Hyperpolarization-activated cyclic nucleotide-gated channel inhibitor in myocardial infarction: Potential benefits beyond heart rate modulation
Abstract
Myocardial infarction (MI) and its associated complications including ventricular arrhythmias and heart failure are responsible for a significant incidence of morbidity and mortality worldwide. The ensuing cardiomyocyte loss results in neurohormone-driven cardiac remodeling, which leads to chronic heart failure in MI survivors. Ivabradine is a heart rate modulation agent currently used in treatment of chronic heart failure with reduced ejection fraction. The canonical target of ivabradine is the hyperpolarization-activated cyclic nucleotide-gated channels (HCN) in cardiac pacemaker cells. However, in post-MI hearts, HCN can also be expressed ectopically in non-pacemaker cardiomyocytes. There is an accumulation of intriguing evidence to suggest that ivabradine also possesses cardioprotective effects that are independent of heart rate reduction. This review aims to summarize and discuss the reported cardioprotective mechanisms of ivabradine beyond heart rate modulation in myocardial infarction through various molecular mechanisms including the prevention of reactive oxygen species-induced mitochondrial damage, improvement of autophagy system, modulation of intracellular calcium cycling, modification of ventricular electrophysiology, and regulation of matrix metalloproteinases.
1 INTRODUCTION
Myocardial infarction (MI) is the leading cause of morbidity and mortality worldwide.1 MI is caused by insufficient oxygen supply to the myocardium, resulting in irreversible damage and cell death. Acute consequences of MI include ventricular arrhythmia and subsequent sudden cardiac death, ventricular dysfunction, and cardiac rupture. Chronic consequences of MI include cardiac remodeling which culminates in heart failure.2
MI sets into motion a complex pathophysiologic process that damages cardiomyocytes, other cardiac resident cells, and the coronary vasculature. Different mechanisms affect the heart and its vasculature at different time points throughout the course of MI. Cellular energy depletion and excessive reactive oxygen species (ROS) production by dysfunctional mitochondria play critical roles in the early phase of MI, resulting in cellular calcium overload and apoptosis.3, 4 Inflammatory process and oxidative stress also trigger non-apoptotic cell death pathways including necroptosis, ferroptosis, and pyroptosis.5-7 The extent to which each of these cell death mechanisms contribute to cardiomyocyte loss in MI remains a subject of extensive investigations. Autophagy, which is the process of lysosomal protein degradation and biomolecular recycling, together with mitophagy, a mitochondria specific form of autophagy,8 is activated to support cardiac cell survival in the early phase (approximately the first 1–3 days) of MI and becomes exhausted in prolonged ischemia.9 There is no consensus defining the exact temporal boundary between acute and chronic phases of MI. However, the chronic phase is characterized by cardiac fibrosis and cardiac remodeling, which are resulted from neurohormonal maladaptation (i.e., overactivation of the rennin angiotensin aldosterone system (RAAS) and sympathetic activation) as well as increased activity of the matrix metalloproteinase system.10-12 These sustained processes change the cellular and extracellular matrix structures, leading to failing myocardium.13, 14 Readers are referred to comprehensive reviews on the pathophysiology of MI3, 4 for more detailed discussion on the topic.
The current standard therapy for MI is coronary revascularization.15 However, restoration of myocardial blood flow also causes additional damage to the myocardium via excessive generation of reactive oxygen species (ROS) and other incompletely understood mechanisms, a phenomenon called reperfusion injury.3, 4 Great efforts have been made to investigate potential additional treatment together with revascularization to reduce ischemia–reperfusion (IR) injury, reduce reinfarction, and ultimately reduce mortality rates. There is solid evidence showing that heart rate is one of the determination factors of clinical outcomes of MI, and heart rate reduction may be beneficial because of the close relationship between heart rate, coronary blood flow, and contractile function.16 Increased heart rate shortens the duration of diastolic phase, thus reducing the myocardial perfusion time of the left ventricle. This is counterbalanced by autoregulatory coronary vasodilation in the normal myocardium, thus maintaining adequate arterial supply. However, in the ischemic myocardium, coronary vessels distal to the occlusion site are already dilated to compensate for the reduced baseline blood flow, and such post-stenotic vascular beds rely on perfusion from collateral vessels. When the heart rate rises and autoregulatory dilation occurs in the non-ischemic myocardium, the driving pressure gradient for ischemic zone perfusion through the collateral vessels is reduced.17 Therefore, heart rate reduction in MI is expected to improve coronary perfusion and improve contractile function of ischemic hearts.
Along these lines, Beta-blockers improved blood flow and cardiac function in MI through their negative chronotropic effect and improvement of cardiac energy metabolism.18 Beta-adrenergic antagonism also mitigates catecholamine-mediated arrhythmogenic processes19 and oxidative phosphorylation uncoupling.20 However, there is some downside of beta-blockade. Beta-blockers are contraindicated in patients with known obstructive pulmonary diseases due to their potential to cause severe bronchoconstriction. In addition, the ability of beta-blockers to improve coronary blood flow is blunted by two major mechanisms. First, even though increasing diastolic duration physiologically results in increased time for coronary blood flow, beta-blockade slows isovolumic ventricular relaxation. Considering the diastolic pressure–time integral as a major determiner for coronary blood flow, the negative lusitropic effect will impede coronary perfusion.21 Second, beta-blockers unmask alpha-adrenergic-mediated vasoconstriction.22 Such disadvantages have prompted the search for more selective bradycardic agents.
Zatebradine (UL-FS 49) is the prototype of selective bradycardic agents that inhibits the hyperpolarization-activated cyclic nucleotide-gated channels (HCN), which are responsible for the funny current (If) that mediates the pacemaking function in the heart.23-25 Under physiologic conditions HCN is expressed only by pacemaker cells (such as sinoatrial and AV nodal cells).26, 27 However, in disease conditions such as MI and heart failure, HCN can also be present in non-pacemaker cells.26, 28, 29 Although zatebradine has been shown to mitigate exercise-induced regional myocardial ischemia/dysfunction in dogs suffering from chronic coronary stenosis30 and attenuated ischemic regional myocardial dysfunction and reduced infarct size in a swine model of cardiac IR,31 its use was discouraged due to its side effects (prolonged repolarization of Purkinje fibers and visual disturbances).32, 33 Ivabradine, another HCN channel inhibitor, was approved by the United States Food and Drug Administration (U.S. FDA) for patients with stable heart failure with reduced ejection fraction (left ventricular ejection fraction; LVEF ≤ 35%) who have a resting HR above 70 bpm on a maximally tolerated beta-blocker, or have contraindications for beta-blockers.34
The pioneering work on pleiotropic effects of ivabradine by Heusch and colleagues35 has paved the way for subsequent preclinical and small-cohort clinical studies that investigated ivabradine's pleiotropism in more details. Ongoing preclinical and small-cohort clinical studies continue to unveil the pleiotropic effects of ivabradine. This review aims to summarize and discuss the mechanisms associated with ivabradine beyond heart rate modulation and the potential benefits of ivabradine as a future complementary drug for MI patients, either with or without complications, such as cardiogenic shock, ventricular arrhythmia, and heart failure.
2 EFFECTS OF IVABRADINE ON CARDIAC ISCHEMIA–REPERFUSION INJURY IN LARGE ANIMAL MODELS
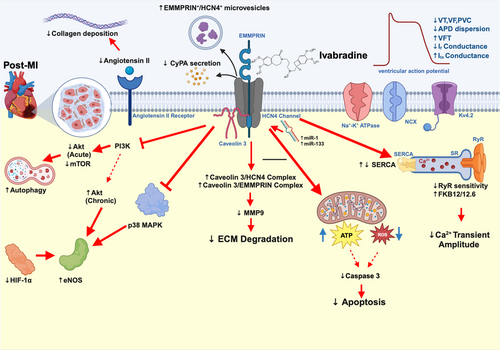
Non-HR related actions of ivabradine in MI was first reported in a porcine IR study, which showed that ivabradine treatment (0.6 mg/kg, IV) at various time points during cardiac IR injury (pretreatment, during ischemia, and upon reperfusion) significantly decreased the infarct size. Myocardial blood flow was increased if the treatment was given before or during ischemia, but not at the time of reperfusion. When pacing was instigated, myocardial blood flow was diminished but the reduction of infarct size still remained.35 In another porcine IR model, ivabradine treatment (0.25 mg/kg, IV) resulted in an increased ventricular fibrillation threshold (VFT), increased duration of monophasic action potential (DMAP), decreased cardiac ultrastructural change, and increased regional myocardial blood flow. When the heart was paced to preclude heart rate reduction, those beneficial effects of ivabradine were eradicated, suggesting that they are HR-dependent.36 In addition to infarct size reduction, ivabradine (0.3 mg/kg, IV) administered 15 min after IR improved left ventricular (LV) function37, 38 through mechanisms that involve the reduction of cyclophilin A (CyPA) release from cardiac mitochondria.39 Since CyPA is a ligand of the extracellular matrix metalloproteinase inducer (EMMPRIN), which can activate the inflammatory cascade,40 the reduction of CyPA by ivabradine resulted in the reduction in inflammation caused by IR injury. Ivabradine also stabilized the EMMPRIN/HCN4/caveolin-3 complex, leading to the downregulation of matrix metalloproteinase 9 (MMP9), which may improve cardiac remodeling in the long term.37 In other porcine IR models treated with inotropic agents or a cardiac assist device, co-treatment with ivabradine effectively reduced the infarct size, decreased myocardial oxygen consumption, and increased the stroke volume without altering blood pressure.41, 42 All of these findings suggested that ivabradine could be a promising pharmacological intervention for use in conjunction with a cardiac assist device or inotropes in MI patients. All of these reports are comprehensively summarized in Table 1.
| Animal model | Condition | Dose/route/time of ivabradine and/or other medications | Pacing | Heart rate reduction | LV function | Infarct size | Relevant findings | Interpretation | Refs | |
|---|---|---|---|---|---|---|---|---|---|---|
| I | R | |||||||||
| Pigs | 90 min | 120 min | 0.6 mg/kg/IV/20 min before ischemia | – | ~18% | – | ↓ | ↑ RMBF | Ivabradine reduced infarct size in a cardiac IR model when given before, or during ischemia, or at the onset of reperfusion in a heart rate independent manner | [35] |
| 0.6 mg/kg/IV/20 min before ischemia | ✓ | – | – | ↓ | ⟷ RMBF | |||||
| 0.6 mg/kg/IV/during ischemia | – | ~18% | – | ↓ | ↑ RMBF | |||||
| 0.6 mg/kg/IV/during ischemia | ✓ | – | – | ↓ | ⟷ RMBF | |||||
| 0.6 mg/kg/IV/onset of reperfusion | – | ~26% | ↑ LOOPaw | ↓ | ⟷ RMBF | |||||
| 0.6 mg/kg/IV/onset of reperfusion | ✓ | – | – | ↓ | ⟷ RMBF | |||||
| Pigs | 1 min | 15 min | 0.25 mg/kg/IV/15 min before ischemia | – | ~32% | – | – |
↑ VFT ↑ DMAP ↓ Ultrastructure change ↑ RMBF |
Ivabradine reduced ventricular arrhythmia and improved change in ultrastructure in a heart rate dependent manner | [36] |
| 0.25 mg/kg/IV/15 min before ischemia | ✓ | – | – | – |
⟷ VFT ⟷ DMAP ⟷ Ultrastructure change ⟷ RMBF |
|||||
| Pigs | 45 min | 7 days | 0.3 mg/kg/IV/onset of reperfusion | – | ~33% |
↑ EF ↑ SV |
↓ |
↓ Necrosis area ↓ Troponin I ↓ MMP-9 expression ↑ caveolin-3/LG-EMMPRIN complex ↑ caveolin-3/HCN4 complex ⟷ EMMPRIN mRNA & protein levels ⟷ Glycosylation protein |
Ivabradine reduced LV cardiac dysfunction and infarct size via reducing ECM-degrading enzymes in a cardiac IR model | [37] |
| Pigs | 45 min | 7 days | 0.3 mg/kg/IV/onset of reperfusion | – | – | ↑ EF | ↓ |
↓ Necrosis area ↑ EMMPRIN+/HCN4+ microvesicles ⟷ endothelial EMMPRIN+ microvesicles ⟷ platelet EMMPRIN+ microvesicles |
Ivabradine reduced LV cardiac dysfunction and infarct size via increasing cardiac EMMPRIN microvesicles in a cardiac IR model | [38] |
| Pigs | 45 min | 7 days | 0.3 mg/kg/IV/onset of reperfusion | – | – | ↑ EF | ↓ |
↓ Necrosis area ↓ CyPA secretion ↑ CyPA/low glycosylated EMMPRIN complex ⟷ Lysosomal degradation |
Ivabradine reduced LV cardiac dysfunction and infarct size via reducing cyclophilin A secretion in a cardiac IR model | [39] |
| Pigs | 60 min | 60 min | Dobutamine 5 μg/kg/min/IV/at the onset reperfusion | – | – |
↑ SV ⟷ Diastolic filling time ↑ CO ⟷ MAP |
– | – | Ivabradine potentiated the effects of dobutamine in improving cardiac function in a cardiac IR model | [42] |
| 60 min | 60 min | Dobutamine 5 μg/kg/min/IV/at reperfusion + Ivabradine 0.5 mg/kg/IV/30 min after reperfusion | ~22% |
↑↑ SV ↑ Diastolic filling time ↑ CO ⟷ MAP |
– | – | ||||
| Dogs | 180 min | 180 min | 1 mg/kg IV/60 min after ischemia + Impella heart pump versus Impella heart pump alone | – | ~10% |
⟷ LVEDP ⟷ LVESP |
↓ |
↓ MVO2 ⟷ Pressure-volume area |
Ivabradine reduced infarct size via decreased myocardial oxygen consumption in a cardiac IR with a ventricular assist device model | [41] |
| Acute cardiogenic shocked pigs | 45 min | 90 min | 0.3 mg/kg/IV/15 min after reperfusion | – | ~23% |
⟷ CO ⟷ PWP ⟷ CVP |
– | ↑ TV | Ivabradine did not alter hemodynamic parameters in a cardiac IR with a cardiogenic shock model | [43] |
| Acute cardiogenic shocked pigs | 45 min | 75 min | 0.3 mg/kg/IV/15 min after reperfusion | – | ~28% |
⟷ SV ⟷ CVP ⟷ CO ⟷PCWP ⟷MPAP |
– | – | Ivabradine did not alter hemodynamic parameters in a cardiac IR with a cardiogenic shock model | [44] |
- Abbreviations: CO, cardiac output; CVP, central venous pressure; CyPA, cyclophilin A; DMAP, duration of monophasic action potential; ECM, extracellular matrix; EF, ejection fraction; EMMPRIN, extracellular matrix metalloproteinase inducer; HCN, hyperpolarization-activated cyclic nucleotide; IR, ischemia/reperfusion; IV, intravenous injection; LG-EMMPRIN, low glycosylated-extracellular matrix metalloproteinase inducer; LooPaw, instantaneous LV pressure-wall thickening product during systole in the anterior wall; LVEDP, left ventricular end diastolic pressure; LVESP, left ventricular end systolic pressure; MAP, mean arterial blood pressure; MMP-9, matrix metallopeptidase 9; MPAP, mean pulmonary artery pressure; MVO2, myocardial volume oxygen; PWP, pulmonary wedge pressure; RMBF, reginal myocardial blood flow; TV, tidal volume; VFT, ventricular fibrillation threshold.
Unlike uncomplicated MI, there are several discrepancies in evidence showing that ivabradine could not improve cardiac function in cardiogenic shock or acute heart failure (Table 1). In studies using a porcine cardiogenic shock model (cardiac IR together with fluid administration to achieve a pulmonary wedged pressure of ≥18 mmHg), ivabradine (0.3 mg/kg, IV) given 15 min after reperfusion resulted in 23%–28% HR reduction without reducing central venous pressure (CVP) but decreased the cardiac output.43, 44 The reason for those non-favorable outcomes might be due to excessive reduction in HR at such a critical period (i.e., during cardiogenic shock). Further studies are needed to investigate whether preload reduction and inotropic support are required to achieve the full benefits of ivabradine under those fluid overload conditions.
3 EFFECTS OF IVABRADINE IN CARDIAC IR IN SMALL ANIMAL MODELS: REPORTS FROM EX VIVO, IN VIVO, AND IN VITRO STUDIES
Several small animal studies showed ivabradine had benefits similar to those shown in the large animal studies, as summarized in Table 2. In IR rat models, ivabradine showed its heart rate independent effect on reducing the infarct size.45 Heusch and colleagues not only did an experiment in rats, but they also did an experiment in isolated mouse cardiomyocytes and isolated mitochondria treated with ivabradine (3 μM), and demonstrated that ivabradine attenuated mitochondrial ROS and preserved mitochondrial function under hypoxia-reoxygenation conditions.45 Moreover, ivabradine also improved systolic cardiac function, reduced cardiac strain, and improved LV geometry.46, 47 In a rabbit MI model, ivabradine not only preserved mitochondrial function, but also improved cellular calcium handling via upregulation of the FK506-binding protein (FKBP) 12 and its homolog, FKBP12.6.48 FKBP12/12.6 interacts with ryanodine receptors (RyR) to reduce diastolic calcium leak from the sarcoplasmic reticulum (SR).51 This improvement in calcium handling by ivabradine could be responsible for enhanced cardiac function.
| Animal/Specimen | Conditions | Dose/route/time of ivabradine and/or other medications | Heart rate reduction | LV function | Infarct size | EP markers | Relevant findings | Interpretation | Refs | |
|---|---|---|---|---|---|---|---|---|---|---|
| I | R | |||||||||
| In vivo study | ||||||||||
| Mice | 30 min | 120 min | 1.7 mg/kg/IV/before IR | ~18% | – | ↓ | – | – | Ivabradine reduced infarct size independently to heart rate reductio | [45] |
| 1.7 mg/kg IV + pacing/before IR | – | – | ↓ | – | – | |||||
| Mice | 60 min | 28 days | 10 mg/kg/d/oral/starting at 1 h after reperfusion/28 days | ~12% |
↑ EF↓ LVEDP ↓ LVESP ↑ Strain ↑ Synchrony ↓ Wall thinning |
– | – | – | Ivabradine attenuated LV remodeling and preserved contraction synchrony | [46] |
| Rats | 60 min | 28/70 days | 10 mg/kg/d/oral/starting at 7 days after I/R/28 days | ~17% |
↑ EF ↑ FS ⟷ LVIDd ⟷ LVIDs At day 28 |
↓ At day 70 | – | – | Ivabradine reduced cardiac dysfunction and infarct size | [47] |
| Rabbits | 20 min | 21 days | 10 mg/kg/day/IP/starting at the onset of reperfusion/21 days | ~20% |
↑ EF ↓ LVEDP ↓ LVESP |
⟷ | – |
↑ FKBP12/12.6 mRNA ⟷ NCX1 ⟷ RyR2 ⟷ SERCA2a ⟷ Phospholamban |
Ivabradine reduced cardiac dysfunction by increasing FKBP12/12.6; a regulator of SR calcium leak | [48] |
| Ex vivo study | ||||||||||
| Langendorff-perfused rabbit hearts | 75 min | 30 min | 0.3 μM/15 min/before ischemia | ~19% |
↓ LVEDP ↓ LVESP |
– | – |
↓ CK ↓ NE ↑ ATP ↑ Mitochondrial RCI ↑ NADPH |
Ivabradine reduced cardiac dysfunction and myocardial damage by improving mitochondrial function in a heart rate dependent manner | [49] |
| 0.3 μM + Pacing 180 bpm/before ischemia | – |
⟷ LVEDP ⟷ LVESP |
– | – |
⟷ CK ⟷ NE ⟷ ATP ⟷ Mitochondrial RCI ⟷ NADPH |
|||||
| Langendorff-perfused rat hearts | 8 min | 10 min | 1 μM/5 min before ischemia | ~30% | – | – |
↓ VT, VF |
⟷ Coronary flow | Ivabradine reduced ventricular arrhythmias in a heart rate dependent manner during ischemia | [50] |
| 1 μM/5 min/before ischemia + Pacing/before ischemia | – | – | – | ⟷ VT, VF | ⟷ Coronary flow | |||||
| 1 μM/5 min/before ischemia + Pacing/at reperfusion | – | – | – | ↓ VT, VF | ⟷ Coronary flow | |||||
| 100 μM/at reperfusion | ~35% | – | – | ⟷ VT, VF | ⟷ Coronary flow | |||||
| Langendorff-perfused heart of mice | 40 min | 5 min | 3 μM/15 min before ischemia + Isoprenaline/10 nM | – |
⟷ CO ⟷ SV |
– | – | ⟷ MVO2 | Ivabradine did not improve cardiac function | [42] |
| In vitro study | ||||||||||
| Isolated cardiomyocytes | 60 min | 5 min | 3 μM/L/30 min before ischemia | – | – | – | – |
↑ Cell viability ↓ intracellular ROS |
Ivabradine increased cell viability via reducing oxidative stress and improving mitochondrial function | [45] |
| 3 μM/L/10 min before reperfusion |
↑ Cell viability ↓ intracellular ROS |
|||||||||
| Isolated mitochondria | 6 min | 3 min | 3 μM/L/during ischemia | – | – | – | – |
↓ ROS ↑ ATP ↑ CRC ⟷ complex I |
||
- Abbreviations: ATP, adenosine triphosphase; CK, creatine kinase; CO, cardiac output; CRC, calcium retention capacity; CVP, central venous pressure; EF, ejection fraction; FKBP, FK506-biding protein; FS, fractional shortening; IR, ischemia/reperfusion; IP, intraperitoneal injection; IV, intravenous injection; LVEDP, left ventricular end diastolic pressure; LVESP, left ventricular end systolic pressure; LVIDd, left ventricular internal diameter during diastole; LVIDs, left ventricular internal diameter during systole; MVO2, myocardial oxygen consumption; NADPH, nicotinamide adenine dinucleotide phosphate; NCX, sodium–calcium exchanger; NE, norepinephrine; RCI, respiratory control index; ROS, reactive oxygen species; RyR2, ryanodine receptor type 2; SERCA2, sarco/endoplasmic reticulum calcium ATPase 2 alpha; SV, stroke volume; VF, ventricular fibrillation; VT, ventricular tachycardia.
Several ex vivo studies showed various benefits of ivabradine.42, 49, 50 In Langendorff-perfused rabbit hearts, ivabradine improved cardiac function by minimizing consumption of cardiac energy and reducing myocardial norepinephrine (NE) release in a HR-dependent manner.49 Ivabradine also reduced ventricular arrhythmias as a consequence of heart rate reduction.50 Remarkably, the ventricular antiarrhythmic effects only occurred when HR was reduced during ischemia.50 Finally, similar to the outcomes of the large animal studies discussed above, the ivabradine-inotrope combination reduced myocardial oxygen consumption (MVO2), but did not improve cardiac efficiency as assessed by the work-MVO2 relationship.42
4 EFFECT OF IVABRADINE IN NON-REPERFUSED MI MODELS: REPORTS FROM IN VIVO STUDIES
Studies in post-MI models reported findings consistent to those found in the IR models (Table 3). One of the mechanisms occurring within cardiomyocytes that could mediate the effects of ivabradine is the activation of autophagy via the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin/ribosomal protein S6 kinase beta-1. (PI3K/AKT/mTOR/p70S6K) pathway.70 Ivabradine at 50 mg/kg/day administered subcutaneously (SC) was shown to reduce cardiac rupture via the reduction of programmed cell death as evidenced by a decrease in cleaved-caspase 3,71 which is a key molecular effector of apoptosis.72 Ivabradine (10 mg/kg, given orally) administered on days 7–90 post-MI resulted in 18% HR reduction, and improved systolic function in rats via ECM modification in a dose-dependent manner in comparison to the control group.53 Ivabradine also improved cardiac function by modification of the renin-angiotensin-aldosterone system (RAAS), resulting in decreased fibrosis and improved coronary perfusion in an MI rat model.54 RAAS is a key mechanism in myocardial ECM remodeling, which leads to cardiac fibrosis and heart failure in ischemic heart disease.73, 74 In a 4-week MI rat model, ivabradine was shown to improve maximal myocardial perfusion and coronary perfusion reserve, and these benefits were associated with a decrease in perivascular collagen without altering the levels of vascular endothelial growth factor (VEGF).55 In an MI rat model, ivabradine 10 mg/kg/day, given PO on days 7–90 post-MI, improved diastolic function and increased microvessel density through increasing the expression of endothelial nitric oxide synthase (eNOS), increasing proliferation of endothelial cells, decreasing collagen density, and decreasing hypoxia-inducible factor 1-alpha (HIF-1α).60 One of the underlying mechanisms of eNOS upregulation by ivabradine in an MI rat model has been shown to be suppression of the p38-mitogen-activated protein kinase (MAPK) pathway.67 Ivabradine also improved myocardial perfusion and increased cardiomyocyte survival to the same extent as atenolol (a beta-blocker used as the standard treatment for MI in clinical practice) in a 4-week MI rat model.56, 59 Treatment with ivabradine (10 mg/kg/day, PO, for 8 weeks starting at 24 h after MI) reduced cardiac dysfunction without affecting the infarct size by improving the activity of sarcoendoplasmic reticulum calcium ATPase (SERCA). These effects were not inferior to those caused by metoprolol.62 When ivabradine treatment was prolonged for up to 12 weeks after MI in rats, there were differential results depending on the age of rats used. In a young MI rat model (aged 10 weeks), 12-weeks of ivabradine treatment improved myocardial capillary density.58 In contrast, the same duration of ivabradine treatment (12 weeks) failed to improve cardiomyocyte survival and capillary densities in middle aged rats (aged 12 months).57 In addition, the abrupt withdrawal of ivabradine after the 12-week treatment period resulted in a deterioration of cardiac function in aged rats.57
| Animal/Specimen | MI | Dose/route/time of ivabradine and other medications | Heart rate reduction | LV function | Infarct size | Ion channels & EP markers | RAA system & fibrosis | Relevant findings | Interpretation | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Duration | ||||||||||
| Mice | 24 h | 10 mg/kg/day/oral/starting at 24 h before MI | ~16% |
↑ EF ↓ LVEDP ↓ LVESP |
⟷ |
↓ VT, VF Incidence ↓ APD dispersion ↑ QT prolonged ↑ MAP amplitude ⟷ MAP velocity ↓ Ca2+ transient ↓ Kv4.2 ↓ KChIP2 mRNA expression ↓ If current ↓ HCN in LV ⟷ SR Ca2+ content ⟷ NCX activity ⟷SERCA ⟷ INa+ ⟷ ICa2+ (L type) |
– |
↓ Arrhythmic mortality ⟷ CaMKII |
Pretreatment with ivabradine reduced ventricular arrhythmias and cardiac dysfunction via modulation of Ca2+ handling and prevention of HCN4 upregulation | [52] |
| Rats | 90 days | 0.3 mg/kg/oral/starting at 7 days after ischemia | 4% |
⟷ LVFS ⟷ SV ⟷ LVSD ⟷ LVEDD ⟷ LV thickness |
⟷ | – | ⟷ LV collagen |
⟷ LV systolic P-V ⟷ LV developed P-V ⟷ LV diastolic P-V ⟷ Plasma NE |
Ivabradine reduced cardiac dysfunction and fibrosis in a dose and heart rate dependent manner | [53] |
| 1 mg/kg/oral/starting at 7 days after ischemia | 7% |
⟷ LVFS ⟷ SV ⟷ LVSD ⟷ LVEDD ⟷ LV thickness |
⟷ | – | ⟷ LV collagen |
⟷ LV systolic P-V ⟷ LV developed P-V ⟷ LV diastolic P-V ⟷ Plasma NE |
||||
| 3 mg/kg/oral/starting at 7 days after ischemia | 10% |
↑ LVFS ↑ SV ⟷ LVSD ⟷ LVEDD ⟷ LV thickness |
⟷ | – | ↓ LV collagen |
↑ LV systolic P-V ↑ LV developed P-V ⟷ LV diastolic P-V ↓ Plasma NE |
||||
| 10 mg/kg/oral/starting at 7 days after ischemia | 18% |
↑ LVFS ↑ SV ↓ LVSD ⟷ LVEDD ↑ LV thickness |
⟷ | – | ↓ LV collagen |
↑ LV systolic P-V ↑ LV developed P-V ⟷ LV diastolic P-V ↓ Plasma NE |
||||
| Rats | 5 months | 10 mg/kg/oral/starting at 2 months after ischemia | 10.4% | ↑ SV | – |
↓ PVC ↑ HRV ⟷ PR ⟷ QRS ⟷ QT |
↓ LV collagen ↓ ACE protein ↓ AT1 receptor protein |
- | Ivabradine reduced cardiac dysfunction and LV remodeling through downregulation of RAAS | [54] |
| Rats | 4 weeks | 10.5 mg/kg/IP/starting at 24 h after ischemia | ~25% | – | ⟷ | – |
↓ interstitial collagen ↓ periarteriolar collagen ⟷ periarteriolar fibroblasts ⟷ VEGF ⟷ Flt-1 ⟷ Flk-1 ↓ Plasma ANG II ↓ ANG II receptor |
↑ maximal myocardial perfusion ↑ coronary perfusion reserve |
Ivabradine reduced cardiac fibrosis and promoted coronary perfusion through the downregulation of RAAS. However, infarct size was not affected by ivabradine | [55] |
| Rats | 4 weeks | 6–8 mg/kg/day/IP/starting at 24 h after ischemia | ~19% | ↑ LV weight | ⟷ | – |
↓ interstitial collagen ↓ periarteriolar collagen ⟷ periarteriolar fibroblasts ⟷ VEGF ⟷ Flk-1 ↓ Plasma ANG II ↓ ANG II receptor |
↑ myocardial perfusion ⟷ baseline myocardial perfusion ↑ coronary perfusion reserve |
Ivabradine reduced cardiac fibrosis to the same extent as atenolol, and ivabradine could improve coronary perfusion reserve | [56] |
| Atenolol 0.6 mg/kg/oral/24 h after ischemia | ~19% | ⟷ LV weight | ⟷ | – |
↓ interstitial collagen ↓ periarteriolar collagen ⟷ periarteriolar fibroblasts ⟷ VEGF ↓ Flk-1 ↓ Plasma ANG II ↓ ANG II receptor |
↑ myocardial perfusion ↑ baseline myocardial perfusion ⟷ coronary perfusion reserve |
||||
| Rats | 12 weeks | 10.5 mg/kg/day/IP/starting at 24 h after ischemia | ~29% |
⟷ EF ⟷ LVESV ⟷ LVEDV ⟷ SV ⟷ CO |
⟷ | – | ↓ interstitial collagen |
⟷ Myocyte density ⟷ capillary density ⟷ arteriole density |
Ivabradine reduced cardiac fibrosis without improving cardiac function or capillary supply | [57] |
| Withdrawal of ivabradine after 12 weeks of treatment for 48 h | ↑ ~ 25% |
↓ EF ↑ LVESV ↑ LVEDV ⟷ SV ⟷ CO |
– | – | – | – | ||||
| Rats | 12 weeks | 10 mg/kg/oral/starting at 24 h after ischemia | ~17% |
⟷ SV ⟷ CO |
⟷ | – | – |
↑ capillary density ⟷ myocyte density |
Although ivabradine increased capillary supply, it did not reduce cardiac dysfunction or infarct size | [58] |
| 10 mg/kg/oral/starting at 3 weeks after ischemia | ~14% |
⟷ SV ⟷ CO |
⟷ | – | – |
⟷ capillary density ⟷ myocyte density |
||||
| Rats | 4 weeks | 6–8 mg/kg/day/IP/starting at 24 h after ischemia | – | ↑ LV thickness | ⟷ | – | – | ↑ cardiomyocyte survival | Ivabradine increased LV wall thickness and cardiomyocyte survival, and cardioprotective effects of ivabradine were not inferior to beta-blocker | [59] |
| Atenolol/0.6 mg/kg/oral/24 h after ischemia | – | ↑ LV thickness | ⟷ | – | – | ↑ cardiomyocyte survival | ||||
| Rats | 90 days | 10 mg/kg/day/oral/7 days after ischemia | ~14% |
↑SV ↓ LVSD ⟷ LVDD ↓ LVEDP ⟷ LVESP |
– | – |
↓ Collagen density ↓ HIF-1α |
↑ Microvessel density ↑ Endothelial cells ↑↑ eNOS expression |
Early treatment with Ivabradine reduced cardiac dysfunction and LV remodeling by promoting tissue oxygenation more effectively than late treatment | [60] |
| 97 days | 10 mg/kg/day oral/93 days after ischemia | ~18% |
↑SV ⟷ LVSD ↑LVDD ↓ LVEDP ⟷ LVESP |
– | – |
⟷ Collagen density ↓ HIF-1α |
⟷ Microvessel density ↑ Endothelial cells ↑ eNOS expression |
|||
| Rats | 90 days | 10 mg/kg/day oral/starting 7 days after ischemia | ~15% | – | – |
↓ If conductance ↓ HCN4 transcription ↑ miR-1 ↑ miR-133 |
– | – | Ivabradine improved EP cardiac remodeling by counteracting HCN channel overexpression | [61] |
| Rats | 8 weeks | 10 mg/kg/day/oral/starting at 24 h after ischemia | ~23% |
↑ EF ↑ dP/dt ↑ wall thickness ↓ wall stress ↓ LVEDP ⟷ LVESP |
⟷ |
⟷ NCX activity ↑SERCA activity ⟷ NCX/SERCA |
– | – | Ivabradine reduced cardiac dysfunction without affecting infarct size by improving calcium handling, and cardioprotective effects of ivabradine were not inferior to beta-blocker | [62] |
| Metoprolol 250 mg/kg/day oral (24 h after MI) | ~30% |
↑ EF ↑ dP/dt ⟷ wall thickness ↓ wall stress ↓ LVEDP ⟷ LVESP |
⟷ |
↓ NCX activity ⟷ SERCA activity ↓ NCX/SERCA |
– | – | ||||
| Rats | 90 days | 10 mg/kg/day/oral/starting at 7 days after ischemia | ~12% |
↑ EF ↓ LVEDP ↓ LVESP |
– |
↓ AP duration ↑ Ito ↑ Kv4.2 ↓ KChIP2 mRNA expression |
↑ CP ↑ ATP ↓ ADP ↓ AMP |
Ivabradine reduced cardiac dysfunction and EP change by promoting cardiac energy production. | [63] | |
| STZ-induced DM rats | 7 weeks | 10 mg/kg/day/IP/after ischemia | ~5% |
↓ LVDD ↓ LVSD ↑ LVFS ↓ LVEDP |
⟷ | – | – |
↓ plasma NE ↓ plasma BNP ↑ NE uptake-1 |
Ivabradine reduced cardiac dysfunction without affecting infarct size via reducing plasma NE and BNP in diabetic rats | [64] |
| Cardio genic shock pigs | 2 h | 0.29 mg/kg/IV interval bolus + Dobutamine 5 μg/kg/min/starting at 30 min after ischemia versus pretreatment | N/A |
↑ EF ↓ LVEDP ↓ LVESP ↑ dP/dt ↑ SV ↑diastolic time ↓ tau |
– | – | – | – | Ivabradine with dobutamine improved cardiac function | [65] |
| Rats | 2 months | 3 mg/kg/IV | ~ 20% |
⟷ CO ⟷ SV ⟷ EF |
– | – | – | – | Ivabradine did not improve cardiac function in post-MI rats in neither normo-volume models nor preload-increased models | [66] |
| 3 mg/kg IV bolus + physiological saline 12 mL/kg versus non-treat + physiological saline 12 mL/kg |
⟷ CO ⟷ SV ⟷ EF |
– | – | – | – | |||||
| Mice | 4 weeks | 10 mg/kg/day/oral | ~ 26% |
↑ EF ↑ FS ↑ LVIDD |
↓ | – | – |
↓ LV myocyte size ↑ capillary density ↑ Akt-eNOS ↓ p38 MAPK |
Ivabradine reduced cardiac dysfunction and LV remodeling by enhancing Akt-eNOS and inhibiting p38 MAPK | [67] |
| Rat | 6 h | 5 mg/kg/oral/starting at beginning of ischemia | ~20% | ⟷ |
↓ VT, VF, PVC ↓ QTc ⟷ MAP Duration ⟷ MAP amplitude ⟷ NCX activity ↓ SERCA ⟷ Ca2+ Transient ⟷ Ca2+ SR content ↓ RyR sensitivity |
– | – | Ivabradine reduced ventricular arrhythmias without affecting infarct size through modulation of intracellular calcium | [68] | |
| Rats | 6 weeks | 7 μg/kg/min/IV + Dopamine 10 μg/kg/min/starting 4 weeks after MI versus Dopamine | ~21% |
↑ EF ↓ LVEDP ↓ LVESP ↑ CO ↑ SV ↑ +dP/dt ⟷ -dP/dt |
⟷ |
↓ VT, VF, PVC ⟷ HCN4 expression |
– | – | Ivabradine prevented cardiac detrimental effects from dopamine, and this effect was not related to HCN4 expression | [69] |
| Rats | 7 days | 10 mg/kg/day/intragastric/starting 48 h after ischemia |
↓ LVEDP ↑ LVSP ↑ +dP/dt ↓ -dP/dt |
↓ | – | – |
↓ Apoptosis, Necrosis ↑ LC3, Beclin1, ATG ↓ TNF, IL-6, IL-1 ↓ PI3K, AKT, mTOR, p70S6K |
Ivabradine reduced infarct size by autophagy activation | [70] | |
| Rapamycin (Autophagy inducer) 2 mg/kg IP 48 h after MI |
↓ LVEDP ↑ LVSP ↑ +dP/dt ↓ -dP/dt |
↓ | – | – |
↓ Apoptosis, Necrosis ↑ LC3, Beclin1, ATG ↓ TNF, IL-6, IL-1 ↓ PI3K, AKT, mTOR, p70S6K |
|||||
| 10 mg/kg/day +3-MA (autophagy inhibitor) 15 mg/kg intraperitoneal 48 h after MI |
↑ LVEDP ↓ LVSP ↓ +dP/dt ↑ -dP/dt |
↓ | – | – |
↑ Apoptosis, Necrosis ↓ LC3, Beclin1, ATG ↑ TNF, IL-6, IL-1 ↑ PI3K, AKT, mTOR, p70S6K |
|||||
| Rats | 10 days | 50 mg/kg/day/SC/starting at 24 h after MI | ~36% |
⟷ BP ⟷ EF |
⟷ | – | – |
↓ Apoptosis ↓ Cleaved-caspase 3 ↓ Cardiac rupture ⟷ CD107b ⟷ LC3 |
Ivabradine reduced cardiac rupture without affecting cardiac function through reducing apoptosis | [71] |
| Ex vivo study | ||||||||||
| Langendorff-perfused of rats | 10 min | 1 μM/5 min before ischemia | – | – | – |
↑ delay onset of loss of electrical excitability ↓ conduction delay |
– | – | Ivabradine improved electrical activity | [50] |
- Abbreviations: ACE, angiotensin-converting enzyme; ADP, adenosine diphosphate; AKT, protein kinase B; AMP, adenosine monophosphate; ANG II, angiotensin II; APD, action potential duration; AT1, angiotensin II type 1; ATP, adenosine triphosphate; BNP, brain natriuretic peptide; CaMKII, Ca2+/Calmodulin dependent protein kinase II; CD, cluster of differentiation; CO, cardiac output; CP, creatinine phosphate; dp/dt, derivative of pressure over time; EF, ejection fraction; eNOS, endothelial nitric oxide synthase; EP, electrophysiological; Flk-1, fetal liver kinase-1; Flt-1, Fms related receptor tyrosine kinase 1; HCN, hyperpolarization-activated cyclic nucleotide; HIF-1α, hypoxia-inducible factor 1-alpha; HRV, heart rate variability; ICa2+, calcium current; If, funny current; IL, interleukin; INa+, sodium current; IP, intraperitoneal injection; Ito, transient outward potassium current; KChIP2, K+ Channel interacting protein 2; Kg, kilogram; Kv4.2, voltage-gated potassium channel 4.2; LC3, microtuble-associated protein light chain 3; LV, left ventricular; LVEDD, left ventricular end diastolic dimension; LVEDP, left ventricular end diastolic pressure; LVESP, left ventricular end systolic pressure; LVFS, left ventricular fractional shortening; LVSD, left ventricular systolic dimension; MAP, monophasic action potential; Mg, milligram; MI, myocardial infarction; miR, micro RNA; mRNA, messenger ribonucleic acid; mTOR, mammalian target of rapamycin; NCX, sodium–calcium exchanger; NE, norepinephrine; p70S6K, ribosomal protein S6 kinase beta-1; PI3K, phosphoinositide 3-kinase; P-V, pressure-volume; PVC, premature ventricular contraction; QTc, corrected QT interval; RAA, renin-angiotensin-aldosterone; RyR, ryanodine receptor; SERCA, sarcoendoplasmic reticulum calcium ATPase; SR, sarcoplasmic reticulum; SV, stroke volume; TNF, tumor necosis factor; VEGF, vascular endothelial growth factor; VF, ventricular fibrillation; VT, ventricular tachycardia.
In non-reperfused MI models, ivabradine also exerted antiarrhythmic effects. Ivabradine was shown to decrease HCN4 transcription and If conductance in the left ventricle and left atrium possibly through increasing HCN4-regulating microRNAs (miR-1 and miR-133) in a rat model.61, 75 Ivabradine also improved cardiac function, optimized cardiac energy consumption and importantly, attenuated the prolongation of the duration of the action potential (APD) in rats.63 APD prolongation is known to be a pathologic electrophysiological finding in failing hearts that could be explained by alterations of multiple transsarcolemmal ionic currents including a decrease of the transient outward current (Ito), a major determinant of the early phase of action potential (AP) repolarization.26 Ivabradine prevented the post-MI reduction of Ito possibly through upregulation of the voltage-gated potassium channel KV4.2 that mediates the fast Ca2+-independent K+ current component of Ito (Ito,f).63 Single-dose oral gavage of ivabradine (5 mg/kg, at 1 min after MI) also showed antiarrhythmic potential as the treatment reduced the incidence of ventricular arrhythmia as well as preventing prolongation of the corrected QT interval (QTc) in rats.68 Despite those reported potential benefits, the underlying electrophysiologic and molecular mechanisms remained unclear as ivabradine did not affect the monophasic action potential (MAP) and even decreased SERCA activity.68 Another ex vivo optical mapping study also showed that ivabradine delayed the onset of the loss of electrical excitability, resulting in a reduction of ischemia induced ventricular arrhythmias.50
In non-reperfused MI rats with streptozotocin-induced diabetes, ivabradine reduced cardiac dysfunction without affecting infarct size by reducing plasma NE.64 In a pig model of post-MI cardiogenic shock, ivabradine administered together with inotropes such as dobutamine, improved cardiac function significantly by improving diastolic time and reducing tau65; the most frequently used established index to describe LV diastolic function.76 Co-treatment of ivabradine with dopamine also reduced cardiac dysfunction and reduced ventricular arrhythmias independent of HCN4 expression in the LV of MI rats.69 However, in the increased preload MI rat model to mimic MI-induced cardiogenic shock, ivabradine treatment did not improve cardiac function throughout the two-month duration of the experimental period.66 This unfavorable result could be explained at least in part by the lack of vasodilatory effect of ivabradine. Vasodilators such as nitroglycerin are the standard treatment for decompensated volume overload in heart failure.77 Therefore, future studies using a combination of ivabradine and a vasodilator may uncover the potential benefits of ivabradine in volume overload post-MI conditions. The potential pleiotropic mechanisms of ivabradine beyond heart rate modulation in reducing cardiac adverse effects under MI condition are summarized in Figure 1.
5 EFFECTS OF IVABRADINE IN ACUTE CORONARY SYNDROME: REPORTS FROM CLINICAL STUDIES
Several clinical reports showed that ivabradine provided great benefits in patients with acute coronary syndrome (ACS), as summarized in Table 4. Heart rate control could be achieved with ivabradine treatment (5 mg twice daily) in patients who could not tolerate beta-blockers.78 Another study showed that continuous oral ivabradine treatment (2.5 mg twice daily starting at 12 h after revascularization and then increased to 5 mg twice daily 24 h after initial dose) for a duration of 60 days improved cardiac function and was non-inferior to metoprolol treatment in STEMI patients with LVEF <50%.80 Ivabradine was shown to be as effective as metoprolol in reducing cardiac dysfunction in patients with acute inferior wall MI, but was more effective in reducing AV block in comparison to metoprolol.81 When ivabradine was given along with a beta-blocker, the benefits were greater when compared to beta-blocker as a monotherapy.82-85 Ivabradine also reduced the inflammatory response in non-ST-elevation myocardial infarction (NSTEMI) patients as demonstrated by a reduced plasma C-reactive protein (CRP) level.82 Ivabradine also improved diastolic function and reduced LV volume when combined with beta-blocker therapy without reducing the infarct size.83-85 However, long-term (180 days) treatment with the ivabradine-metoprolol combination did not provide greater benefits in terms of cardiac function, when compared to metoprolol alone.86 Nevertheless, these findings demonstrated the potential benefits of ivabradine in ACS. These findings indicate that further randomized controlled trials are warranted to justify the clinical use of this regimen.
| Patients | IVA group dose/route/onset/duration of treatment | Control group dose/route/onset/duration of treatment | R (% reduction from baseline) | LV functions | Biomarkers | Other relevant findings | Interpretation | Refs |
|---|---|---|---|---|---|---|---|---|
|
ACS patients who could not tolerate carvedilol
|
5 mg bid/oral/NA/24 h |
Baseline | ~16% | – | – | ↓ HR | Ivabradine provided effective control of heart rate in ACS patients | [78] |
|
Age: 65 ± 14 N = 75 |
||||||||
|
AMI cardiogenic shocked patients
|
2.5 mg bid/oral, nasogastric tube/NA/6 months | Placebo | ~32% | ↑ EF | ↓ Pro-BNP |
⟷ Mortality ↑ SBP ↑ DBP |
Ivabradine improved cardiac function but not the mortality rate in AMI CS patients | [79] |
|
Age: 57 ± 9.7 N = 30 |
Age: 55 ± 10.4 N = 28 |
|||||||
| Ivabradine versus beta-blocker | ||||||||
|
Anterior STEMI patients
|
2.5 mg bid/oral/12 h after PCI/60 days |
Metoprolol 25 mg bid/oral/12 h after PCI/60 days | ~28% |
↓ ESV ↓ EDV ↑ EF |
↓Pro-BNP ⟷ plasma CK ⟷ plasma Troponin I |
↓ HF admission | Treatment with ivabradine for 60 days improved cardiac function and reduced HF admission to a greater effect than metoprolol in STEMI patients | [80] |
|
Age: 62 ± 8 N = 79 |
Age: 62 ± 7.5 N = 76 |
|||||||
|
Patients with acute inferior wall MI
|
2.5 mg bid/oral/NA/30 days |
Metoprolol tartrate 25 mg bid/oral/NA/30 days | ~24% |
⟷ ESV ⟷ EDV ⟷ EF |
– |
⟷ Mortality ⟷ MACE ↓ AV Block |
Treatment with ivabradine for 30 days was as effective as metoprolol in reducing clinical outcomes, and it reduced AV blocks to a greater extent than metoprolol in AMI patients | [81] |
|
Age: 55 ± 10.64 N = 232 |
Age: 55 ± 9.03 N = 232 |
|||||||
| Ivabradine add-on beta-blocker versus beta-blocker alone | ||||||||
NSTEMI patients within 24-h onset
|
5 mg bid/oral/NA/30 days | Placebo | – | – | ↓ hsCRP | Ivabradine was negatively associated with level of hsCRP | Ivabradine reduced the inflammatory response in NSTEMI patients | [82] |
| -with beta-blockers | - with beta-blockers | |||||||
|
Age: 71 ± 24 N = 12 |
Age: 52 ± 15 N = 11 |
|||||||
|
STEMI post-PCI patients within 6 h of onset
|
5 mg/IV/1 h after PCI/8 h | placebo IV |
~25% 8 h after treatment |
↓ ESV ↓ EDV ⟷ EF ~1 day after treatment |
⟷ Trop T ⟷ CKMB 1 day after treatment |
⟷ infarct size by MRI 4 months after treatment |
Ivabradine intravenously reduced LV volume, but it did not reduce infarct size | [83] |
| −60% of patients | −33% of patients | |||||||
| use B-blockers | use B-Blockers | |||||||
|
Age: 60 ± 10.8 N = 82 |
Age: 58 ± 11.6 N = 42 |
|||||||
|
STEMI post-PCI patients
|
5 mg bid/oral/1–3 h after PCI/90 days | Placebo | ~28% |
↓ ESV ↓ EDV ↑ EF |
– |
⟷ infarct size ⟷ wall thickness |
Ivabradine in conjunction with a beta-blocker improved cardiac function but it did not reduce infarct size in STEMI post-PCI patients | [84] |
| - bisoprolol titrated to 10 mg for 90 days | - bisoprolol titrated to 10 mg for 90 days | |||||||
|
Age: 57 ± 11.7 N = 62 |
Age: 59 ± 10.5 N = 62 |
|||||||
|
CCS post-PCI patients with chronic stable angina
|
5 mg bid/oral/NA/30 days | Placebo | ↓ 11% at rest |
⟷ ESV ⟷ EDV ⟷ EF |
↑ Chrono-tropic reserve ↑ Exercise time ↑ workload ↑ MVO2 ↑ Δ E' ↓ E/E' ⟷E/A ↓ ET |
Ivabradine improved diastolic function without affecting systolic function in CCS post-PCI patients | [85] | |
| - with beta-blockers | - with beta-blockers | |||||||
|
Age: 63 ± 8.8 N = 14 |
Age: 62 ± 7.8 N = 14 |
|||||||
|
STEMI post-PCI patients within 12-h
|
2.5 mg bid/oral/12 h after PCI/180 days | Placebo | ↓ 22% |
↓ ESV ↓ EDV ↑ EF at 90 days ⟷ ESV ⟷ EDV ⟷ EF at 180 days |
⟷ pro-BNP ⟷ Trop T ⟷ CRP |
⟷ SBP ⟷ DBP |
Ivabradine did not improve cardiac function during long-term follow-up in STEMI post-PCI patients | [86] |
| - metoprolol 12.5 mg bid/oral | - metoprolol 12.5 mg bid/oral | |||||||
|
Age: 52 ± 9.42 N = 32 |
Age: 52 ± 9.55 N = 34 |
|||||||
- Abbreviations: A, peak velocity flow in late diastole caused by atrial contraction; ACS, acute coronary syndrome; AMI, acute myocardial infarction; AV, atrioventricular; B-Blocker, beta-blocker; Bid, twice daily; Bpm, beats per minute; CK, creatinine kinase; CKMB, creatine kinase-myoglobin binding; CS, cardiogenic shock; DBP, diastolic blood pressure; E, peak velocity blood flow from left ventricular relaxation in early diastole; E', early diastolic mitral annulus velocity; EDV, end diastolic volume; EF, Ejection fraction; ESV, end systolic volume; ET, ejection time; HF, heart failure; HR, heart rate; hsCRP, highly sensitive C-reactive protein; IVA, ivabradine; LV, left ventricular; MACE, major adverse cardiac events; MRI, magnetic resonance imaging; N, number; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST elevated myocardial infarction.
6 PERSPECTIVE AND CONCLUSION
The preclinical studies discussed above have provided valuable insights into the cardioprotective mechanisms of ivabradine against MI. Schematic summary of how heart-rate independent effects of ivabradine led to improved outcomes of MI is shown in Figure 2. It is likely that the full benefit of ivabradine in this context comprises both HR-dependent (see Introduction) and HR-independent effects. Mitochondrial oxidative stress mitigation has been identified as an important component of ivabradine's HR-independent actions, especially during the acute phase of MI and reperfusion where mitochondria are a well-known gatekeeper of cell survival.87 The redox status of subcellular compartments, including mitochondria, is the result of free radical production (by various metabolic reactions) and free radical scavenging (by enzyme-dependent and enzyme-independent mechanisms). It remains to be investigated in details how ivabradine interacts with the molecular machinery of those two competing processes (free radical production and scavenging) to promote redox rebalancing, which holds potential to induce profound biological effects.88
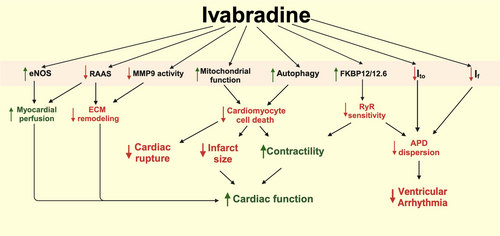
It is important to note that in a non-remodeled heart (e.g., healthy heart or acutely pathological heart), an increase in heart rate can augment contractility (up to a certain limit), a phenomenon known as force-frequency relationship.89 This improvement of contractile function at a higher heart rate is mediated by an increase in cytoplasmic Ca2+ due to reduced Ca2+ reuptake by the sarcoplasmic reticulum secondary to a reduction in the diastolic duration. On the other hand, such augmentation is absent in failing hearts, which exhibit a flat force-frequency graph due to defects of excitation-contraction coupling and calcium handling.89 Therefore, major long-term benefits of bradycardic agent in chronic ischemic heart failure are likely to be mediated by a HR-independent process, and the underlying mechanisms may be different from those in acute MI. Such chronic mechanisms include sympatholytic action, as evidenced by reduced plasma norepinephrine49, 53, 64; RAAS system inhibition, which eventually reduces cardiac collagen deposition53-57, 60; improved coronary perfusion due to increased nitric oxide60, 67; enhanced autophagy70; and improved cellular Ca2+ handling.52, 62, 68 In addition, ivabradine exerts antiarrhythmic effects in post-MI hearts through at least two mechanisms. First, it attenuates APD prolongation by preventing post-MI reduction of Ito.63 Second, it blunts post-MI overexpression of HCN channels, thereby suppressing abnormal automaticity in non-pacemaker cardiomyocytes.61 However, it is not known whether and how ivabradine affects other arrhythmogenic mechanisms at the cellular level (e.g., early and delayed afterdepolarizations) and tissue level (e.g., conduction block and reentry) in ischemic hearts.
Large animal models such as pigs play a pivotal role toward clinical translation since they better reflect human anatomy and (patho)physiology compared to rodents.90 Thus far there are three porcine acute IR studies that support the benefits of ivabradine in terms of cardiac functional improvements and attenuation of cardiac remodeling.37-39 As discussed in the previous section (“Effects of ivabradine in acute coronary syndrome: reports from clinical studies”; also see Table 4), several clinical trials also confirm some functional improvements in ivabradine-treated MI patients. In addition to those coronary disease trials, ivabradine has also been tested in the heart failure study “SHIFT”, which demonstrated that ivabradine reduced the rate of hospitalization but not mortality in patients with LVEF < 35% and HR > 70 bpm.91 It is worth noting that the SHIFT study was primarily designed to test the effects of ivabradine on heart failure regardless of the causes. Hence only 68% of the patients in the SHIFT study had ischemic heart failure, and the duration between MI/revascularization and initiation of ivabradine treatment was not a controlled factor. Furthermore, humans have much lower heart rates (60–100 bpm) compared to rodents (400–600 bpm). Such rapid heart rates in small animals may provide more room for the bradycardic action of ivabradine with better cellular energetic protection. These could be the reasons for the less promising results in clinical trials compared to the those reported in the preclinical studies.
In summary, current evidence suggests that ivabradine has a potential to be an add-on therapy in MI patients who cannot tolerate beta-blockers (for example, patients with hypotension or obstructive pulmonary diseases) and also in MI patients with persistent tachycardia despite beta-blocker treatment. However, more translational studies are required to define optimal regimens (dosages and timing relative to the disease course) and appropriate patient subgroups in which maximal benefits and least adverse effects will be achieved. In addition to MI, possible benefits of ivabradine should also be further investigated in chronic stable angina patients (see Ref. [92] for detailed discussion), Patients who have inappropriate sinus tachycardia or supraventricular tachycardia,93-95 or hypertensive patients with elevated HR.96, 97 Intriguingly, life-long ivabradine treatment in otherwise healthy mice (starting at 12 weeks of age) to achieve 15% HR reduction prolonged the life span by 6.2%.98 The extent to which HR-dependent and HR-independent effects of ivabradine contribute to the demonstrated longevity remains to be further studied. Nevertheless, this promising result suggests that ivabradine may confer even more benefits beyond mitigating cardiovascular diseases.
AUTHOR CONTRIBUTIONS
Nipon Chattipakorn: Conceptualization; funding acquisition; writing – review and editing; supervision; resources; project administration; validation; visualization; methodology. Adivitch Sripusanapan: Investigation; methodology; validation; visualization; writing – original draft; formal analysis. Panat Yanpiset: Methodology; validation; visualization; writing – original draft. Sirawit Sriwichaiin: Writing – original draft; methodology; validation; visualization. Natthaphat Siri-Angkul: Writing – review and editing; investigation; formal analysis; validation. Siriporn C. Chattipakorn: Conceptualization; funding acquisition; writing – review and editing; visualization; validation.
ACKNOWLEDGMENTS
This work was supported by the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand and the National Research Council of Thailand (NC), the Distinguished Research Professor Grant from the National Research Council of Thailand (N42A660301 to SCC), and the Chiang Mai University Center of Excellence Award (NC).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




