteroides fragilis |
mTOR (Pro) |
↑ Proliferation, tumor size, tumor growth, BFAL13, RHEB |
Male BALB/c nude mice Human colon cancer cell line HCT116, DLD1, and normal human colon epithelial cell line FHC |
|
Iftekhar et al. 2021 [26] |
Pks+ Escherichia coli |
Wnt, p53 (Pro) |
↑Proliferation, megalocytosis, formation of multinucleated cells, Wnt independence | Murine colon organoid culture (mouse strains mTmG, Trp53flox/flox, miR-34a KO, miR-34b/c KO, and miR-34a/b/c KO) |
|
Cougnoux et al. 2014 [76] |
Pks+ Escherichia coli |
p53 (Pro) |
↑ Tumor growth, senescence, SUMOylation of p53 |
AOM/DSS colon cancer mouse model with HTC-116 cells (C57/B6 mice) Human colon cancer cell line HTC-116 Human colon cancer biopsies |
|
Zhang et al. 2018 [78] |
CNF1 cyclomodulin from Escherichia coli |
p53 (Pro) |
↑ Reversible senescence, multinucleated polyploidization, de-polyploidization, genomic instability in progeny | Human colon cancer cell line HCT116 |
|
Graillot et al. 2016 [80] |
CDT-I from Escherichia coli |
p53, Wnt (Pro) |
↑ Tumor growth, impaired DNA damage response, | Unspecified human colon cancer cell line deficient in p53 and APC |
|
Gamallat et al. 2016 [81] |
Lactobacillus rhamnosus GG |
p53 (Anti) |
↑ Apoptosis, p53, Casp3, Bax ↓Tumor progression, Bcl-2, β-catenin |
DMH colon cancer rat model (Female Sprague-Dawley rats) |
|
Walia et al. 2018 [82] |
Lactobacillus rhamnosus Lactobacillus plantarum |
p53 (Anti) |
p53 and Bcl-2 levels normalized ↑ Apoptosis, p21, Bax, Casp3, Casp9 |
DMH colon cancer rat model (Female Sprague-Dawley rats) |
|
An et al. 2019 [83] |
Protein p8 isolated from Lactobacillus rhamnosus |
p53 (Anti) |
↑ Cell-cycle arrest at G2, p53, p21 ↓ Colony formation, migration, proliferation |
Human colon cancer cell lines, DLD1, were transfected with plasmid DNA containing codon-optimized p8 gene segments |
|
Sharma et al. 2020 [84] |
Metabiotic extract from Lactobacillus rhamnosus MD 14 |
p53, Wnt (Anti) |
↑ Apoptosis, p53 ↓ Cox2, β-catenin, NF-kB, KRAS |
DMH colon cancer rat model (Male Sprague-Dawley rats) |
|
El-Deeb et al. 2018 [85] |
EPS from Lactobacillus acidophilus |
p53 (Anti) |
↑ Apoptosis, p53 | Human colon cancer cell line Caco-2 |
|
Brandi et al. 2019 [86] |
Lactobacillus kefiri SGL |
p53 (Anti) |
↑ Bax ↓ mutant p53, Casp3, IL-8 |
Human colon cancer cell line HT29 |
|
Wang et al. 2016 [87] |
Bifidobacterium infantis and Ganciclovir |
p53 (Anti) |
↑ Apoptosis, p53, Fas, FasL, IGF-1, IGF-2, Casp3, Casp3 | Human colo320 intestinal xenograft tumor model (Balb/c-nu and Balb/c mice) |
|
Meng et al. 2020 [91] |
Protein Amuc_1434 from Akkermansia muciniphila |
p53 (Anti) |
↑ G0/G1-phase cell-cycle arrest, apoptosis, p53, ROS, TRAIL, Casp3, Casp8 ↓ Proliferation |
Human colon cancer cell line LS 174T expressing Muc2 |
|
Mahyar-Roemer et al. 2001 [10] |
n-butyrate produced by anaerobic gut bacteria |
p53 (Anti) |
↑ Apoptosis (independent of p53), mitochondrial proliferation, p21 | Human colon cancer cell line HCT116 (wild-type p53 and p21 or inactivation of p53 and p21) |
|
Dadfarma et al. 2021 [88] |
Metallopeptidase from Lactobacillus casei |
p53 (Anti) |
↑ Apoptosis, p53, MAP2K1 | Human colon cancer cell lines, SW480, were transfected with plasmid DNA containing the metallopeptidase gene |
|
Yaghoubi et al. 2021 [92] |
Ribosomal protein from Helicobacter pylori |
p53 (Anti) |
↑ Apoptosis, p53, BAX ↓Tumor growth, BCL24 |
Human colon cancer cell line HT29 and murine colon cancer cell line CT26 Isograft tumor model (Female Balb/c mice) with CT26 cells |
- 1 The affected cell behavior (cell response) might be due to the method/model and not the bacteria per se.
- 2 Bacteria were detected in samples subjected to 16s rRNA sequencing.
- 3 lncRNA AK096729 named BFAL1 by the authors.
- 4 Co-administration with 5-fluorouracil and/or tumor-homing peptide iRGD further increased the effects. ↓ = Decreased. ↑ = Increased. Italic = genes. Non-italic = proteins. Pro- or anti-tumorigenic refers to the bacteria or product from bacteria.
NORMAL EPITHELIUM (WNT)
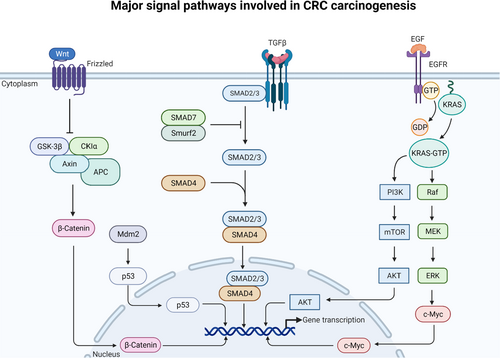
When the Wnt protein binds to its receptor, frizzled, it dissembles the tumor suppressor gene, APC, and its complex (GSK3-β and β-catenin), thereby increasing the amount of free cytoplasmic β-catenin that subsequently translocates to the cell nucleus and increases transcription (Fig. 2). In addition, if mutations are present in the APC, it prevents β-catenin from being suppressed despite the absence of Wnt [19-21]. There are 19 members of the Wnt gene family playing fundamental roles in cell regulation, including cell proliferation and tissue homeostasis [22]. A comprehensive review covering the influence of pathogenic bacteria on the Wnt//β-catenin pathway in various diseases, including mechanisms and consequences, has recently been published [23].
Pro-tumorigenic bacteria
Several bacterial virulence factors have been described to target the Wnt/β-catenin pathway, resulting in inflammatory responses. One example is colonization with anti-virulence factor A (AvrA) expressing Salmonella enterica serovar Typhimurium, which significantly reduced the proto-oncogene Wnt1 at the protein level in intestinal epithelial cell lines and tumor tissue from mice [24]. Similarly, the same organism can influence the Wnt/β-catenin pathway in the intestinal epithelial cells of mice, where several proteins of the Wnt family were induced by AvrA, including Wnt2 and Wnt3 Wnt6, Wnt9a, and Wnt11 [25]. Furthermore, in a study utilizing organoids, healthy murine colon epithelial cells were exposed to a short-term infection of colibactin-producing (pks+) Escherichia coli, leading to Wnt independence due to mutations in the Wnt signaling pathway and resulting in constant Wnt signaling [26].
The involvement of Fusobacterium nucleatum in CRC carcinogenesis has been extensively studied, and its relation to CRC seems indisputable [27, 28]. However, whether cancer is the cause or the consequence of F. nucleatum colonization in CRC tissue is an unanswered question [29]. In terms of F. nucleatum-associated CRC, it has been reported that cell proliferation was dependent on an F. nucleatum adhesion molecule, FadA, and mediated through attachment and invasion of epithelial cells via the epithelial cell-surface receptor E-cadherin. When FadA was bound to E-cadherin, it resulted in activation of β-catenin-regulated transcription and an increased expression of transcription factors and the oncogenes c-Myc and Cyclin D1 [30]. Furthermore, it was determined that this F. nucleatum-mediated oncogenic process was modulated by the phospholipid-binding protein, Annexin A1, which was upregulated in cancerous cells by binding FadA to E-cadherin, affecting β-catenin signaling [31]. Another study found that F. nucleatum activated the Wnt/β-catenin pathway through the upregulation of cyclin-dependent kinase 5 (Cdk5), promoting proliferation and migration of cells [32]. In addition, administration of F. nucleatum and bone mesenchymal stem cells (BMSCs) to ApcMin/+ mice resulted in an upregulation of the Wnt signal pathway and accumulation of β-catenin in the nuclei [33]. The combined effect of F. nucelatum and BMSCs was more significant than the administration of F. nucleatum alone, and crosstalk between these might be involved in the F. nucleatum-related CRC. These results emphasize the complexity of CRC carcinogenesis and the multitude of mechanisms whereby F. nucleatum exerts its carcinogenic effect.
Some studies have found that lipopolysaccharide (LPS), connected through the transmembrane toll-like receptor 4 (TLR4), activates phospho-p21-activated kinase 1 (P-PAK1), leading to phosphorylation and nuclear accumulation of β-catenin [34, 35]. Others have also established evidence supporting the carcinogenic potential of LPS through TLR4. Interestingly, they found that TLR4 signaling activated the β-catenin pathway through PI3K, thus being nondependent on Wnt secretion and new protein synthesis [36]. In addition, the same group has previously identified TLR4 as an essential contributor to CRC pathogenesis in mice, further emphasizing the importance of this receptor as a mediator of bacteria-induced cancer [37].
Anti-tumorigenic bacteria
Probiotics are live microorganisms considered beneficial for human health when administered through food and dietary supplements [38]. Lately, probiotics have received much attention due to the acknowledged link between dietary lifestyles, gut microbiota, and CRC development [39]. In addition, metabolites, signaling molecules, or components of probiotic microorganisms have been isolated and investigated in studies on CRC, as it has been suggested to be a potentially safer strategy than administrating live probiotic microorganisms [40].
Some studies have reported bacterial anti-tumorigenic effects via the Wnt/β-catenin signaling pathway. For example, in one study, in vitro results showed that a cocktail of Lactobacillus spp. (refer to Table 1 for the complete list of bacterial species) had an anti-tumorigenic effect on cell lines [41]. When administered, downregulation of the gene-encoding β-catenin, CTNNB1, and an increased expression of genes controlling degradation of the β-catenin complex were observed in a time-dependent manner. The beneficial effect of lactobacilli was further tested in a CRC-induced mouse model (azoxymethane/dextran sulfate sodium-induced CRC), where reduced inflammation and tumor development were observed in the treatment group [41]. Similarly, dietary supplementation of Bifidobacterium bifidum and Lactobacillus gasseri, in combination with the flavonoid quercetin, was administered to ApcMin/+ mice, resulting in downregulation of CTNNB1, reduced numbers of aberrant crypt foci and adenomas, and alteration of genes involved in the Wnt signal pathway such as Wnt5b, Wnt6, and Wnt11 [42].
A few studies have investigated the anti-tumorigenic role of bacteria that, in some cases, are considered pathogens. In one study, they investigated the role of the butyrate-producing strain Clostridium butyricum on two cancer cell lines [43]. Results showed that butyrate-treated cells displayed increased apoptosis and decreased proliferation. In addition, they tested the effect of supplementation with C. butyricum on a diet for mice, where mice fed with the bacteria had an increased β-catenin level and decreased proportion of cyclin D1-positive cells. In the second study, a cytotoxin from Vibrio cholera, motility-associated killing factor A (MakA), induced apoptosis in several cancer cell lines [44]. The mechanistic functions of MakA involved suppression of β-catenin, and importantly, their results showed that MakA had less toxicity towards normal cells.
DYSPLASTIC ACF AND EARLY ADENOMA (PI3K/AKT)
KRAS is a monomeric guanosine triphosphatase (GTPase) that transmits signals from a membrane-bound epidermal growth factor receptor (EGFR) when epidermal growth factor (EGF) is bound to the EGFR. KRAS is activated when guanosine triphosphate (GTP) is bound and consequently activates the phosphatidylinositol 3-kinase (PI3K), which through the mammalian kinase target of rapamycin (mTOR) activates the serine/threonine kinase AKT by phosphorylation (Fig. 2). This activation leads to the translocation of AKT to the nucleus, which regulates the transcription of genes involved in cell survival [45-47].
Pro-tumorigenic bacteria
Only one study was found to investigate the pro-tumorigenic properties of bacteria in the PI3K/AKT pathway of CRC; here, it was shown that Peptostreptococcus anaerobius increased tumor burden in mice. The authors hypothesized that P. anaerobius increased the gene expression and protein level of PI3K and AKT. Additionally, they showed that the proliferating cell nuclear antigen, a proliferation marker, was increased when P. anaerobius was present. These results indicated that P. anaerobius could drive CRC by increasing the proliferation through the PI3K-AKT pathway [48].
Anti-tumorigenic bacteria
A group of researchers investigated the effect of the potent, cytotoxic compound, Salternamide A (SA), on the hypoxia-inducible factor-1 α (HIF-1α) level in CRC cell lines. HIF-1 is a vital protein involved in angiogenesis, upregulated by low oxygen levels in the tumor. SA was extracted from Streptomyces sp. and added to cell lines where it suppressed the level of HIF-1α under hypoxic conditions. The authors concluded that SA acted as a signaling molecule through the PI3K-AKT-mTOR pathway, as these pathways were suppressed in a time- and concentration-dependent manner [49]. Another study showed that Leuconostoc mesenteroides, isolated from dairy products, induced apoptosis and DNA fragmentation in a CRC cell line, and downregulation of AKT was observed in cells treated with bacterial conditioned media [50].
INTERMEDIATE ADENOMA (MAPK)
Besides signaling through the PI3K/AKT pathway, activated KRAS also signals through a cascade of kinases called the mitogen-activated protein kinase (MAPK) module. First, the GTPase protein, RAS, activates the RAF kinase, leading to MEK activation, activating MAPKs such as ERK (Fig. 2). These proteins will then activate transcription factors, which can translocate to the nucleus and regulate the transcription of target genes involved in cell phenotypic functions such as migration and differentiation [51].
Pro-tumorigenic bacteria
Porphyromonas gingivalis, known for its association with periodontitis and recently found to be enriched in fecal and mucosa samples from patients with CRC, promoted CRC proliferation through activation of the MAPK signaling pathway as well as the PI3K/AKT signaling pathway [52].
Anti-tumorigenic bacteria
In a study, the synergetic effect of secreted exopolysaccharides (EPS) from Lactobacillus rhamnosus was investigated in combination with radiation in a rat model. CRC was induced by administering 1.2-dimethylhydrazine (DMH) to the rats. As a result of either EPS or radiation treatment, the concentration of β-catenin was reduced in the rats. This effect was further enhanced when combinational treatment was given. Since increased β-catenin is involved in increased proliferation, a reduction in the amount of β-catenin is expected to result in a reduced proliferation rate. The authors confirmed this by Western blot analysis [53]. In addition to the anti-tumorigenic effect of L. mesenteroides demonstrated under the AKT pathway, it was also demonstrated that MAPK was upregulated when treated with conditioned media from L. mesenteroides [50].
LATE ADENOMA – TRANSFORMING GROWTH FACTOR-β (TGF-β)
The growth factor TGF-β is involved in the differentiation of intestinal stem cells and is implicated in proliferation, apoptosis, angiogenesis, and inflammatory responses [54, 55]. When TGF-β binds to its receptor, it activates receptor-activated SMADs (suppressors of mothers against decapentaplegic), referred to as R-SMADs, and causes the formation of a complex between SMAD2/3 and SMAD4 (Fig. 2). When this complex is translocated to the nucleus, it binds to transcription sites and regulates the expression of genes involved in proliferation and differentiation [55, 56].
Pro-tumorigenic bacteria
Ever since a causal role between Helicobacter pylori and gastric cancer was discovered, there has been an interest in examining the relation of this bacteria to other types of cancers, including CRC [57]. Concerning TGF-β, an association between Helicobacter spp. (see Table 1 for the complete list of species) and CRC was found in a study where they developed a SMAD3-deficient (SMAD3−/−) mouse model to investigate bacteria-induced CRC. Their results showed that SMAD3−/− mice were in a pro-inflammatory state; however, they maintained free of CRC over nine months if averting infection with Helicobacter spp. On the contrary, CRC was present in 50 – 66% of the Helicobacter-infected animals with an exacerbated inflammatory response prior to tumor development, suggesting Helicobacter spp. as an essential trigger in CRC development through the TGF-β pathway in rodents [58]. Whether this translates into humans is still unknown. This relationship was further explored in Helicobacter-infected SMAD3/Rag2-double knockout mice, where Rag2 denotes mice deficient in B- and T-lymphocytes. The authors found that an inflammatory response was created due to impaired T-regulatory cell function; in addition, they discovered that cell proliferation/survival of epithelial cells was mediated by an increased expression of both pro-tumorigenic (c-Myc) and anti-apoptotic proteins, such as Bcl-xL and Bcl-2 [59]. These findings are consistent with the conclusions made by others where mice deficient in Rag2 and transforming growth factor beta 1 (TGF-β1) only developed CRC when Helicobacter hepaticus was present [60]. These results indicate that Helicobacter spp. may also be important drivers of CRC pathogenesis.
In a study by Gu et al. they found that the expression of carcinoembryonic antigen-related cell adhesion molecules (CECAMs), especially CEACAM5, was inversely correlated with the expression of TGF-β pathway genes (TGFBR1, TGFBR2, and SMAD3) [61]. CEACAM proteins are known to serve as mucosal receptors for microbes, including Escherichia coli and H. pylori, resulting in colonization of the gut mucosa, with inflammation as a result [62, 63]. The authors also found that an impaired TGF-β signal pathway increased the presence of pro-tumorigenic bacteria, such as Clostridium septicum, decreased supposedly beneficial bacteria, such as Bacteroides vulgatus and Parabacteroides distasonis, and proposed a relationship between the TGF-β pathway activity, CEACAMs, and the microbiome in the development of CRC [61]. Along with the above findings, a study found that LPS stimulated the production of TGF-β1 in CRC cell lines [64], and another study found an association between F. nucleatum and a subtype of CRC tumors with the high expression of TGF-β1 [65]. Interestingly, a study has shown that patients with a high abundance of F. nucleatum were associated with higher mutation rates in the TGF-β pathway [66]. These results further implicate F. nucleatum as one of the main drives of CRC and indicate a connection between it and alterations in the TGF-β pathway.
Anti-tumorigenic bacteria
The anti-tumorigenic effect of bacteria through the TGF-β pathway has less frequently been reported; however, one study reported a lower incidence of colonic tumors in a non-inflammatory mouse model when the diet was supplemented with P. distasonis (Pd). Mice receiving a Pd-supplemented diet were divided into early and late groups, where late denoted >19-week-old mice. Mice receiving Pd supplementation at a late stage displayed an increased TGF-β expression compared with mice receiving no supplementation. Surprisingly, an elevated TGF-β expression was not observed in the early Pd-supplemented group of mice. The authors hypothesized that this was due to a diminished transient effect at sampling [67].
CARCINOMA (EGFR, mTOR, and p53)
The transcription factor p53 regulates the transcription of genes involved in apoptosis, cell-cycle arrest, and DNA repair. Under normal conditions, p53 levels are low because of constant degradation by the ubiquitin ligase murine double minute 2 (Mdm2) (Fig. 2). However, if DNA damage inhibiting Mdm2 has occurred, p53 will remain stable and increase. High levels of p53 will lead to cell-cycle arrest, prevent passing on mutations to daughter cells, and prevent cancer origination. It is, therefore, said to be a tumor suppressor protein [68]. Although EGFR and mTOR were described under the PI3K/AKT pathway, they were included in this section due to their role in the progression from an adenoma to carcinoma.
EGFR
Pro-tumorigenic bacteria
Only one study investigated the pro-tumorigenic properties of bacteria regarding the epidermal growth factor receptors (EGFR). This study showed that expression levels of ErbB2 and ErbB3 were higher in CRC cells when exposed to Listeria monocytogenes supernatants [69].
Anti-tumorigenic bacteria
Bacillus polyfermenticus, a probiotic, has been shown to reduce tumor size when injected peritumorally and inhibit the growth of tumor cells and suppress colony formation. The molecular mechanism for the reduced tumor growth may have been a reduced expression of ErbB2 and ErbB3 and an inhibition of the cell-cycle regulator, cyclin D1 [70]. The study used conditioned media of B. polyfermenticus, suggesting a bacterial, soluble compound(s) as the biological anti-carcinogenic substance; however, it was not further identified. Another study investigated the effect of the thermostable direct hemolysin (TDH) produced by Vibrio parahaemolyticus. TDH had an anti-tumorigenic effect as it downregulated CRC cell line proliferation. The researchers suggested that this effect was exceeded via MEK, a serine/tyrosine/threonine kinase, by downregulating epidermal growth factor receptor (EGFR) tyrosine kinase activity in a dose-dependent manner [71].
mTOR
Pro-tumorigenic bacteria
Long noncoding RNAs (lncRNAs) have gained much attention due to their role in CRC tumorigeneses. Besides interacting with DNA, RNA, and proteins, LncRNAs can act as oncogenes or tumor suppressor genes, affect patient outcomes, and induce drug resistance [72]. In one study, lncRNAs were found to be involved in bacteria-induced CRC [73]. The presence of enterotoxigenic Bacteroides fragilis (ETBf) resulted in upregulation of the lncRNA AK096729 (named Bf-associated lncRNA1, BFAL1) in CRC cell lines. Compared to adjacent normal tissue, the protein, BFAL1, was upregulated in ETBf-colonized CRC tissue in a dose-dependent manner. The mechanism by which BFAL1 promoted CRC was further explored, and the authors found that RHEB was upregulated in cell lines exposed to ETBf. As a protein, RHEB can bind directly to the mTOR complex and regulate the mTOR-signal pathway; thus, the authors proposed a signaling cascade of ETBf–BFAL1–RHEB/mTOR that promoted tumor growth in CRC.
p53
Pro-tumorigenic bacteria
pks+ E. coli has been reported in 50% of human colorectal tumors; however, a direct bacteria-host cell contact is required for the toxic effect [74, 75]. In a study utilizing organoids, also described under the Wnt pathway, healthy murine colon epithelial cells were exposed to a short-term infection of pks+ E. coli. Epithelial cells developed a pre-malignant state represented by DNA damage and characteristics of CRC cells, such as enhanced proliferation and Wnt independence. Colibactin may have disrupted the Wnt and the p53 pathway by inducing a mutation, or a combination of mutations, as heterozygous loss of the micro-RNA-34a gene was seen through exome sequencing in the Wnt-independent organoids [26]. In another study utilizing pks+ E. coli, enhanced tumor growth was observed after a short-term infection. In the following analyses, this growth was found to be sustained by cellular senescence through colibactin exposure. Furthermore, it was shown that the presence of pks+ E. coli resulted in small ubiquitin-like modifier attachment (SUMOylation) of p53, promoting this cellular senescence [76]. These findings are interesting and in contrast to the general observation, where cellular senescence is a state of cell-cycle arrest most common in pre-malignant tumors, affecting proliferation and restricting tumor progression [77].
Another study investigated the effect of a cytotoxic toxin, necrotizing factor-1 (CNF1), produced by a uropathogenic E. coli strain. It was shown that CNF1 induced cellular senescence and that colon cancer cells had an increased expression of p53 [78]. The effect of other E. coli toxins has been investigated concerning CRC. In a study, cytolethal distending toxin (CDT) exposure to a colonic cell culture with p53-deficient cells led to an impaired DNA damage response. Thus, the loss of the tumor suppressor p53 made the cells more sensitized to the virulent effect of CDT, indicating that CDT can promote CRC but not initiate it [79, 80].
Anti-tumorigenic bacteria
Several studies have investigated the beneficial role of probiotics regarding the p53 pathway, primarily Lactobacillus spp were used. For example, Lactobacillus rhamnosus GG (LrGG) was orally administered in a DMH-induced CRC rat model, and it was found to decrease tumor incidence, increase p53 expression, and induce apoptosis in colonic cells [81]. Similar, in another study, Lactobacillus rhamnosus and Lactobacillus plantarum were orally administered to rats in a DMH-induced CRC model. The administration of these probiotic strains normalized the levels of p53, which were overexpressed during DMH treatment [82]. Also, the endogenous p8 protein from L. rhamnosus was expressed by CRC cells through a constructed plasmid and inhibited the p53-p21-Cyclin B1/Cdk1 signal pathway and induced cell-cycle arrest [83]. In another study, p53 was upregulated in rats with early DMH-induced CRC, where a meta-biotic extract (containing various short fatty acids among active compounds) from L. rhamnosus MD 14 was administered, leading to an almost normalization of the colon histology [84]. Similarly, other studies have found lactic bacteria to affect the expression of p53 (refer to Table 1 for specification of bacteria), either mediated through the synthesis of EPS or via non-specified mechanisms [85-88]. In addition, in vitro studies have shown that n-butyrate, a common food derivative metabolized by anaerobic bacteria, such as lactobacilli, can induce apoptosis in CRC cell lines by stimulating p21 [10].
The mucus layer primarily comprises Mucin 2, whose expression is regulated by p53 [89]. Akkermansia muciniphila is a bacteria that resides in the mucosa-associated mucus layer [90], and a protein derived from it, AMUC_1443, was able to degrade Mucin 2, and in an in vitro study, AMUC_1443 induced apoptosis and upregulated the expression of p53 in CRC cells [91].
While the Helicobacter spp. have mainly been investigated for their pro-tumorigenic properties, one study suggests that a ribosomal protein of H. pylori has anti-tumorigenic activity by increasing p53 gene expression and apoptosis in in vitro models and by reducing tumor growth in an isograft mouse model when co-administered with 5-fluorouracil and tumor-homing peptide iRGD [92].
DISCUSSION
The human body harbors more bacteria than human nucleated cells, with the highest proportion of bacteria in the colon [93]. This mutual, symbiotic relationship between the human host and the microbiota has co-existed for thousands of years, ultimately affecting our health and immune system [94]. However, this beneficial partnership is only maintained when the gut microbiota is not altered, as a microbial imbalance is linked to numerous diseases, including CRC [95]. Recently, molecular insights have highlighted numerous ways bacteria or phyla can initiate and drive CRC carcinogenesis [96]. Furthermore, evolutionary studies in mice have shown that the microbiota is altered in the malignant transgression from normal epithelium to carcinoma [97, 98]. As a result, cancer research is now addressing whether the microbiota or specific bacterial drivers are involved in CRC carcinogenesis.
Summary of main findings
Several microorganisms have a potential role in the development of CRC through the alteration of signal pathways. For example, S. enterica and F. nucleatum were found to be pro-tumorigenic bacteria affecting the Wnt signaling pathway, while some Helicobacter spp. were found to have pathogenic properties affecting the TGF-β signaling pathway. Also, E. coli, and its related toxins, were found to have pro-tumorigenic properties regarding the p53 and Wnt signaling pathway. Moreover, LPS was reported to provoke alterations in both the TGF-β and Wnt signaling pathways, highlighting the difficulty in pointing out single bacteria as the driver of CRC carcinogenesis since LPS is a compound of the cell wall in Gram-negative bacteria. Most of the pro-tumorigenic bacteria found in this review are well-known CRC drivers; however, a bit surprisingly, no literature has reported the involvement of Streptococcus spp., other known CRC drivers [96, 99].
As mentioned, the application of probiotics is a fast-moving field, and not surprisingly, several known probiotic strains were found to have anti-tumorigenic properties. However, mainly lactobacilli were investigated, and their beneficial role as food additives has been investigated for CRC and other diseases, such as diverticular disease, without clear cumulative evidence supporting beneficial outcomes [39, 100, 101]. Surprisingly, in a few cases, the anti-tumorigenic effect was induced from metabolites of bacteria typically considered pathogenic ex: Vibrio, Helicobacter, and Clostridium species. These results emphasize the caution needed when classifying healthy and unhealthy microbiomes. However, careful considerations should be taken when classifying healthy and unhealthy microbiomes since this is a complex matter with variations between healthy people, across age, gender, and ethnicity, and because diet greatly influences it [102].
Heredity, ethnicity, CRC, and gut microbiota
While the current review focused on sporadic CRC, about 5-7% of patients with CRC have an inherited syndrome. Hereditary CRC can be divided into two groups depending on the etiology: hereditary non-polyposis CRC (HNPCC) and hereditary polyposis CRC (HPCC) [103]. Lynch syndrome is associated with mutations of DNA mismatch repair proteins and is an example of HNPCC, and is also the most common hereditary cancer [104]. In contrast, familial adenomatous polyposis (FAP) is an example of HPCC, where numerous polyps develop in the colon due to a germline mutation in the APC gene [105]. A recent study found the colon mucosa of patients with FAP to harbor patchy biofilms composed primarily of known bacterial drivers, E.coli and B. fragilis [106]. Thus, modulation of the gut microbiota may be a therapeutic target in patients with FAP [107]. In line with this, patients with FAP were treated with the antibiotic erythromycin for four months in a clinical trial, resulting in a decrease in adenoma size and number and the cumulative adenoma burden. Interestingly, the treatment also reduced cell proliferation and the number of somatic APC mutations [108].
Patients with Lynch syndrome suffering from cancer have a different fecal microbiota than patients with Lynch syndrome without cancer [109]. Therefore, prospective microbiome monitoring through fecal samples from cancer-free patients with Lynch syndrome has been suggested as a surveillance strategy for this group of patients [110]. Since the initiation of the bowel cancer screening programs, there has been an increasing interest in developing non-invasive methods for early diagnosis, where 16s rRNA sequencing of fecal samples might be a promising tool [111]. Also, bacterial drivers, such as F. nucleatum, have been suggested as possible prognostic biomarkers [29].
In line with these observations, differences in the microbiota composition are also observed across geographical locations of patients, as shown in a study comparing the gut microbiota of patients and controls from Finland and Iran [112]. The study found a significant difference in the gut microbiota compositions in both geographical groups and between patients and controls. Thus, it is crucial to validate the biomarkers in ethnically and geographically different cohorts when developing fecal microbial biomarkers for CRC [113].
Clinical perspectives – modulation of the microbiota and CRC
Recent big data research, such as molecular pathological epidemiology, has combined pathology and data science to investigate associations between diet, gut microbiota, the expression profiles of CRC cells, and patient outcome [114]. Lifestyle changes and especially diet significantly influence CRC prognosis, suggesting modulation of the gut microbiota as a promising area in CRC treatment and prevention [115]. Lifestyle changes such as increased aerobic exercise and changes in diet, such as increased intake of omega-3 fatty acids, are associated with a lower risk of recurrence and mortality in patients with CRC [116, 117]. In addition, a higher intake of dietary, soluble fibers (prebiotics) that are metabolized by the microbiota into short-chain fatty acids, the main fuel for colonocytes, is associated with a reduced risk of incident distal colorectal adenoma [118]. These beneficial outcomes might be due to improvements in our microbial diversity, where a blooming of beneficial bacteria is associated with protection against CRC through pathway regulation. For example, increased intake of fibers is associated with an increase in butyrate-producing bacteria, which has shown an anti-carcinogenic effect in in vivo models through p21 [10, 119].
Probiotics, in combination with conventional treatment of CRC, has also been suggested as a safe tool to impact the microbiota and one clinical trial demonstrated that administration of probiotics resulted in a reduction of F. nucleatum in the mucosal microbiota in patients with CRC, indicating that probiotics might be helpful in both prevention and treatment of CRC [120]. Lastly, recent evidence suggests that the gut microbiota is implicated in immune and chemotherapy resistance, where the presence of specific species, such as A. muciniphila, B. fragilis, Bifidobacterium spp., and Faecalibacterium spp., is associated with favorable anti-tumorigenic immune responses, indicating that a shift towards these bacteria is preferred [121]. These findings again highlight the caution needed when defining healthy and unhealthy microbiota, as B. fragilis in this review is primarily associated with a pro-tumorigenic environment.
Besides infections, antibiotics are the most common manipulators of the gut microbiota, and their long-term use should be avoided if possible. A recent meta-analysis of retrospective studies found that long-term antibiotic exposure increased the risk of CRC [122]. However, in the future, new classes of antibiotics such as biofilm and quorum sensing inhibitors may become essential tools to manipulate the virulence and resistance of specific bacterial drivers and thereby bacterial-driven carcinogenesis [123]. The use of aspirin and COX2 inhibitors have also been investigated as chemopreventive drugs in CRC, and recent studies have shown that aspirin has an antibiotic effect against F. nucleatum, inhibiting its pro-tumorigenic effect in mouse models and, in addition, patients with daily aspirin use had a lower abundance of the bacterial driver in their colon adenoma tissue [124-126]. However, the relationship between CRC development and COX's is complicated, and the anti-tumorigenic mechanisms of anti-inflammatory drugs are still not fully understood. The COX enzymes (mainly COX2) degrade phospholipids and trigger the release of prostaglandin E2 (PGE2). PGE2 release results in increased mitogenic signaling, genomic instability, and suppression of apoptosis [127]. Anti-inflammatory drugs like aspirin and other NSAIDs include non-selective, irreversible COX inhibitors (aspirin) or selective, reversible COX2 inhibitors (coxibs). Although the biochemical mechanisms of aspirin are not fully understood, they include both cyclooxygenase (COX)-dependent and COX-independent mechanisms [128, 129]. COX2 levels in colonic cancers correlate with tumor size and degree of invasion, and there is evidence that NSAIDs, particularly aspirin, have preventive effects on the development of colorectal adenomas and cancer [130]. A recent 5-year follow-up meta-analysis found COX2 inhibitors to reduce the overall risk of colorectal cancer, but when stratifying the risk by years, they found that the effect attenuated over time [131]. An important caveat regarding COX2 inhibitors is that they are linked to mortality from gastrointestinal bleeding and cardiovascular events, meaning that the safety profile of COX2 needs to be firmly defined prior to broader clinical use [132].
When summarizing the above findings, it seems plausible to prevent the development of CRC and improve treatment efficacy or prognostic outcomes by modifying the gut microbiota; however, challenges exist. In terms of probiotics, defining the ideal bacterial species or a combination of several species with the highest benefit and no pathogenic or biohazard capacity is still a challenge [121]. Also, the cumulative evidence supporting the beneficial effect is still lacking [39, 101].
However, in a meta-analysis, 34 randomized controlled trials included 2723 patients undergoing elective abdominal surgery. Patients were randomized to probiotics or synbiotics (combination of prebiotics and probiotics) vs standard care. Administration of either probiotics or synbiotics significantly reduced the risk of infections (relative risk 0.56, p < 0.00001). Synbiotics showed a more significant effect on infections compared with probiotics (p < 0.0001) and also led to a shorter hospital stay (p = 0.005) [133]. Another randomized trial on patients undergoing surgery for CRC demonstrated a significant reduction in the levels of inflammatory cytokines following postoperative administration of probiotics consisting of six viable strains of lactobacilli and bifidobacteria [134].
Lastly, in terms of antibiotics, or other medications, targeting only one species might be challenging, and we do not yet know the downstream effect of this regarding the bacterial equilibrium in the gut.
Limitations of included studies and future studies
Mice and cell lines have proven valuable models to study CRC, and this is also the case concerning the bacterial role in CRC. However, to explore causality, pathways need to be assessed in models where the pathway is not already altered, though the time factor makes this approach difficult in mice and cell lines. Moreover, mice may not reflect the evolution of CRC in humans, as CRC is a notoriously slow-developing cancer resulting from years of inflammatory stress and the accumulation of mutations [135]. In addition, in vitro cell lines are very limiting as they are only composed of one cell type and do not include the tumor microenvironment (TME), which plays a vital role in cancer development and progression [136]. Lately, organoids have been used since they maintain tissue and donor characteristics; thus, they allow for the investigation of diseases and treatment strategies in a more realistic setting [6]. An organoid is still an in vitro model, and a limitation of this model is that the TME is not conserved compared with in vivo models [137]. Only one study has used this model to investigate how bacteria affect the cell signalings; however, organoids were only exposed to pks+ E. coli for a few hours [26].
It should be noted that some studies might not have been included in this review due to different nomenclature of pathways or because main pathways were not included in the text; thus, not all bacterial altered pathways might have been described in this review. Also, our search strategy did not include specific genes, as our focus was on the major signaling pathways in the ACS model of CRC. Thus, we have not covered the role of bacteria concerning mutations in APC, a frequently mutated gene in CRC, where recent results have shown a mutational signature due to the presence of pks+ E. coli in an organoid model [138]. Hopefully, future studies can add to these findings to generate a more comprehensive picture of the bacterially altered pathways in CRC. In this review, we chose to focus on the bacterial influence on CRC pathways. However, the gut microbiota also consists of the mycobiome and virome, which may have a role to play in carcinogenesis [139, 140].
Future studies investigating the bacterial influence on CRC should employ methods such as fluorescent in situ hybridization, targeting specific CRC drivers, and RNA sequencing, targeting specific pathways known to be altered in CRC, in combination with organoid and in vivo models, to explore this relationship further and strengthen the conclusions from the reviewed literature. Lastly, new models that can capture the complexity of the TME during CRC carcinogenesis, where pro- and anti-tumorigenic bacteria are present simultaneously, are warranted to accelerate our understanding of the complex CRC environment.
CONCLUSION
The microbial agent(s) that contribute to cancer-related inflammation in CRC needs further investigation before the impact of the microbiota on the signal pathways is fully understood. However, this review highlights some of the mechanisms by which bacteria can alter major molecular pathways in the ACS model. It also identifies points of particular interest for future research and possible preventive interventions to modulate the gut microbiota and improve clinical outcomes.
Figures were created with BioRender.com.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING
No funding was necessary for the conduction of this study.




