Chronic heart failure induces early defenestration of liver sinusoidal endothelial cells (LSECs) in mice
See related editorial: McCourt, P, 2024. Go with the flow: Hemodynamic changes affect liver ultrastructure. Acta Physiol. (Oxf). e14141.
Abstract
Aim
Chronic heart failure (CHF) is often linked to liver malfunction and systemic endothelial dysfunction. However, whether cardio-hepatic interactions in heart failure involve dysfunction of liver sinusoidal endothelial cells (LSECs) is not known. Here we characterize LSECs phenotype in early and end stages of chronic heart failure in a murine model.
Methods
Right ventricle (RV) function, features of congestive hepatopathy, and the phenotype of primary LSECs were characterized in Tgαq*44 mice, with cardiomyocyte-specific overexpression of the Gαq protein, at the age of 4- and 12-month representative for early and end-stage phases of CHF, respectively.
Results
4- and 12-month-old Tgαq*44 mice displayed progressive impairment of RV function and alterations in hepatic blood flow velocity resulting in hepatic congestion with elevated GGT and bilirubin plasma levels and decreased albumin concentration without gross liver pathology. LSECs isolated from 4- and 12-month-old Tgαq*44 mice displayed significant loss of fenestrae with impaired functional response to cytochalasin B, significant changes in proteome related to cytoskeleton remodeling, and altered vasoprotective function. However, LSECs barrier function and bioenergetics were largely preserved. In 4- and 12-month-old Tgαq*44 mice, LSECs defenestration was associated with prolonged postprandial hypertriglyceridemia and in 12-month-old Tgαq*44 mice with proteomic changes of hepatocytes indicative of altered lipid metabolism.
Conclusion
Tgαq*44 mice displayed right-sided HF and altered hepatic blood flow leading to LSECs dysfunction involving defenestration, shift in eicosanoid profile, and proteomic changes. LSECs dysfunction appears as an early and persistent event in CHF, preceding congestive hepatopathy and contributing to alterations in lipoprotein transport and CHF pathophysiology.
1 INTRODUCTION
The liver receives up to 25% of the total cardiac output; approximately two-thirds of the blood flow is supplied by the portal vein, and one-third by the hepatic artery. Therefore, hemodynamic stability is essential for proper liver function. Passive congestion and/or diminished perfusion may result in various liver pathologies including such diverse entities as Budd–Chiari syndrome, hepatic veno-occlusive disease, or postoperative jaundice Fontan-associated liver disease.1
In particular, impaired liver function is present in heart failure (HF) as a result of hemodynamic perturbation with the dominant role of alterations in the right ventricle (RV), rather than left ventricle (LV) failure.2 Indeed, right-sided HF is transmitted directly to the hepatic veins, resulting in an increase in venous pressure and hepatic congestion that may also be associated with decreased forward hepatic blood flow as well as, decreased liver perfusion and oxygenation.3 Hepatic congestion linked to altered liver function may entail further consequences including hypoalbuminemia, micronutrient deficiencies, and inflammation.1, 3-5 Furthermore, most drugs' pharmacokinetics are primarily determined by the liver function; thus, congestive hepatopathy may be associated with impaired drug absorption from the gastrointestinal tract and with altered hepatic metabolism of drugs.5 Altogether, it is believed that altered blood flow in the hepatic circulatory system is an important factor determining liver dysfunction and progression of HF.2, 6 However, in many patients, congestive hepatopathy often remains subclinical and undiagnosed, with no transaminase elevations (ALT, AST), although an increase in cholestasis markers such as GGT and ALP may be indicative.7, 8 Serum bilirubin was also reported to correlate with right atrial pressure, tricuspid regurgitations, or pulmonary hypertension4 and was shown to represent a strong predictor of mortality in patients with HF.5
Despite numerous experimental and clinical studies,1, 2 the mechanisms of cardio-hepatic interactions are not well understood. In particular, little is known about how liver sinusoidal endothelial cells (LSECs) respond to the progression of chronic HF (CHF).
LSECs represent a highly specialized population among other endothelial cells. Importantly, LSECs display unique morphology, including lack of basal lamina and the presence of fenestrae9 that ensure bi-directional exchange of the lipoproteins, macromolecules, and solutes between the intravascular space and hepatocytes.10 They are involved in the multiple aspects of liver function, and their malfunction may promote liver disease.11, 12 Loss of fenestrae represents a hallmark of LSECs dysfunction in various liver pathologies13 and aging.14 Defenestration leads to impaired transport of lipoproteins as well as hyperlipidemia15; it also affects drug retention in systemic circulation.14 Alternations in intracellular crosstalk between LSECs and other liver cells are often linked to liver steatosis, fibrosis, and inflammation.16, 17 Dysfunctional LSECs may display pro-inflammatory phenotype,16, 18 altered production of LSEC-derived vasodilators and vasoconstrictors (e.g., NO, thromboxane A2, prostaglandins)10, 19 reduced endocytic capacity, changes in LSECs bioenergetics and other alterations in LSECs phenotype.18 Given the versatility of LSECs, hemodynamic disturbance in hepatic microcirculation due to HF, may induce multiple changes in their function and morphology, which can be also transmitted to the liver parenchyma via paracrine communication.
In this work, we took advantage of the unique murine model of slowly developing CHF, Tgαq*44 mice with cardiomyocyte-specific overactivation of Gαq protein.20 The overactivation of Gαq protein mimics excessive neurohormonal stimulation of the heart by key neurohormones—norepinephrine, endothelin-1, and angiotensin II, reflecting the pathophysiology of HF in humans.21, 22 Tgαq*44 mice represent a unique model of slowly developing CHF by progressively decreased cardiac performance23, 24 with concomitant cardiac fibrosis,21, 24 significantly impaired physical activity22, 25 and systemic,26 coronary,27 and brain endothelial dysfunction.28
Here, we show that the loss of LSECs fenestrae and alterations in LSECs phenotype appeared as an early and persistent event in CHF, preceding the development of congestive hepatopathy and alterations in liver function.
2 RESULTS
2.1 Right ventricle dysfunction and altered hepatic blood flow in Tgαq*44 mice
To assess whether left-sided chronic heart failure developed by Tgαq*44 mice promotes right-ventricular dysfunction followed by the alternations in hepatic blood flow, RV function, and hepatic vein blood flow velocity were evaluated by MRI and Doppler method, respectively. In 4-month-old Tgαq*44 mice, representing early-stage of HF, the RV ejection fraction was significantly attenuated and further deteriorated in 12-month-old Tgαq*44 mice (representative age for end-stage HF) (Figure 1A) with concomitant impairment in stroke volume and cardiac output (Figure 1A–C). End systolic volume of RV was increased in both 4- and 12-month-old Tgαq*44 mice (Figure 1D), while the ratio of end diastolic volumes of RV/LV was substantially increased in 12-month-old Tgαq*44 mice (Figure 1F). The impairment of RV function in Tgαq*44 mice was accompanied by an increase in RV mass (27.82 ± 2.18 vs. 19.07 ± 3.11 and 30.90 ± 5.77 vs. 18.81 ± 2.80 for 4- and 12-month-old Tgαq*44 vs. age-matched FVB mice, respectively).
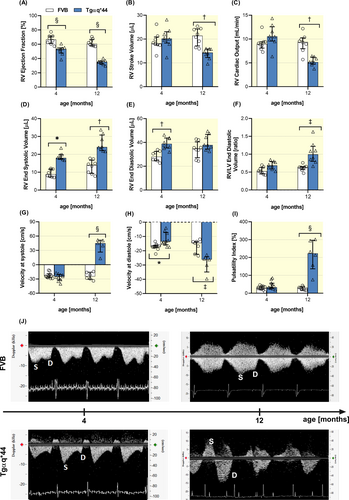
Right-sided HF in Tgαq*44 was associated with an altered hepatic blood flow velocity pattern. In FVB mice, the hepatic vein blood flow velocity revealed the presence of distinct two peaks that correspond to the systolic and diastolic phases of RV function (Figure 1J). In 4-month-old Tgαq*44 mice, peak blood flow velocity in the diastolic phase was decreased with no changes in systolic peak blood flow velocity when compared with age-matched FVB mice (Figure 1G). The prominent feature of hepatic flow in 12-month-old Tgαq*44 mice was that the hepatic vein flow velocity in the systolic phase revealed retrograde flow, whereas in the diastolic phase, the flow velocity was increased (Figure 1G,H). Moreover, the Doppler-derived spectra from 12-month-old Tgαq*44 mice showed typical fusion of retrograde systolic blood flow with atrial and tricuspid valve (naturally) backward-flow, which altogether resulted in widened retrograde blood flow wave (Figure 1J). Calculated pulsatility index was not changed for 4-month-old Tgαq*44 mice, but was significantly increased for 12-month-old Tgαq*44 mice as compared to age-matched FVB mice (Figure 1I).
2.2 Biochemical and ultrastructural markers of hepatic congestion in Tgαq*44 mice
In 12-month-old, but not in 4-month-old Tgαq*44 mice, albumin concentration in plasma significantly decreased, whereas GGT plasma concentration was significantly higher in comparison with age-matched FVB mice. TBIL tended to be higher in 4-month-old Tgαq*44 mice; this difference however reached statistical significance in 12-month-old Tgαq*44 mice (Table 1). In contrast, plasma aminotransferases (AST, ALT) were largely preserved. Of note, there was a significant decrease in liver mass in Tgαq*44 mice as compared with FVB mice.
| 4-month-old | 12-month-old | |||
|---|---|---|---|---|
| FVB | Tgαq*44 | FVB | Tgαq*44 | |
| Body weight [g] | 24.86 ± 1.8 | 25.42 ± 1.6 | 27.10 ± 3.1 | 27.43 ± 2.8 |
| Liver mass [mg] | ||||
| Wet | 1288 ± 133.4 | 1237 ± 126.3 | 1448 ± 162.6 | 1197 ± 99.6§ |
| Dry | 440.2 ± 66.4 | 417.9 ± 46.5 | 493.7 ± 73.3 | 396.1 ± 35.7§ |
| Wet/dry ratio | 2.96 ± 0.31 | 2.96 ± 0.14 | 2.95 ± 0.24 | 3.03 ± 0.20 |
| ALB [mg/mL] | 55.27 ± 7.61 | 62.20 ± 5.54* | 46.52 ± 4.23 | 39.85 ± 5.92* |
| GGT [ng/mL] | 1.02 ± 0.37 | 1.76 ± 0.63 | 1.64 ± 0.80 | 6.28 ± 2.91‡ |
| ALP [U/L] | 81.42 ± 19.05 | 82.81 ± 16.48 | 96.72 ± 16.73 | 89.22 ± 19.60 |
| ALT [U/L] | 40.12 ± 4.64 | 63.75 ± 11.85* | 60.60 ± 18.98 | 49.80 ± 9.98 |
| AST [U/L] | 225 ± 60.28 | 209.4 ± 113.7 | 131.8 ± 30.78 | 106.9 ± 28.98 |
| TBIL [μmol/L] | 1.05 ± 0.47 | 1.52 ± 0.48 | 0.81 ± 0.43 | 1.47 ± 0.52† |
| TG [mmol/L] | 2.11 ± 0.35 | 2.89 ± 0.53* | 2.81 ± 0.69 | 2.85 ± 0.81 |
| TC [mmol/L] | 2.74 ± 0.24 | 2.87 ± 0.22 | 2.65 ± 0.36 | 2.82 ± 0.63 |
| HDL [mmol/L] | 1.38 ± 0.11 | 1.34 ± 0.14 | 1.31 ± 0.17 | 1.25 ± 0.23 |
| LDL [mmol/L] | 0.17 ± 0.04 | 0.16 ± 0.04 | 0.17 ± 0.03 | 0.21 ± 0.07* |
- Note: Body weight and liver mass (n = 11–17), ALB and GGT (n = 7–8), blood biochemistry (n = 10–16) in Tgαq*44 mice at early (4 months old) and end (12 months old) stages of HF progression compared with age-matched FVB mice. Normality was assessed using a Shapiro–Wilk test. Results are presented as mean ± SD, *p ≤ 0.05, †p ≤ 0.01, ‡p ≤ 0.001, §p ≤ 0.0001 significantly different versus age-matched FVB compared using two-way ANOVA following Bonferroni post hoc test or non-parametric Kruskal–Wallis ANOVA (body weight and TC).
- Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TBIL, total bilirubin; TC, total cholesterol; TG, triglycerides.
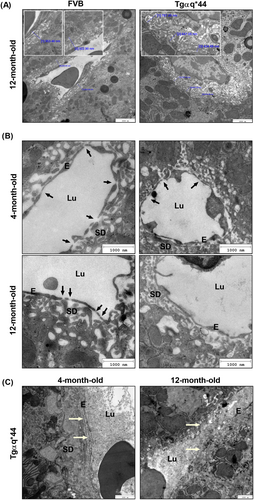
Despite altered plasma biomarkers of congestive hepatopathy (GGT, TBIL, and albumin), liver histology neither at 4- nor at 12-month-old Tgαq*44 mice revealed steatosis, fibrosis, or any other gross pathology (Figure S1). Furthermore, neither the expression of collagen IV nor the expression of αSMA (smooth muscle actin) and vimentin—markers of endothelial to mesenchymal transition were increased (Figure S2). However, sinusoidal dilatation was invariably observed in histological cross section of Tgαq*44 mouse livers (Figure S1C) and was confirmed on the ultrastructure level by TEM (Figure 2). Although the overall liver parenchyma ultrastructure was well preserved in Tgαq*44 mice, the Disse space and hepatic microvilli were altered as compared with age-matched FVB mice. In Tgαq*44 mice, in addition to collapsed space of Disse and visibly reduced hepatic microvilli, LSECs displayed a lower number of fenestrae (Figure 2A,B). Furthermore, the deposition of basement membrane material was detected in some sections of liver obtained from a single 4- as well as 12-month-old Tgαq*44 mice (Figure 2C).
2.3 Loss of fenestrae in LSECs in Tgαq*44 mice
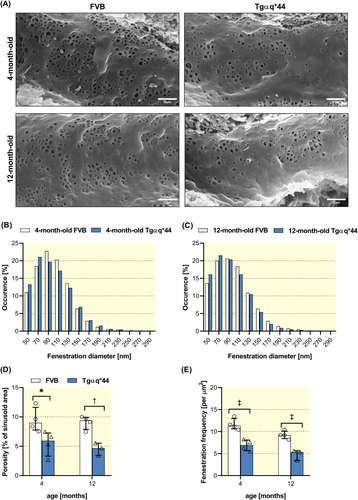
To quantify the number of fenestrae/μm2 using in vivo and in vitro experimental approach, SEM and AFM techniques were used, respectively. Analysis of SEM images of hepatic sinusoids in situ (Figure 3A) revealed no significant changes in distribution of fenestration diameters, although smaller fenestrae (with mean diameter 50–70 nm) occurred slightly more frequent in both, 4-month-old (Figure 3B) and 12-month-old Tgαq*44 mice (Figure 3C) as compared with age-matched FVB mice. However, the HF development in Tgαq*44 mice induced a significant loss of porosity (Figure 3D) and decreased number of fenestrae (Figure 3E) clearly observed in 4- and 12-month-old Tgαq*44 mice.
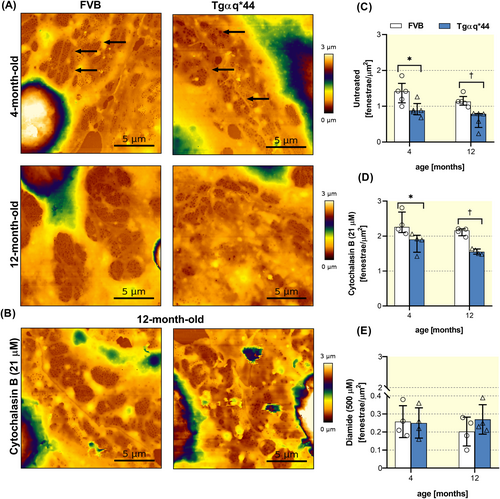
Similar results were obtained based on in vitro quantification of LSECs fenestrae by AFM measurements. LSECs isolated from 4- and 12-month-old Tgαq*44 mice displayed decreased number of fenestrae as compared with age-matched FVB mice (Figure 4A,C). Noteworthy, age-dependent decrease in the number of fenestrae was also observed in LSECs isolated from FVB mice. Importantly, LSECs isolated from Tgαq*44 mice also presented a diminished functional response to cytochalasin B, which normally strongly stimulates the formation of fenestrae (Figure 4B,D). In contrast, no differences between Tgαq*44 and FVB groups were noted for the effects of diamide on the number of fenestrae, and in all experimental groups, diamide efficiently closed fenestrae (Figure 4E).
2.4 No changes in endocytic capacity and preserved bioenergetics in LSECs in Tgαq*44 mice
Despite defenestration, the uptake assay of AcLDL by scavenger receptors (SR-A and SR-H) did not detect any changes in LSECs isolated from Tgαq*44 mice (Figure 5A). However, there was an impairment of the LSECs ability to form a tight barrier as evidenced by lower total resistance values in LSECs isolated from 4-month-old Tgαq*44 mice (Figure 5B), whereas LSECs isolated from 12-month-old Tgαq*44 mice displayed barrier integrity comparable to that of LSECs from age-matched FVB (Figure 5C). Of note, there was a clear-cut age-related impairment of the LSECs ability to form a tight barrier in LSECs isolated from older Tgαq*44 mice and FVB mice, which was approximately five times lower than for LSECs in young mice (as evidenced by low TEER values of approximately 200 Ω cm2 as compared with 1000 Ω·cm2, respectively).
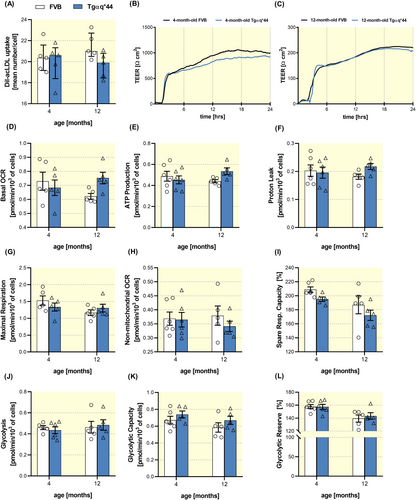
LSECs bioenergetics status was not affected and was largely comparable among LSECs isolated from Tgaq*44 mice and age-matched FVB mice (Figure 5D–L) with the notable exception of a slight increase in the basal oxygen consumption rate (OCR) in 12-month-old Tgαq*44 mice (Figure 5D). This suggests that LSECs from old Tgαq*44 mice were slightly, but not significantly, metabolically more active than age-matched FVB mice. Other parameters including ATP production–linked OCR (Figure 5E), proton leak (Figure 5F), maximal respiration (Figure 5G) and non-mitochondrial oxygen consumption (Figure 5H), as well as glycolysis (Figure 5J) and glycolytic reserve (Figure 5L) were not changed.
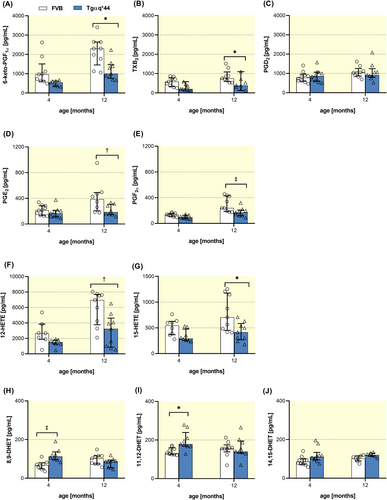
2.5 The shift in COX-, LOX-dependent, and CYP450-derived eicosanoid biosynthesis by LSECs in Tgαq*44 mice
LSECs isolated from 4-month-old Tgaq*44 mice were featured by increased biosynthesis of 8,9-, 11,12- and 14,15-DHET (Figure 6H–J) (biologically active metabolites of vasoprotective CYP450-derived EETs) as compared with age-matched FVB mice; however, these differences disappeared in 12-month-old mice. In turn, the biosynthesis of COX-dependent prostanoids—in particular 6-keto-PGF1α (a metabolite of PGI2), PGF2α, TXB2 (a metabolite of TXA2), PGE2 as well as LOX-derived 12-HETE and 15-HETE (Figure 6A–G)—were significantly lower in 12-month-old Tgaq*44 mice versus age-matched FVB mice.
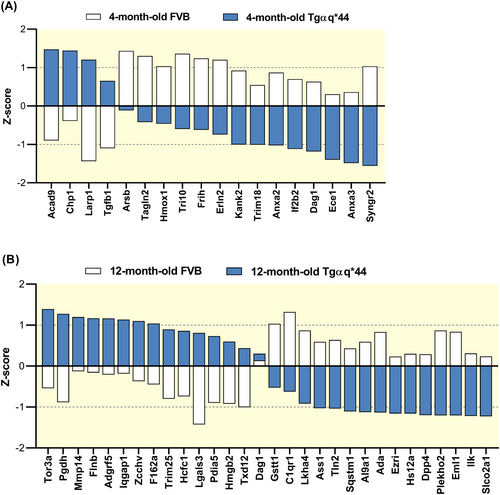
2.6 Changes in the proteomic phenotype of LSECs in Tgαq*44 mice
LSECs isolated from 4-month-old Tgαq*44 mice displayed 18 differently expressed proteins (DEPs) as compared with age-matched FVB mice, that included a number of proteins that could be linked to remodeling of the cytoskeleton: dystroglycan 1 (Dag1), transforming growth factor-beta receptor-associated protein 1 (Tgfb1), trangelin 2 (Tagln2), and KN motif and ankyrin repeat domain-containing protein 2 (Kank2) (Figure 7A). Some of these proteins also appeared as DEPs in 12-month-old Tgαq*44 versus age-matched FVB mice (Figure 7B) together with other proteins also linked to cytoskeleton remodeling: ezrin (Ezri), filamin beta (Flnb), matrix metalloproteinase-14 (Mmp14), complement component C1q receptor (C1qr1), and echinoderm microtubule-associated protein-like 1 (Eml1). Among identified DEPs, there were also proteins linked to endothelial vasoprotective/proinflammatory or barrier function: heme oxygenase 1 (Hmox1), adenosine deaminase (Ada), dipeptidyl-peptidase 4 (Dpp4), 15-hydroprostaglandin dehydrogenase (Pgdh), soluble carrier organic anion transporter family member 2A1 gene (Slco2a1), leukotriene A4 hydrolase (Lkha4), and high mobility group protein B2 (Hmgb2), as well as others related to the regulation of vascular phenotype: annexin 2 (Anxa2), annexin 3 (Anxa3), calcineurin B homologous protein 1 (Chp1), la ribonucleoprotein domain family, member 1 (Larp1) or associated with redox regulation: protein disulphide-isomerase A5 (Pdia5), thioredoxin domain containing 12 (Txd12), glutathione s-transferase, theta 1 (Gstt1) or, finally, with shear stress-dependent regulation: sequestosome-1 (Sqstm1), integrin-linked protein kinase (Ilk), Iq motif containing gtpase activating protein 1 (Iqgap1), and endothelin-converting enzyme 1 (Ece1) (Figure 7B).
2.7 Alterations in postprandial clearance in Tgαq*44 mice
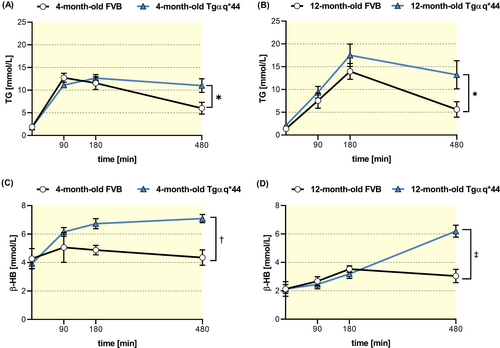
Because defenestration of LSECs could contribute to prolonged TG clearance in postprandial hyperlipidemia,15 fasting, and postprandial plasma TG concentrations were measured in Tgαq*44 mice compared to age-matched FVB mice. Interestingly, 4- but not 12-month-old Tgαq*44 mice, showed elevated fasting plasma TG levels with no changes in HDL, LDL, or TC (Table 1).
However, in both 4-month-old and 12-month-old Tgαq*44 mice, postprandial hypertriglyceridemia induced by olive oil gavage was significantly potentiated, suggesting prolonged TG clearance as compared with age-matched FVB mice (Figure 8A,B). At the same time, postprandial β-HB concentration was significantly higher in 4- and 12-month-old Tgαq*44 mice (Figure 8C,D) indicating increased fatty acid oxidation in liver Tgαq*44 mice’ liver.
2.8 Changes in the proteome of hepatocytes in Tgαq*44 mice
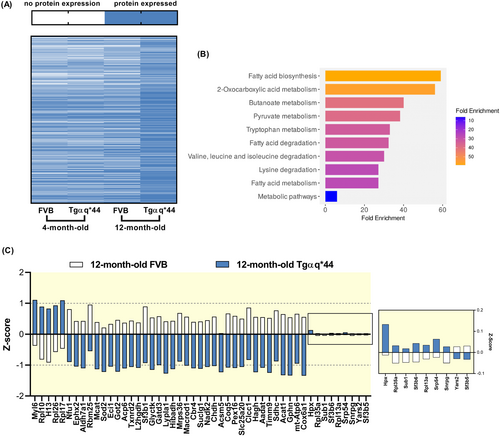
To further assess a possible alterations in liver lipid metabolism, the proteomes of isolated hepatocytes from 4- and 12-month-old Tgαq*44 and age-matched FVB mice were analyzed. In 4-month-old Tgαq*44 mice, hepatocyte proteome was largely preserved, while hepatocytes isolated from 12-month-old Tgαq*44 mice displayed the most pronounced alterations in protein expression, and the highest overall number of detected proteins compared to all other groups (Figure 9A). Analysis of DEPs in 12-month-old mice versus age-matched FVB mice revealed a number of proteins mainly related to metabolic processes including downregulation of fatty acid biosynthesis, metabolism, and degradation (Figure 9B) represented among others by carbonyl reductase 4 (Cbr4), succinate dehydrogenase cytochrome (Sdhc), malonyl-CoA-acyl carrier protein transacylase (Mcat), mitochondrial aspartate aminotransferase (Got2), and acetyl-CoA C-acetyltransferase (Acat1) (Figure 9C). Additionally, dysregulation of amino acid metabolism and degradation pathways were observed as evidenced by changes in the expression of aspartate transaminase (Got2), alpha-aminoadipic semialdehyde dehydrogenase (Aldh7a1), kynurenine/2-aminoadipate aminotransferase (Aadat), and choline dehydrogenase (Chdh) (Figure 9C).
3 DISCUSSION
In this work, we have demonstrated that LSECs defenestration represents an early and persistent event in the development of CHF in Tgαq*44 mice. Importantly, this unique and clinically relevant model of chronic left-sided HF, driven by cardiomyocyte-specific alterations in Gαq-dependent signaling and characterized by prolonged pathophysiology,22, 24, 25 also presented right-sided HF. The impaired RV function in Tgαq*44 mice led to progressive attenuation of venous hepatic blood outflow and hepatic congestion, although it was not accompanied by a gross liver pathology, or fibrosis. The salient finding of this work was an early LSECs dysfunction in Tgαq*44 mice involving partial loss of fenestrae, shift in eicosanoid profile, and proteomic changes. On the functional level, LSECs defenestration was associated with prolonged postprandial triglyceride clearance in early and end stage of HF in Tgαq*44 mice and with prominent changes in proteome of hepatocytes at the end stage of HF, consistent with altered metabolism in dysfunctional liver.
Altogether, data showed that LSECs have an essential contribution to the heart-liver crosstalk in HF, and appeared much earlier than dysfunction of hepatocytes and biomarkers of congestive hepatopathy. Accordingly, LSECs dysfunction represents an early and persistent event in CHF, preceding liver dysfunction and potentially contributing to alterations in lipoprotein postprandial metabolism in HF pathophysiology.
Much of our understanding of the mechanisms of congestive hepatopathy was previously based on descriptive pathological human studies or canonical models of partial ligation of the inferior vena cava (pIVCL)29 inducing severe liver disease. However, the present study was based on clinically relevant murine model of CHF linked to excessive neurohormonal drive, that allowed us to trace the development of pathophysiology of congestive hepatopathy from the early to the end stage of HF, in Tgαq*44 mice.
Previous studies in Tgαq*44 mice extensively characterized various pathophysiological elements of cardiac pathology26, 28, 30, 31 as well as mechanisms of endothelial dysfunction in coronary circulation linked to cardiac oxidative stress,27 systemic endothelial dysfunction in aorta associated with erythropathy,26 and altered brain endothelial function associated with cognitive impairment.28 The endothelial involvement in the pathophysiology of various organs alongside the progression of HF was here extended by demonstrating that LSECs dysfunction occurred in 4-month-old Tgαq*44 mice and, therefore, at an early stage of HF and prior to systemic endothelial dysfunction.26 These results confirm the notion that the liver is very sensitive to the perturbation of systemic hemodynamics8 and LSECs dysfunction may occur very early during HF progression, in particular, if right-sided HF is involved. Notably, right-sided HF often occurs simultaneously to left-sided HF in clinical patients32 and apparently, this was also the case as shown here for Tgαq*44 mice and could be linked to primary and secondary involvement of RV.
Signs of congestive hepatopathy in response to right-sided HF were reflected by increased GGT and TBIL as well as a fall in plasma albumin concentration without alterations in ALT or AST levels in 12-month-old Tgαq*44 mice. Interestingly, no gross liver pathology was revealed along HF progression in contrast to canonical model of congestive hepatopathy such as pIVCL.29, 33 In fact, LSECs defenestration without fibrosis appears to be in striking contrast with the findings of many other studies reporting LSECs defenestration in aging,15 or in various liver diseases including cirrhosis,34 hepatitis,35 and alcoholic injury,36 which have been linked with liver fibrosis.
Apparently, although defenestration is a hallmark of LSECs dysfunction previously reported in various pathologies,34-36 in Tgαq*44 mice it had a distinct pathophysiology. LSECs dysfunction in Tgαq*44 mice was not associated with hepatic stellate cells (HSCs) activation, nor with pro-fibrotic LSECs response as there were no markers of endothelial to mesenchymal transition. Furthermore, LSECs dysfunction in Tgαq*44 mice was also not causally associated with systemic inflammation, in contrast to studies in a murine model of hepatic congestion induced by pIVLC.33 Altogether, our work provided a novel insight into pathophysiological sequence of events in congestive hepatopathy development in CHF with the early appearance of LSECs defenestration without fibrosis, nor inflammation.
The phenotype of dysfunctional LSECs observed in young Tgαq*44 mice was rather not progressive in nature, showing a similar extent of LSECs defenestration at early and end stage of HF, despite the progression of right-sided HF. Therefore, we speculate that even mild changes in hepatic blood wave velocity and right ventricle systolic and diastolic cardiac dysfunction observed in 4-month-old Tgαq*44 mice may represent a major pathomechanism of LSECs dysfunction driven by right-sided HF in accordance with pathophysiology of congestive hepatopathy postulated for HF patients.37
Interestingly, collapsed space of Disse and diminished hepatocyte microvilli detected by TEM in 12-month-old Tgαq*44 mice were compatible with the previous study on RV failure model.2 Of note, concomitantly decrease in forward hepatic blood flow, and diminished liver perfusion may also play a role in the pathophysiology of hepatic congestion, in end stage of HF.4-6, 8
In 12-month-old but not 4-month-old Tgαq*44 mice, there was a systemic upregulation of ACE/Ang II pathway and downregulation of ACE2/angiotensin-(1–7),23 while LSECs defenestration remained at approximately the same level in early and end stage of HF progression. Therefore, the systemic activation of ACE and the excessive activity of Ang-II, the major driver of HF progression and involved in the pathogenesis of liver cirrhosis or portal hypertension38, 39 was unlikely to determine LSECs function and defenestration in Tgαq*44 mice.
On the other hand, our functional results are compatible with the notion of dysregulated actin cytoskeleton-dependent mechanisms responsible for maintaining LSECs fenestrae.40, 41 Depolymerization of filamentous actin by cytochalasin B normally rapidly increases the number of LSECs fenestrae.40, 41 However, this response was impaired in LSECs from Tgαq*44 mice, indicating functional alternations in actin cytoskeleton. It was an important finding that, diamide efficiently closed fenestrae in LSECs from both Tgαq*44 and FVB mice, suggesting that the loss of LSECs fenestrae in Tgαq*44 mice was not linked to the response distortion of redox-dependent fenestrae closure.42 The dysregulated actin cytoskeleton-dependent mechanisms of LSECs fenestrae40, 41 could have been directly due to right-sided HF, altered hemodynamics, and venous congestion. In fact, low shear stress, affected endothelial expression of cytoskeletal-related proteins, thereby inducing reorganization of cytoskeleton in endothelial cells from other vascular beds.43, 44 Proteomic analysis of isolated LSECs supported the notion that venous congestion resulted in cytoskeleton remodeling as evidenced by—the altered expression of the number of cytoskeletal-related proteins or of proteins involved in the direct interaction with actin (Flnb, Iqgap1, Dag1, Tagln2, Sqstm1) and regulating actin polymerization (Kank2). Interestingly, the downregulation of SQSTM1 protein was detected in 12-month-old Tgαq*44 mice, which corroborates previously reported results for endothelial cells in low shear-stress conditions45; similarly, the upregulation of IQGAP1 was detected in endothelial cells in static conditions without flow.46 Altogether, LSECs defenestration in Tgαq*44 mice documented here using SEM of hepatic sinusoids in situ and AFM in primary LSEC in vitro, was attributed to venous congestion and functional alterations in cytoskeleton organization.
Noteworthy, comparable results of quantification of LSECs defenestration in Tgαq*44 based on in vivo and in vitro approaches represent an important validation of AFM-based methodology used previously not only to study dynamics of LSECs in vitro40, 47 but also to quantify LSECs defenestration in murine models of disease.18
The phenotype of LSECs dysfunction apart from defenestration is usually linked to alterations in vasoactive mediators48-50 and this also occurred in LCES from Tgαq*44 mice. LSECs from 4-month-old Tgαq*44 mice showed upregulation of the CYP450-dependent pathway, but in 12-month-old Tgαq*44 mice, epoxyeicosatrienoic acid (EET) metabolites recovered to levels similar to those of age-matched FVB mice. The conversion of active EETs to less-active dihydroxyeicosatrienoic acids (DHETs) is catalyzed by soluble epoxide hydrolase (sEH), therefore, high levels of vasoprotective EET metabolites suggest a compensatory hepatoprotective mechanism in 4-month-old Tgαq*44 mice. In fact, sEH is considered a possible therapeutic target for amplifying the beneficial effects of EETs, that have been associated with, for example, liver regeneration.51 On the other hand, biosynthesis of COX-derived eicosanoids in LSECs, including the major vasoprotective eicosanoid PGI2 was downregulated in 12-month-old Tgαq*44 mice. Furthermore, LSECs proteome profiling revealed that expression of HMOX-1, ADA, and ECE-1 was decreased, suggesting an altered function of carbon monoxide (CO), adenosine, and endothelin (ET-1) in liver microcirculation during HF progression. Apparently, the profile of vasoactive mediators produced by LSECs was altered in hepatic congestion in Tgαq*44 mice, albeit with a completely distinct profile of these changes as, for example, in portal hypertension.50
LSECs dysfunction in 4-month-old Tgαq*44 mice was also featured by impaired ability to form a functional barrier under in vitro conditions but the difference between older Tgαq*44 mice and FVB mice disappeared. However, there was a clear age-dependent impairment compatible with profound age-dependent loss of barrier tightness.52 Interestingly, LSECs in 4-month-old Tgαq*44 mice displayed altered expression of laminin-binding DAG1 reported to be involved in blood–brain barrier formation. An assessment using Evans blue dye showed that DAG1 KO mice had increased barrier permeability53; DAG1 is essential for the formation of the astroglial endfeet architecture to maintain physiological blood–brain barrier function.54 These findings may correspond with weaker LSECs barrier integrity in the early stage of HF in 4-month-old Tgαq*44 mice.
LSECs dysfunction in Tgαq*44 mice was not associated with alterations in functional cellular bioenergetics. Similarly, in our previous study, LSECs showed remarkable metabolic plasticity in mice that were fed high-fat diet18 suggesting LSEC metabolism is well sustained under pathological conditions. Similarly, LSECs endocytic capacity of AcLDL did not display any changes, which may reflect the maintenance of this important physiological mechanism of liver circulation clearance, when LSECs are dysfunctional.55 Taken together, in Tgαq*44 mice LSECs dysfunction involved loss of fenestrae, shift in eicosanoid profile, and proteomic changes, however without changes in LSECs endocytic capacity of AcLDL, barrier function, and bioenergetic functional profile.
To find functional consequences of the LSECs dysfunction with a prominent defenestration, we analyzed possible alterations in lipid postprandial clearance that may occur when LSECs lose fenestrae.56 The important finding of this work, was to show that partial defenestration of LSECs in Tgαq*44 mice correlates with prolonged clearance of TG-rich lipoproteins during postprandial hypertriglyceridemia after olive oil administration, which strongly suggests important functional consequences of LSECs defenestration occurring in CHF. Moreover, no fat accumulation in the liver and the lack of significant alternations in the hepatocyte proteome (first changes occur in 12-month-old Tgαq*44 mice) indicates that the prolonged postprandial clearance of TG observed in 4-month-old Tgαq*44 mice could have been indeed related to less effective chylomicrons transport to the liver rather than impaired hepatocyte function.
Our results of prolonged clearance of TG-rich lipoproteins during postprandial hypertriglyceridemia in HF in Tgαq*44 mice may have clinical relevance because increased hypertriglyceridemia 3 and 4 hours after oral triglyceride load was demonstrated in HF patients.57 Given the function of fenestrae for the passage of lipoproteins between sinusoids and liver parenchyma, defenestration may indeed contribute to hampered lipoprotein clearance and hyperlipoproteinemia.12 Indeed, it was demonstrated that in plasmalemma vesicle-associated protein (PLVAP)-deficient mice, there was a pronounced reduction in the number of fenestrae in LSECs, which resulted in a severe hyperlipoproteinemia caused by the massive accumulation of chylomicron remnants in the plasma.58 Therefore, open fenestrae in LSECs are crucial for the undisturbed passage of lipoproteins,11, 12, 56 including TG-rich chylomicrons.
Interestingly, prolonged clearance of TG-rich lipoproteins during postprandial hypertriglyceridemia was associated with increased ketone bodies formation in 4- and 12-month-old Tgαq*44 mice. These results may suggest the activation of adaptive and functional changes in fatty acid liver metabolism in the early phase of CHF, resulting in increased production of ketone bodies. Ketone bodies display not only liver protective effects59 but also pronounced cardioprotective effects,60 thus excessive ketone release in postprandial phase might represent an important mechanism by which liver regulates cardiac metabolism. Of note, downregulation of ACAT1 together with changes in other proteins related to lipid metabolism in isolated hepatocytes in 12-month-old Tgαq*44 mice indicated a downregulation of fatty acid synthesis. As reduced fatty acid synthesis is usually counterbalanced by activation of fatty acid oxidation, these changes in hepatocytes in lipid metabolism might provide a basis for increased ketone bodies formation. However, other mechanisms could also be involved, and further studies are needed to elucidate pathophysiological basis for increased ketogenesis in Tgαq*44 mice in more detail.
4 MATERIALS AND METHODS
4.1 Animals
Tgαq*44 mice, initially developed by Mende et al.20 as well as wild-type control mice (FVB), were bred in the Institute of Experimental and Clinical Medicine of the Polish Academy of Sciences in Warsaw. Mice were housed in a temperature-controlled environment (22 ± 2°C) with a 12 h light/dark cycle, fed a standard laboratory diet, and water given ad libitum. All experiments were performed on Tgαq*44 and age-matched FVB female mice at the ages of 4 and 12 months, representing early- and end-stages of HF development, respectively. Tgαq*44 and age-matched FVB mice were euthanatized with ketamine (100 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally. All procedures involving animals were conducted in accordance with the Guide for the Care and Use of Laboratory Animals Directive 2010/63/EU of the European Parliament and approved by the 2nd Local Institutional Animal Care and Use Committee in Krakow (agreement no. 174/2021).
4.2 MRI-based assessment of right ventricle function
The RV volume and mass were evaluated at end-diastole using the 9.4 T MRI scanner (Bruker BioSpin, Ettlingen, Germany). The bright-blood cine images were collated in six to seven contiguous slices covering the whole ventricle volume using a retrospectively gated cine FLASH sequence (IgFLASH) as previously described.23, 24 The detailed protocol method has been described in Supplementary Information. Data were segmented manually using ImageJ 1.51 k (https://imagej.nih.gov/ij/) and summed up to obtain end-systole (ESV) and end-diastole (EDV) ventricle volumes. Right ventricle mass was calculated at end-diastole. Stroke volume (SV) was defined as SV = EDV-ESV, cardiac output (CO) as CO = SV × heart rate/60, and ejection fraction (EF) as EF = 100% × (EDV-ESV)/EDV.
4.3 Assessment of hepatic blood flow velocity by Doppler
A Doppler Flow Velocity System (Indus Instruments, Texas, USA) equipped with a single-transceiver 20 MHz Doppler probe was used to assess hepatic vein flow according to the protocol as previously described.23, 24 Pulsatility index (PI) was defined as the difference between maximal and minimal venous blood flow velocities during a single cardiac cycles, divided by the maximal velocity during the same cycle, express as a percentage. The detailed protocol method has been described in Supplementary Information.
4.4 Blood biochemistry and liver mass
Plasma samples were obtained by collecting blood from the left ventricle of the mouse heart, followed by centrifugation at 1000 × g for 10 min, and were used for the following measurements of liver enzyme activity: ALT (alanine transaminase), AST (aspartate transaminase), ALP (alkaline phosphatase) and concentrations of triglycerides (TG), total cholesterol (TC), HDL (high-density lipoprotein), and LDL (low-density lipoprotein) and TBIL (total bilirubin). All parameters were measured using the biochemical analyzer ABX Pentra 400 (Horiba Medical, Kyoto, Japan) according to the manufacturer's instructions. Albumin (ALB) and GGT (gamma-glutamyltransferase) concentrations in plasma were measured using commercially available ELISA assays (Bethyl Laboratories and LifeSpan BioSciences, USA, respectively). Livers were excised, weighed, and dried overnight at 60°C. Afterward, organs were weighed again and the wet-to-dry mass ratio was calculated.
4.5 Transmission electron microscopy (TEM) analysis of liver ultrastructure
The liver ultrastructure was visualized with electron microscope JEOL JEM-2100 HT (Tokyo, Japan) at voltage of 80 kV. The detailed protocol method has been described in Supplementary Information.
4.6 Scanning electron microscopy (SEM) analysis of LSECs fenestrae in hepatic sinusoid in situ
Liver was perfused and fixed according to the protocol developed by Cogger and O'Reilly at al.61 Fixed tissue was cut into 1–2 mm3 blocks, and left at 4°C for 72 h. Afterward, specimens were washed in cacodylate buffer (Sigma Aldrich, Germany), incubated with 1% osmium tetroxide, dehydrated in ethanol, and dried using HMDS (Sigma Aldrich, Germany). Tissue specimens were mounted onto SEM stubs, covered by gold particles, and visualized by electron microscope Helios 5 DualBeam (Thermo Scientific, USA) at 20 000 × magnification. Images were analyzed according to the previously described protocol,61 using ImageJ v.1.54d—fenestrae diameter, porosity, and fenestrae frequency data were obtained from 6 to 10 sinusoids per mouse.
4.7 Isolation of primary hepatocytes and LSECs
LSECs were isolated according to the previously described protocol18, 40 with subsequent improvements. Briefly, utilizing the universal perfusion system and setup for an isolated perfused liver (Hugo Sachs Electronics, Germany), primary liver cells were isolated. Mice were euthanized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) mixture. The portal vein was cannulated and an incision in the inferior vena cava was made to allow blood to drain from the circulatory system. To flush out the blood, the liver was first perfused with the perfusion buffer (142 mM NaCl, 6.708 mM KCl, 9.6 mM HEPES, 6 mM NaOH) at 37°C. Next, the digestion buffer with 0.02 mg/mL Liberase (Roche, Switzerland) was applied. The liver was carefully removed after digestion, and LSECs population was purified using endothelial-specific CD31-based immunoseparation on magnetic MicroBeads (MACS, MiltenyiBiotec, Germany). After isolation, cells were seeded at the desired density and incubated overnight at 37°C under hypoxic conditions containing 5% CO2 and 5% O2 (recommended for LSECs62) in EGM-2 cell culture medium (Lonza, Basel, Switzerland), unless otherwise stated.
Murine hepatocytes were separated from non-parenchymal cells by filtering with cell strainers (70 μm) and centrifuged at 50 × g for 2 min. Then, using 90% of Percoll solution (Sigma Aldrich, Germany) dead hepatocytes were discarded, and cell viability (>90%) was determined by trypan blue exclusion. Next, hepatocytes were plated on 6-well plates (400 000/well) precoated with collagen I. After an initial 3-4 h attachment period, Williams' E medium (Sigma Aldrich, Germany) was added.
4.8 Assessment of LSEC's fenestrae number using atomic force microscopy (AFM)
LSECs were treated with either 21 μM Cytochalasin B (Sigma Aldrich, Germany) or 500 μM Diamide (Sigma Aldrich, Germany) for 30 or 60 min, respectively, or remained untreated. Next, LSECs were fixed with 1% glutaraldehyde and processed using a Nanowizard 3 AFM system (JPK Instruments, Germany). The total area covered by the collected AFM maps was 2500 μm2 or more. However, for the fenestrae analysis, we selected a specific region that excluded the nuclear area and glass surface. This way, we focused on a membrane area of at least 1000 μm2, which included multiple adjacent cells, ensuring a comprehensive representation for the analysis. The number of fenestrae was determined by taking advantage of a purposely designed neural network. The whole methodology was previously described in detail.63 Briefly, a fully convolutional neural network was utilized comprising 12 layers, where the input consisted of two features extracted from the force spectroscopy map: height at 90% of the maximal indentation depth and stiffness (slope) at the last 10 nm of the indentation curve. Next, the neural network was trained using the Adam optimizer and a loss function that incorporated both L1 (least absolute deviations) and L2 (least square errors) terms.
4.9 Measurement of the trans-endothelial electrical resistance of LSECs
The barrier formation potential of LSECs was assessed with a real-time ECIS system using the 8W10E+ plate with electrode chamber arrays and ECIS Z- Theta system (Applied Biophysics, USA) with associated software v.1.2.126 PC. Immediately after cell seeding, resistance measurements (in ohms) were started and the increase in resistance with respect to the time denoted that cells were forming contacts among one another. The cell-free wells served as a negative control to provide the baseline changes in impedance for all experiments.
4.10 Assessment of LSECs bioenergetic
Metabolic analyses of isolated LSECs were performed using a Seahorse XFe96 Analyzer (Agilent Technologies, USA) which enables real-time, simultaneous measurement of OCR (reflecting mitochondrial respiration) and ECAR (reflecting glycolysis) as previously described.18 Data were normalized for the cell number assessed by nuclei staining using Hoechst 33342 (Thermo Scientific, USA).
4.11 LC–MS/MS quantification of eicosanoids released by LSECs
The eicosanoids released by LSECs were quantified in EGM-2 medium (Lonza, Basel, Switzerland). The method and instrument operation parameters were previously described in detail.64 Data were normalized for the cell number assessed by nuclei staining using Hoechst 33342 (Thermo Scientific, USA).
4.12 Postprandial hypertriglyceridemia and ketone bodies
Tgαq*44 and age-matched FVB mice were fasted for 16 hours. Blood samples were collected from tail veins prior (0 min) to olive oil gavage (10 mL/kg body weight) (Sigma Aldrich, Germany) and at 90, 180, and 480 min after olive oil administration into K2EDTA-containing tubes. Samples were centrifuged (1000 × g, 10 min at 4°C), plasma TG and β-Hydroxybutyrate (β-HB) concentration was measured using an automatic biochemical analyzer ABX Pentra 400 (Horiba Medical, Kyoto, Japan), and commercially available assay, respectively (Sigma-Aldrich, Germany).
4.13 Primary hepatocytes and LSECs proteome profiling
Hepatocytes and LSECs were lysed in lysis buffer containing 7 M urea (BioShop, Burlington, Ontario, Canada), 2 M thiourea (BioShop, Burlington, Ontario, Canada), and 30 mM Tris–HCl (pH 8.0) (BioShop, Burlington, Ontario, Canada), sonicated on ice, centrifuged (16 000 × g, 15 min, 4°C), and after which the supernatants were collected. Samples were proceeded according to the in-solution protocol (hepatocytes)65 or to the modified FASP procedure (LSECs)66 and analyzed by LC–MS system. The detailed protocol method has been described in Supplementary Information.
4.14 Statistical analysis
A detailed description of the data presentation and statistical analysis has been provided in the legends to the figures. Calculations were performed using GraphPad v. 8.0.1; a value of p ≤ 0.05 was considered to be statistically significant. Data from the ECIS system illustrating barrier formation changes were subjected to the analysis of covariance (ANCOVA) based on their parallel course, which was confirmed with the equal slopes analysis model in Statistica v. 13.0 software (StatSoft, Inc., Krakow, Poland).
5 CONCLUSION
In Tgαq*44 mice with chronic left-sided HF, the presence of RV failure followed by alternations in hepatic blood flow and hepatic congestion, was associated with defenestration of LSECs with altered LSECs phenotype and prolonged clearance of TG-rich lipoproteins during postprandial hypertriglyceridemia. Therefore, quite surprisingly, LSECs dysfunction and defenestration represent an early event in CHF pathophysiology, and may become an additional therapeutic target in HF, the reversal of which may have a beneficial effect on cardio-hepatic interactions and HF prognosis determined by liver physiological status.3, 67
AUTHOR CONTRIBUTIONS
Kamila Wojnar-Lason: Conceptualization; investigation; writing – original draft; writing – review and editing; funding acquisition; visualization; formal analysis; methodology. Urszula Tyrankiewicz: Investigation; writing – review and editing; visualization. Agnieszka Kij: Investigation; writing – review and editing; methodology. Anna Kurpinska: Investigation; writing – review and editing; formal analysis; methodology. Patrycja Kaczara: Investigation; writing – review and editing. Grzegorz Kwiatkowski: Investigation; writing – review and editing. Natalia Wilkosz: Investigation; writing – review and editing; methodology. Magdalena Giergiel: Investigation; writing – review and editing; formal analysis. Marta Stojak: Investigation; writing – review and editing. Marek Grosicki: Writing – review and editing; investigation; formal analysis. Tasnim Mohaissen: Writing – review and editing; investigation. Agnieszka Jasztal: Investigation; writing – review and editing. Zuzanna Kurylowicz: Investigation; writing – review and editing. Marek Szymonski: Writing – review and editing; resources. Izabela Czyzynska-Cichon: Supervision; investigation; writing – review and editing. Stefan Chlopicki: Supervision; resources; writing – review and editing; conceptualization; funding acquisition; project administration.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Olga Woznicka (Institute of Zoology and Biomedical Sciences, Jagiellonian University) and Michal Pacia (The Jagiellonian Center of Innovation) for their assistance and technical support in electron microscopy image acquisition. The authors thank Agata Malinowska from Mass Spectrometry Laboratory, Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland for LC–MS proteomic data acquisition. The used equipment was sponsored in part by the Centre for Preclinical Research and Technology (CePT), a project co-sponsored by European Regional Development Fund and Innovative Economy, The National Cohesion Strategy of Poland. The open access publication of this article was funded by the programme 'Excellence Initiative-Research Univiersity' at the Jagiellonian University in Krakow.
FUNDING INFORMATION
This work was supported by the National Science Centre, Poland (grant no. 2019/35/N/NZ5/03568 to K.W-L. and grant no. 2021/42/A/NZ4/00273 to S.C.).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




