Calcineurin inhibition, cardiovascular consequences, vascular resistance, and potential responses
Abstract
Aim
To place the consequences of calcineurin inhibition in a cardiovascular context.
Methods
Literature review coupled with personal encounters.
Results
Calcineurin is a calcium-binding and calmodulin-binding protein that is conserved across evolution from yeast to mammals. The enzyme functions as a calcium-dependent, calmodulin-stimulated protein phosphatase. Its role in regulating physiology has largely been elucidated by observing calcineurin inhibition. Calcineurin inhibition transformed organ transplantation from an experiment into a therapy and made much of general immunotherapy possible. The function of this phosphatase and how its inhibition leads to toxicity concern us to this date. Initial research from patients and animal models implicated a panoply of factors contributing to hypertension and vasculopathy. Subsequently, the role of calcineurin in regulating the effective fluid volume, sodium reabsorption, and potassium and hydrogen ion excretion was elucidated by investigating calcineurin inhibition. Understanding the regulatory effects of calcineurin on endothelial and vascular smooth muscle cell function has also made substantial progress. However, precisely how the increase in systemic vascular resistance arises requires further mechanistic research.
Conclusion
Calcineurin inhibition continues to save lives; however, options to counteract the negative effects of calcineurin inhibition should be vigorously pursued.
1 CALCINEURIN AND TOXICITY
Calcineurin is a calcium- and calmodulin-dependent serine/threonine protein phosphatase, also known as protein phosphatase 3.1, 2 The heterodimeric protein consists of a catalytic subunit, termed calcineurin A, containing an active site dinuclear metal center, and a tightly associated, myristoylated, Ca2+-binding subunit, termed calcineurin B. Together, the subunits function as a calcium-dependent serine–threonine phosphatase. The enzyme has ubiquitous functions but is particularly important in clinical medicine because it activates T cells and can be blocked by drugs. This discovery converted modern solid organ transplantation from an experiment into an effective treatment.3 Calcineurin is targeted by a drug class called calcineurin inhibitors, which include cyclosporine, voclosporin, pimecrolimus, and tacrolimus.
When an antigen-presenting cell interacts with a T-cell receptor on T cells, there is an increase in the cytoplasmic calcium level, which activates calcineurin by binding the calcineurin regulatory subunit and activating calmodulin. Calcineurin then induces nuclear factors of transcription (NFATs) to induce the transcription of interleukin-2 (IL-2) genes.4 IL-2 in turn activates T-helper lymphocytes and induces the production of other cytokines. In this way, calcineurin governs the action of cytotoxic lymphocytes. The amount of IL-2 being produced by the T-helper cells significantly influences the extent of the immune response. However, calcineurin has essential roles above and beyond the immune response.5, 6 These functions impact many other bodily systems and remain imperfectly defined. Insights into calcineurin function, activation, substrate recognition, and targeting have recently been reviewed.7 We focus here on cardiovascular problems and perhaps opportunities for protective interventions.
2 CALCINEURIN INHIBITION, HYPERTENSION, AND TARGET ORGAN DAMAGE
Although calcineurin inhibition made modern solid organ transplantation as a routine therapy possible, clinical problems soon arose. Du Toit et al. drew attention to the fact that, although the use of cyclosporine led to significant outcome improvements, deleterious side effects including nephrotoxicity, hepatotoxicity, hirsutism, gingival hyperplasia, tremors, and tumors were associated with the therapy.8 Animal studies directed at mechanisms were the result. These efforts focused on vascular and glomerular morphological injuries.9
Rosen et al. developed an early utilitarian rat model of cyclosporine-induced nephrotoxicity.10 The thick ascending limb of Henle's loop (with high oxygen requirements) was particularly affected. Subsequent investigations implicated the renin–angiotensin system, vasculopathy, immune cells, and transforming growth factor-β (TGF-β). These pathways converged on fibrotic mechanisms, any and all of which result in renal injury and an increase in blood pressure (or in reverse order).11 The calcineurin inhibition model identified hypertension and vasoconstriction. Primarily implicated were angiotensin (Ang II), osteopontin, various immune cells, TGF-β, fibrosis, and vasculopathy. Renal endothelial cell injury, independent of NFAT signaling, was also reported.12 The endothelin B receptor was observed to be markedly increased in human biopsies with cyclosporine toxicity.13 Much early research was done.14 The role of sodium and salt intake was also carefully considered in association with cyclosporine nephrotoxicity.15
Clinicians and pathologists were confronted with major decisions when judging renal biopsies in calcineurin inhibitor-treated patients.16 The patients can develop delayed graft function for various reasons such as toxic tubulopathies, arteriopathies, or vascular fibrinoid necrosis, as well as acute rejection. Furthermore, other causes of acute kidney injury or unexpected renal disease could be present.17 Calcineurin toxicity was a confounding variable in judging rejection in post-transplant renal biopsies. Mihatsch, colleagues, and other pathologists were confronted with the problem of differentiating between acute renal graft rejection and cyclosporine toxicity.18 They described the clinical and pathological differentiating features. Also apparent was the fact that thrombotic microangiopathy was a common feature. This condition could be related to humoral rejection or could be a consequence of calcineurin inhibitor toxicity.19 Thrombotic microangiopathy has many causes; however, an association with calcineurin inhibition was soon secured.20 Investigators indicated that calcineurin inhibitors led to vasoconstrictor effects that were related to interference within the balance of vasoactive substances. They also speculated on possible sodium-retaining calcineurin inhibitor effects that were at that time unknown. An integrated (limited) list of factors contributing to calcineurin inhibitor toxicity was proposed (Figure 1).
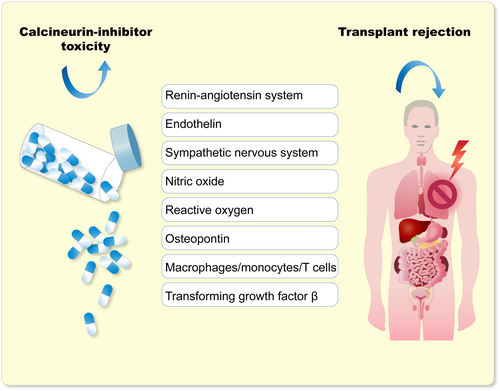
Salt intake intervention studies suggested that dietary salt reduction could possibly even increase cyclosporine nephrotoxicity and thereby blood pressure.21 A vexing dilemma was clinically separating vasculopathy from hypertension, from transplant rejection, and from various external factors (diet, salt, etc.), in transplanted patients. Furthermore, these patients are commonly prescribed 10 or more separate medications for various justifiable reasons. A factorial risk for undesirable side effects has been described, and reliable compliance to these gargantuan regimens is nearly impossible.22
Clinicians observed that cyclosporine-treated patients commonly developed metabolic acidosis with hyperkalemia. Attention was primarily drawn to potassium, which could cause sudden death. The evolving cyclosporine syndrome appeared to be a form of renal tubular acidosis (RTA), namely the so-called type 4 RTA. This condition occurs when the tubules are unable to remove excess potassium; simultaneously, the kidney's ability to remove acid from the blood is impaired. Diminished aldosterone function is generally held to be responsible for type 4 RTA.23 The recognition proved to be a major impetus in further research on cyclosporine nephrotoxicity and on the function of calcineurin in the distal renal tubule and collecting duct.24 Animal studies by Mohebbi et al. demonstrated that tacrolimus can impair urinary acidification especially in the setting of acid load by dysregulation of many acid–base transporters in the proximal and distal nephron including H+-ATPase, sodium bicarbonate cotransporter, and anion exchanger (AE1).25
3 CALCINEURIN AND VOLUME REGULATION
Side effects of calcineurin inhibitors drew attention to the constellation – hypertension, hyperkalemia, hypercalciuria, and non-anion gap metabolic acidosis.26 Blood pressure regulation and maintenance became an early problem after the introduction of calcineurin inhibitors, and the mechanisms were investigated. Meister and Aperia suggested that altered catecholamine regulation of sodium transport could be responsible.27 They reasoned that the interplay between dopaminergic and adrenergic signaling might be a key explanation. Dopamine inhibits tubular Na+, K+-ATPase activity and increases sodium excretion. Noradrenaline stimulates Na+, K+-ATPase activity and decreases urinary sodium excretion. The signaling pathway by which two opposite first messengers regulate Na+, K+-ATPase activity involves the dopamine-specific protein phosphatase-1 inhibitor (DARPP-32) and calcineurin. The authors suggested that aberrations in the renal dopamine/noradrenaline system may be the cause of alterations in the regulation of sodium excretion during ontogeny and in salt-sensitive hypertension.27 Renal sensory nerves also are related to calcineurin-induced blood pressure responses.28 The multifactorial effects are related to actions in the nervous system, vasculature, and the kidney.29
Mechanistically, the most appealing connection was identified between calcineurin inhibitors and the Na/Cl cotransporter (NCC) activity. This transporter functions in the distal convoluted tubular portion of the nephron. A Mendelian genetic disorder featuring hypertension, metabolic acidosis (generally mild), and hyperkalemia, termed familial hyperkalemic hypertension (Gordon's syndrome, familial hyperkalemic hypertension, FHHt, or pseudohypoaldosteronism type 2, PHA2), drew attention to similarities between FHHt and calcineurin inhibitor-induced hypertension. FHHt is not a simple genetic disorder. Four causative genes have been identified, namely (with-no-lysine kinases) WNK1, WNK4, Cullin 3 (CUL3), and Kelch-like protein 3 (KLHL3). The latter two genes encode for ubiquitin ligases responsible for WNK elimination. For the first 3 genes, inheritance is autosomal dominant. For KLHL3, inheritance is mostly dominant (presumably variable penetrance). A few cases with autosomal recessive disease were also described.30
WNK1 and WNK4 were first identified as responsible for FHHt. Their importance in the regulation of NCC, as well as other relevant effects, followed shortly.31 The finding that tacrolimus activates NCC and causes hypertension had important implications. Hoorn et al. showed that in wild-type mice, tacrolimus caused salt-sensitive hypertension and increased the abundance of phosphorylated NCC, as well as the NCC-regulatory kinases WNK3, WNK4, and the SPS1-related proline/alanine-rich kinase (SPAK).32 The authors found that NCC-deficient (SLC12A3) knockout mice were not influenced by tacrolimus; however, mice overexpressing this gene were particularly susceptible to blood pressure-related tacrolimus actions. The effects, also elaborated elsewhere, include not only a direct action on NCC, but also on SPAK, KLHL3, and the protein phosphatase-1 inhibitor 1.33 The latter protein regulates calcium cycling. Presumably, calcineurin inhibitor-induced hypertension should be particularly responsive to NCC blockers, such as thiazide diuretics or thiazide-like chlorthalidone. There are some data available to support this idea.34 Even a report of a transplanted kidney from a Gitelman's syndrome patient (loss-of-function defect in NCC), whose donor kidney protected from hypertension, inspires confidence in this model (Figure 2).35
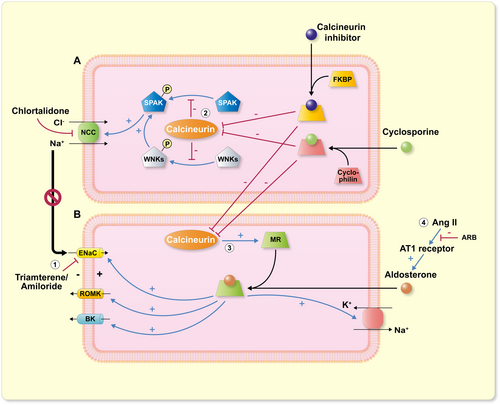
4 CALCINEURIN AND SYSTEMIC VASCULAR RESISTANCE
Calcineurin inhibitor-treated patients develop decreased regulatory T cells, endothelial dysfunction, and hypertension, which results in an increase in systemic vascular resistance. This effect must necessarily take place within the vessel wall. Endothelial cells, and then vascular smooth muscle cells, must be involved. Chiasson et al. showed that mice treated with tacrolimus for 1 week dose-dependently exhibited decreased splenic CD4+/FoxP3+ (regulatory T cells), increased splenic CD4+/IL-17+ (T-helper 17) cells, developed endothelial dysfunction, and became hypertensive.36 Tacrolimus binds to the FKBP (previously called FK506—after “fujimycin-binding protein”). The FKBPs, or FK506-binding proteins, constitute a family of proteins that have prolyl isomerase activity and are related to the cyclophilins in function, although not in amino acid sequence. Both the FKBP–tacrolimus complex and the cyclosporine–cyclophilin complex inhibit the calcineurin phosphatase, thus blocking signal transduction in the T-lymphocyte transduction pathway.
The investigators then crossed “floxed” FKBP12 mice with Tie2-Cre mice to generate offspring lacking FKBP12 in only endothelial and hematopoietic cells, creating FKBP12EC knockout (KO) mice. These mice exhibited increased transforming growth factor-β (TGF-β) receptor activation and SMAD2/3 signaling. FKBP12EC KO mice also developed endothelial dysfunction and hypertension. These data suggest that calcineurin inhibition leads to enhanced TGF-β activity, increased endothelial cell activation, and inflammation. The implication is that calcineurin is an inhibitory regulator of TGF-β signaling in endothelial cells. Blocking calcineurin lifts this inhibition. The studies document toxic effects of calcineurin inhibition directly at the level of the endothelial wall, which is the site where flowing blood deals with blood vessels.
Systemic vascular resistance is largely regulated by the production of nitric oxide (NO), which occurs in endothelial cells where the generating enzyme, endothelial nitric oxide synthase (eNOS), resides. Yuan and colleagues showed that maintenance of normal blood pressure is dependent on inositol 1,4,5-trisphosphate receptor (IP3R1)-mediated regulation of eNOS.37 The investigators found that the calcineurin pathway was less active and eNOS levels were decreased in IP3R-deficient endothelial cells. Furthermore, calcineurin inhibition with cyclosporine reduced eNOS activity and vasodilation following acetylcholine stimulation in their model. The data suggest that the pivotal systemic vascular resistance regulator, NO, is under calcineurin regulation. Blocking calcineurin would result in less NO production and less vasodilatation. The studies indicate that calcineurin is pivotal for normal endothelial cell function. Inhibition could cause TGF-β-related injuries and decreased vasodilatory signaling (Figure 3).
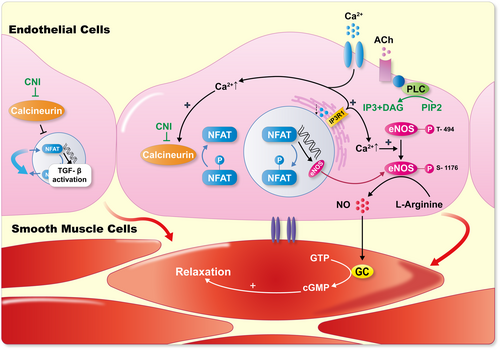
5 CALCINEURIN IN VASCULAR SMOOTH MUSCLE
Vascular smooth muscle contains numerous calcineurin-dependent mechanisms.38 The number of human calcineurin substrates (currently about >100) increases continuously.7 Contraction involves calcium signaling, a function of voltage-gated, dihydropyridine-sensitive CaV1.2 channels. In heart and vascular smooth muscle, localized calcium signals are termed “sparklets” and their activation is protein kinase dependent. The major cAMP effector is protein kinase A (PKA). Cardiac smooth muscle cell cAMP signaling and vascular smooth muscle cell cAMP signaling; however, are not the same. However, the kinases are located on A-kinase anchoring proteins (AKAPs).39 Recent findings show how AKAP-enhanced calcineurin activity enables suppression of PKA without altering cAMP levels by increasing PKA catalytic subunit capture rate.40
Novel recent findings involving T cells and calcineurin may give additional insights. An essential regulator of T lymphocytes, B-cell leukemia 11b (BCL11b), exhibits genetic variations associated with clinical vascular stiffness. Valisno et al.41 used mouse models with absent in BCL11b in vascular smooth muscle cells. Calcineurin activity within the cells was directly affected. Furthermore, the vasodilator-stimulated phosphoprotein (VASP) was diminished and the actin cytoskeleton impaired, all in the direction of stiffness. The study brings genetic findings into the clinical arena and implicates calcineurin.
For example, an investigated genetic approach involved CACNA1D mutations. This gene encodes the α1 subunit of the Cav1.3 L-type calcium channel that regulates (in part) intracellular Ca2+ stability. Wang et al.42 generated, with Crispr-Cas9 technology, a series of rats with CACNA1D variants. In mutant rats, Cav1.3 protein expression and calcineurin phosphatase activity in vascular smooth muscle cells were increased. These studies connect calcineurin activity to a specific cytosolic calcium-regulatory protein.
Effector proteins of cAMP, exchange proteins activated by cAMP (EPACs), have been shown to play vital roles in a plethora of pathways in different cell systems. The role of EPAC in VSMCs, namely its regulation of the vascular tone and phenotypic switching is complex. Mechanisms likely involve reactive oxygen species (ROS) to impact targets and effectors.43 In vascular smooth muscle, PKA is a key regulator and phosphodiesterase 3A controls the cAMP/cGMP levels locally at AKAPs. A direct connection between AKAP79 and calcineurin's ability to suppress PKA activity has been described.40 A genetic syndrome affecting phosphodiesterase 3A activity and effects on PKA could eventually be relevant in this regard.44 The syndrome features severe hypertension resulting in stroke at early ages and also impacts VASP. In affected persons, VASP expression was diminished, compared to non-affected individuals. These studies further connect calcineurin to a variety of intracellular vascular smooth muscle cell-associated events.
The role of protein kinases in pulmonary vascular smooth muscle tone is well established.45 Systemic vascular resistance has received less direct attention. Murthy et al. reported on the inhibition of sustained smooth muscle contraction by PKA and protein kinase G (PKG). PKG is preferentially mediated by phosphorylation of Ras homolog family member A (RhoA).46 Ang II and endothelin, both implicated in calcineurin toxicity, are directly involved in RhoA signaling.47 RhoA inhibitors are being developed and have therapeutic potential.46 Rho-associated coiled-coil-containing protein kinase (ROCK) is a protein serine/threonine kinase. ROCK has a diverse range of functions in the body. The kinase is a key regulator of actin–myosin contraction, stability, and cell polarity.48 Calcineurin also interacts with vascular smooth muscle cells on several additional planes. Smooth muscle cell contraction is dependent upon, not only calcium channels, but also intracellular sarcoplasmic stores. These effectors are regulated by ryanodine receptors (RyRs). Calcineurin upregulates local Ca2+ signaling through RyR vascular smooth muscle effects.49 Possibly, myosin light-chain phosphatase could be a point of intervention.50 Since ROCK could intervene here, a coupling event could be expected. Subsequently, we learned that AKAP79 enables calcineurin to directly suppress PKA activity.40 Vascular smooth muscle cell research is difficult for many reasons, and a clear-cut picture regarding calcineurin's role currently is not possible. A highly hypothetical schema is presented (Figure 4).
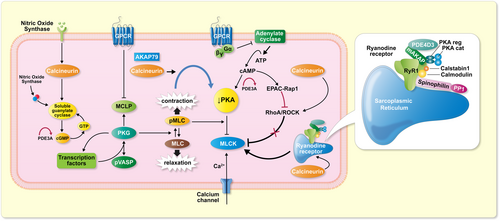
6 THE CLINICAL ARENA
Aside from hypertension, calcineurin inhibition has an influence on cardiovascular disease, particularly in heart transplantation patients. Cardiac allograft vasculopathy tends to be caused by accelerated intimal hyperplasia resulting in diffuse small vessel disease. Sudden cardiac death is a risk in these patients, and calcineurin inhibition-related effects could contribute.51 Calcineurin inhibition does not necessarily facilitate cardiac allograft vasculopathy, but in contrast to mTOR inhibition, it does not prevent progression of disease.52
Calcineurin inhibition and neural toxicity are fairly well defined. Altered mental status, seizures, headaches, paresthesia, and tremor are common side effects.53 A rare but important complication to be aware of is reversible posterior leukoencephalopathy syndrome, also known as posterior reversible encephalopathy syndrome (PRES). This diagnosis requires both clinical and radiographic findings. Clinical symptoms are hypertension in combination with headache, altered mental status, seizures, and visual disturbances. Neuroimaging will show posterior-predominant vasogenic edema best captured by MRI because of the posterior location. PRES can occur at any time.54
Not quite settled is the issue of whether or not calcineurin inhibitors differ in terms of toxicity. Tacrolimus was touted to induce less nephrotoxicity because of improved potency and lower likelihood of off-target effects.55 However, Stegall et al. followed patients for over a decade and reached a different conclusion.56 Perhaps a less-appreciated issue is the development of diabetes after calcineurin inhibition. Yang et al. performed a meta-analysis.57 They evaluated 13 long-term studies. The incidence of rejection, drug conversion, and dyslipidemia were significantly lower in the tacrolimus comparison group. Glomerular filtration rates and graft survival rates in the tacrolimus group were better than in the cyclosporine group. Patient survival, incidence of infection, hypertension, and new tumors were evaluated. However, the use of tacrolimus was associated with a high risk of a new onset of diabetes. Cyclosporine and tacrolimus cause post-transplant diabetes mellitus by a number of mechanisms, including decreased insulin secretion, increased insulin resistance, or a direct toxic effect on the beta cell.58 Calcineurin inhibitors, by inducing tubulopathy, also cause magnesium deficiency that could contribute to side effects. Mechanisms regarding this state of affairs have been elucidated in detail elsewhere.59 Targeted immune interventions have been entertained for type 1 diabetes with mixed results.60 Including a calcineurin inhibition strategy could induce remission in type 1 diabetes.61
7 OPTIONS
The idea that complete avoidance of calcineurin inhibitors could be a possibility has been tested. Patients were randomized to the mammalian target of rapamycin (mTOR) inhibitor sirolimus or tacrolimus after kidney transplantation. The acute rejection episodes, graft survival, and renal function two years later were not different.62 Belatacept is a synthetic fusion protein composed of the Fc fragment of a human IgG1 immunoglobulin linked to the extracellular domain of cytotoxic T-lymphocyte-associated protein-4 (CTLA-4). CTLA4 is a molecule crucial in the regulation of T-cell costimulation, selectively blocking the process of T-cell activation (checkpoint inhibition). Belatacept provides extended graft and transplant survival while limiting the toxicity generated by standard immune-suppressing regimens, such as calcineurin inhibitors.63 A very similar compound, abatacept, is also available. Belatacept may provide sufficient immunosuppression, while avoiding unwanted side effects of other immunosuppressant drugs. However, high rates of post-transplant lymphoproliferative disease (PTLD) have been reported when belatacept is used in particular kidney transplant recipients at high dosage. Belatacept has been promulgated as a calcineurin inhibitor-sparing agent. A recent Cochrane review suggested that treatment with belatacept is associated with less chronic kidney scarring and better long-term kidney transplant function.64 Treatment with belatacept was also associated with better blood pressure, improved lipid profiles, and a lower incidence of diabetes mellitus, compared to calcineurin inhibition.
Sawinski et al.65 assessed four calcineurin inhibitor immunosuppression strategies (minimization, conversion, withdrawal, and avoidance) designed to reduce calcineurin inhibitor exposure and assessed the impact of each on patient and allograft survival, acute rejection, and renal function in the framework of a meta-analysis. They compared clinical outcomes with standard-dose regimens; moderate-strength evidence indicates that conversion to a mammalian target of rapamycin inhibitor or belatacept was associated with improved renal function but increased rejection risk. In addition, they inspected moderate- to high-strength evidence suggesting that planned calcineurin inhibitor withdrawal could result in improved renal function despite an association with increased rejection risk. Unfortunately, meaningful conclusions could not be drawn from this study. Conversion from calcineurin inhibition to other options may present other risk factors. As a result, 446 renal transplant recipients were randomized to belatacept conversion or calcineurin inhibitor continuation. After 24 months, few differences could be discerned since, all did well (Figure 5).66
8 THE JURY IS STILL “OUT”
Renal transplantation is associated with improved survival compared with maintenance dialysis. In the United States and worldwide, post-transplant outcomes have steadily improved over the last several decades, with current 1-year allograft and patient survival rates well over 90%. These results were inconceivable before the cyclosporine era.67 Calcineurin toxicity can be considered the “fly in the ointment.” Investigating the mechanisms has been enlightening for all of medicine. The vast amount of investigations into calcineurin inhibition toxicity has resulted in a profound increase in scientific knowledge about that condition and much else. That being the case, let this state of affairs not result in a “hung jury.” Biomarkers of calcineurin inhibitor nephrotoxicity in transplantation made transplantation evaluable in the first place (Figure 5).68 The surely awaited introduction of xenotransplantation will amplify our attention to all these strategies.
AUTHOR CONTRIBUTIONS
Friedrich C. Luft: Conceptualization; writing – original draft; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
None.




