Neuropeptides and neurohormones in immune, inflammatory and cellular responses to ultraviolet radiation
Funding information
Internal Funds, Weill Cornell Medical College, Department of Dermatology.
Abstract
Humans are exposed to varying amounts of ultraviolet radiation (UVR) through sunlight. UVR penetrates into human skin leading to release of neuropeptides, neurotransmitters and neuroendocrine hormones. These messengers released from local sensory nerves, keratinocytes, Langerhans cells (LCs), mast cells, melanocytes and endothelial cells (ECs) modulate local and systemic immune responses, mediate inflammation and promote differing cell biologic effects. In this review, we will focus on both animal and human studies that elucidate the roles of calcitonin gene-related peptide (CGRP), substance P (SP), nerve growth factor (NGF), nitric oxide and proopiomelanocortin (POMC) derivatives in mediating immune and inflammatory effects of exposure to UVR as well as other cell biologic effects of UVR exposure.
1 INTRODUCTION
Sunlight that escapes absorption by earth's atmosphere is comprised of varying wavelengths of ultraviolet radiation (UVR), visible light and infrared light. The UVR spectrum is divided into several wavebands: ultraviolet C (UVC) (100-280 nm), ultraviolet B (UVB) (280-320 nm) and ultraviolet A (UVA) (320-400 nm), which can be further categorized into UVA1 (340-400 nm) and UVA2 (320-340 nm). While UVC radiation is completely absorbed by the atmosphere, both UVB and UVA radiation reach human skin. Photons of UVB radiation have a higher energy than those of longer wavelength UVA radiation. However, they penetrate less deeply into the skin than photons of longer wavelengths. Macromolecules in the epidermis, such as DNA, proteins, lipids and urocanic acid, efficiently absorb photons in the UVB spectrum.1 These macromolecules able to absorb light energy are termed chromophores, and their downstream immunomodulatory, inflammatory and cell biologic effects are discussed extensively elsewhere.2
UVA radiation is less efficiently absorbed by such chromophores and penetrates more deeply, affecting the epidermis and the dermis, indirectly causing damage through the creation of reactive oxygen species (ROS).3 The generation of these ROS can be created directly by a chromophore's absorption of UVA radiation or indirectly via secondary interaction with an excited chromophore.3 UVA radiation comprises more than 90% of solar UV radiation reaching earth, and the human population has received increased exposure over the past several decades resulting from the advent of efficient UVB-absorbing sunscreens and increasing popularity of UVA tanning beds.4 Since UVB radiation is the primary cause of sunburn, sunscreens that block UVB radiation but not UVA radiation allow individuals to spend a much greater amount of time in the sun, leading to previously unprecedented levels of UVA radiation exposure.
UVR exhibits a duality in medicine and public health. Too much sunlight leads to sunburn and mutations in DNA that promote skin tumourigenesis.5, 6 Too little sunlight leads to deficiency in vitamin D and can precipitate mood disorders in predisposed individuals.7 Latitude gradients are found for several autoimmune diseases, such as multiple sclerosis and type I diabetes, with increasing prevalence found with higher latitudes suggesting a role for the interplay between UVR and vitamin D status.8 Furthermore, phototherapy is used to treat a number of skin disorders including psoriasis, atopic dermatitis, polymorphic light eruption, vitiligo, alopecia areata and lichen planus, among others, suggesting that the immunomodulatory effects of UVR regulate some autoimmune disorders.9-11 Recent preprints (not yet peer-reviewed) even suggest that living in a geographic location with higher UVR exposure decreases incidence and mortality of COVID-19.12, 13 However, UVR exposure can induce and exacerbate lesions in cutaneous lupus erythematous,14 and the prevalence of dermatomyositis has been shown to increase with decreasing latitude in Europe.15 Blood pressure16 and metabolic function17-19 appear to be influenced by UVR exposure, and frequent users of tanning beds often meet criteria for addiction.20, 21 Evidence shows that neuropeptides and neurohormones mediate many of these effects.
Neuropeptides such as CGRP and substance P (SP) originate in small, unmyelinated c-fibres and myelinated Aδ-fibres of sensory nerves within the skin, lymph nodes, muscle and viscera.22, 23 The enzyme NO synthase (NOS) resides with CGRP and SP in nerve terminals and histochemical localization shows widespread distribution in both the peripheral and central nervous systems, including afferent fibres of sensory nerves of the dorsal root ganglia.24, 25 NO appears to be necessary for induction of immunomodulatory effects of neurogenic mediators, especially in UVA exposure.26 Although proopiomelanocortin (POMC) and its derivates were initially discovered as pituitary hormones, skin cells such as keratinocytes, melanocytes, microvascular endothelial cells (ECs) and immune cells also synthesize the peptides.27 Similarly, cutaneous neurotrophins, such as NGF, are expressed by sensory and sympathetic neurons, mast cells and keratinocytes.28 UVR increases the expression of these peptides and hormones in animals and humans. In the following review, we discuss the role of these neuromessengers in mediating components of the immunomodulatory, inflammatory and cell biologic responses found after UVR exposure. To better understand the following discussion, we first start with a consideration of the methods and findings of experiments examining UVR and its individual components.
2 BACKGROUND ON EXPERIMENTS EXAMINING BIOLOGIC EFFECTS OF ULTRAVIOLET RADIATION
Two primary basic science models have been used to study the effects of UVR on cell-mediated immunity. Contact hypersensitivity (CHS) occurs when antigen is applied topically to skin, while delayed-type hypersensitivity (DTH) results from intradermally injected antigen. This distinction is important because epidermal Langerhans cells (LCs), because of their location, may play a lesser role in DTH.29 Although the CHS and DTH models are robust for evaluation of cellular immune responses, one important difference between murine and human physiology should be noted. Human skin is significantly thicker than murine skin with studies demonstrating that the stratum corneum is approximately 5 µm in mice compared to 10-20 µm in humans.30 This is important in interpreting UVR effects. The increased thickness of human skin may alter the relative penetration of UVB radiation to UVA radiation compared to mice, which may account for differences in immunologic observations between human and murine studies. Longer wavelengths (ie UVA radiation) penetrate more deeply than shorter wavelengths (ie UVB radiation).31 Nonetheless, immunomodulatory effects of both UVA and UVB radiation have been shown in human skin,32, 33 although the locus of the relevant chromophores remains unknown.
Early research discovered that UVB radiation alters immunologic responses in two ways. UVB radiation modulated the murine immune system such that routine rejection of transplanted highly antigenic skin tumours failed,34 and skin exposed to UVB radiation exhibits loss of ability to be contact sensitized to haptens locally with low doses and systemically at high doses.35, 36 In humans, it has been noted that transplant recipients undergoing immunosuppression exhibit increased incidence of skin cancer.37, 38 In addition, failure to develop CHS to topical dinitrochlorobenzene after low-dose UVB irradiation in humans has been reported to be a risk factor for skin cancer in humans, although the data in this study could also be interpreted to show that patients are more susceptible to UVB radiation-induced immune suppression after development of skin cancers.39 Loss of CHS in mice can be induced both locally at areas of UVB radiation exposure and systemically in areas that were not directly exposed. Local and systemic immune suppression induced by UVB radiation were found to differ in several ways. At low doses of UVB radiation given for four consecutive days, insufficient to cause gross changes in the skin and equivalent of 0.5 minimal erythemal dose or 10 mJ/cm2 on untanned human skin,40 mice fail to become effectively sensitized to chemical haptens applied to the irradiated site along with the development of antigen-specific tolerance and transferable regulatory T cells.35, 41, 42 In this model, immunization at a non-irradiated site leads to a normal CHS response. In the systemic, high-dose model of UVB radiation-induced immune suppression, a dose of radiation equivalent to 250 mJ/cm2 applied for four consecutive days and sufficient to produce gross changes in the skin is given; in this model, immunization even at a non-irradiated site leads to a failure of CHS and delayed type hypersensitivity to an intradermally injected protein antigen along with the development of antigen-specific tolerance and transferable regulatory T cells.35, 43 Interestingly, when the cumulative UVB dose of 1000 mJ/cm2 is given in a single day or spread out into two doses over two consecutive days, there is a weaker suppressive effect.43 In addition, chronic, very low-dose UVB radiation exposure, defined as a cumulative 50 mJ/cm2 spread out over 6 weeks with three doses per week (~2.77 mJ/cm2 per dose), is also capable of suppressing a CHS response.44 The cellular and humoral mechanisms behind this suppression of immunity may be related to impairment of antigen-presenting capability, reduction of LCs epidermal density, induction of mast cell degranulation and formation of regulatory T cells (Tregs).45, 46 The molecular pathways behind these cellular changes are reportedly dependent on photoactivation of chromophores leading to transition of nucleotide bases to cyclobutyl pyrimidine dimers (CPDs), isomerization of trans-urocanic acid to cis-urocanic acid (UCA), release of platelet-activating factor (PAF) and PAF analogs,47 peroxidation of membrane lipids, formation of ROS,48 formation of tryptophan photoproducts, which activate aryl hydrocarbon receptors,49 activation of C350 and, perhaps, α-MSH release.51 A seminal study provided evidence of the role of CPD formation in UVB radiation-induced immunosuppression in humans.52 Loss of CHS to nickel after UVB was prevented by post-exposure application of DNA repair enzyme photolyase, which decreased CPDs by 40%-45%. In the model of local immunosuppression, polymorphisms in the genetic loci for lipopolysaccharide (LPS) response and TNF-α production determine susceptibility.53 In humans, a single nucleotide polymorphism identified in the aryl hydrocarbon receptor loci, which is activated by UVB radiation and leads to an anti-apoptotic effect in keratinocytes, appears to increase the risk for developing squamous cell carcinoma.54 There also appears to be a role for calcitonin gene-related peptide (CGRP) and nerve growth factor (NGF) in UVB radiation-induced immune suppression, as described below.
While the immunosuppressive effects of UVB radiation appear to operate in a dose-dependent manner, UVA exhibits different properties. Low-dose UVA irradiation (defined as 840 mJ/cm2) of mice does not modulate systemic CHS responses, while medium dose (1680 mJ/cm2), but not higher doses, is immunosuppressive.26 In humans, UVA radiation could suppress the elicitation of CHS to nickel using a dose of 233 mJ/cm2 of UVA2 radiation combined with 1709 mJ/cm2 of UVA1 radiation.55 However, this was seen only with exposures to UVA radiation over 5 days prior to challenge but not with fewer or more exposures. Of note, high-dose UVA radiation up to 24 hours prior or immediately after UVB radiation has been shown to protect from UVB-induced immunosuppression, possibly by upregulating INF-γ and IL-12 which inhibits release of IL-10.56-58 Further studies combined high-dose UVA and high-dose UVB radiation to effectively produce an ultraviolet spectrum that simulates sunlight at midday on a cloudless, spring day in Sydney, Australia, referred to as solar-simulated UV radiation (ssUVR).59 The dose-response immunosuppression curve (measured by the absence of CHS response) of ssUVR parallels the bell-shaped UVA dose-response curve indicating that the effect of UVA predominates in the immune response to sunlight.26 A subsequent study examining recall sensitivity to nickel in nickel-immune human subjects showed that UVA contributes roughly threefold more than UVB to immunosuppression in a ssUVR dose matching incidental sun exposure, roughly 20 minutes under the midday summer sunlight.32 At longer exposures, UVB radiation is likely to contribute more to immunosuppression. These studies highlighted the need for UVA protection, in addition to UVB, to be conferred by sunscreens.60 Many of the same cellular, humoral and molecular mechanisms associated with UVB immunosuppression are also implicated in UVA immunosuppression.61 The cellular changes are thought to be reduction of epidermal LC density, formation of Tregs,62 release of histamine from mast cells63 and defect of CD8+ T-cell development in long-term memory cells.64 The molecular alterations may be production of ROS,65 production of nitric oxide (NO) most likely leading to reactive nitrogen species (RNS),66 formation of cyclobutene dimers (CPDs),67 activation of the alternate complement pathway68 and inhibition of oxidative phosphorylation in keratinocytic mitochondria leading to depletion of intracellular adenosine triphosphate (ATP).69 Studies in humans utilizing nicotinamide, an amide form of vitamin B3 shown to prevent ATP depletion induced by UVR and enhance DNA repair,70 have shown that topical application prevents immunosuppression (measured by impaired Mantoux reaction in Mantoux-positive volunteers) after suberythemal ssUVR exposure.71 Indeed, a subsequent phase 3 randomized clinical trial showed that oral nicotinamide supplementation reduced the incidence of non-melanoma skin cancers and actinic keratoses in a high-risk cohort.72
3 CALCITONIN GENE-RELATED PEPTIDE
Alternative splicing of the calcitonin gene produces a 37 amino acid peptide aptly named CGRP.73 Existing in two isoforms (α- and β-CGRP), the peptide exhibits immunoreactivity across both the central and peripheral nervous systems and exerts a variety of biological effects, chiefly vasodilation, on several tissues and organs.74 CGRP-containing nerve fibres are found abundantly in the skin and are often colocalized with SP or somatostatin (SOM).75 Within the skin, CGRP fibres are intimately associated with LCs, and exposure of LCs to CGRP inhibits antigen presentation for Th1-type immunity while enhancing presentation for Th2 response.76, 77 It was recently found that CGRP can regulate the outcome of antigen presentation by LCs or bone marrow-derived dendritic cells through effects on microvascular ECs. CGRP increases IL-6 release from ECs which serves as an intermediate mediator, which is at least partly responsible for biasing the outcome of antigen presentation to responsive T cells towards a Th17 outcome as shown by increased expression of IL-17A and decreased expression of INF-γ, IL-4 and IL-22.78 Thus, while a direct effect on LCs stimulates a Th2 response, an indirect pathway through ECs may stimulate a Th17 response. CGRP has also been shown to have anti-inflammatory effects in some situations. Experiments have demonstrated that systemic CGRP pretreatment inhibits oedema effects of inflammatory molecules in murine and human skin in vivo79 and reduces the number of neutrophils present in mouse blood and the peritoneal cavity after injection with LPS (inflammatory, membrane component of gram-negative bacteria).80 Interestingly, CGRP inhibits the stimulated production of some chemokines by ECs81 and, perhaps this explains, at least in part, this anti-inflammatory effect. In addition to prominent microvascular vasodilation resulting from activation of endothelial NOS,82 CGRP promotes EC proliferation and subsequent angiogenesis.83 This effect is important in wound healing,84 but may promote the persistent angiogenesis seen in chronic inflammation.85
CGRP is synthesized and released by nerves after UVR exposure. Acutely there is a temporary decrease in CGRP mRNA expression in rat dorsal root ganglia and skin CGRP protein levels after UVR at less than 320 nm.86, 87 The decreased CGRP levels in acutely irradiated skin represented increased nerve terminal release of CGRP which had been rapidly metabolized. Experiments with a subacute time course demonstrated an increase in the number of epidermal nerve fibres (ENFs) immunoreactive for CGRP without alteration in total number of ENFs after extended, subinflammatory UVR exposure in mice, suggesting that UVR induced synthesis and/or transport of neuropeptides.88-90 This increase in CGRP content could be blocked by sunscreen application,89 and neurogenic inflammation was increased in UVB-exposed mice compared in control mice as a consequence of increased neuropeptide content.90 Human skin samples also displayed upregulation of CGRP after UVB radiation in a dose-dependent manner.91 Keratinocyte proliferation has also been noted independently after both UVB radiation and CGRP treatment.90
CGRP plays an essential role in immunosuppression after UVB radiation exposure, in both the local and systemic models, as demonstrated by loss of CHS induction and modulation of LC density by triggering mast cells to release TNF-α.86, 92, 93 Neuropeptide depletion of mice via pretreatment with capsaicin inhibited UVB-induced immunosuppression of the early and late swelling responses to hapten92 indicating release of CGRP, and other neuropeptides were necessary for the immunomodulatory effects seen. Pretreatment with a CGRP antagonist prior to antigen exposure restored the capacity to respond to hapten in UVB- and UVA2-exposed skin.63, 86, 89, 92 UVB irradiation and topical CGRP application both reduce the density of LC in the epidermis.86 CGRP has been shown to induce mast cells to release TNF-α, and injection of anti-TNF-α antibodies prior to CGRP application prevented loss of LC density and restored CHS induction.93 Conflicting evidence exists regarding experiments with mast cell-deficient mice. Application of CGRP to mast cell-deficient mice failed to impair CHS induction in one experiment93 and induced tolerance to hapten in another experiment.94 Mast cells have been shown to release IL-10 after chronic UVB irradiation, which plays an immunosuppressive and anti-inflammatory role.95 Earlier research showed that IL-10 plays a role in UVB-induced immunosuppression independent of mast cells.94 CGRP can augment the production of IL-10 as evidenced by studies showing that CGRP elevates IL-10 production in a murine dendritic cell line stimulated with LPS and granulocyte-macrophage colony-stimulating factor (GM-CSF)96 and in human peripheral blood mononuclear cells stimulated with inactivated Staphylococcus aureus.97 CGRP mixed with anti-IL-10 antibodies failed to promote tolerance to hapten.94 Additional experiments with IL-10-/- mice demonstrated the immunosuppressive role of IL-10 in relation to photocarcinogenesis.98 Splenic Treg cells from UV-irradiated IL-10-/- mice showed impaired suppressor function and produced increased amounts of IFN-γ. In addition, IL-10−/− mice that were chronically UVB-irradiated were protected against the induction of skin tumours compared to IL-10+/+ and IL-10+/− mice. The role of CGRP in UVR-induced immunosuppression is shown in Figure 1.
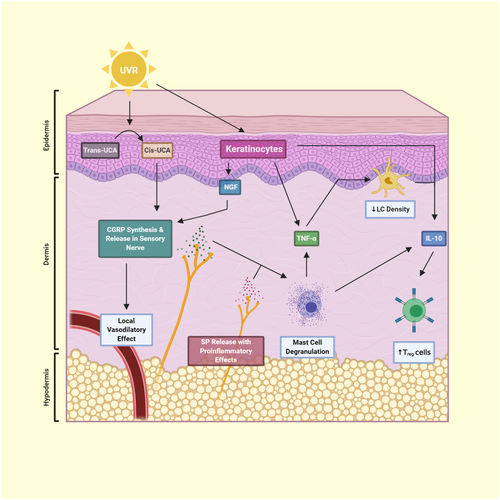
Cis-UCA can impair induction of CHS and alter LC morphology similarly to UVB radiation99; these effects appear to be mediated by local TNF-α release by keratinocytes induced by cis-UCA. Cis-UCA, at least partially, depends on neuropeptides, such as CGRP and SP, for its immunosuppressive and microvascular effects. In neuropeptide-depleted mice, cis-UCA was unable to suppress systemic CHS responses100; the authors propose that CGRP plus SP release by nerves induces mast cell degranulation that leads to immune suppression. Another experiment examining blood flow in a rat hind paw blister base noted that cis-UCA increased microvascular blood flow, and that this effect was significantly diminished but not abolished by anti-CGRP and/or anti-SP antibodies.100
Nociceptor release of CGRP helps drive the immune response in the later stage of inflammation (sunburn) after UVB exposure. Chemical depletion of nociceptor terminals or CGRP knockout in mice did not alter the early phase (defined as first 48 hours) of the inflammatory response but, after 5 days, increased CD45+ cells were observed because of increased numbers of dendritic cells (DCs) and αβ+ T cells.101
UVB stimulation of CGRP expression appears to modulate the systemic neuroendocrine regulation of adipose tissue.102 Mice exposed to UVB and sacrificed 24 hours later showed increased CGRP expression in epidermis, liver and hypothalamus. The increased CGRP expression in the liver lead to increased IL-6 which stimulated serum amyloid A to act in an endocrine manner and decrease adiponectin in adipose tissues decreasing serum adiponectin levels.102 This study provides evidence that epidermal release of CGRP after UVB radiation acts as a signal to influence other organs and leads to a systemic effect.
Hyperalgesia after UVB radiation is mediated in part by CGRP and is thought to be a behavioural response to minimize future exposure to an external irritating cue.103 Injection of capsaicin produces a similar hyperalgesic effect as UVB radiation. Pretreatment with antagonists to either CGRP, SP or mast cells prevented capsaicin-induced hyperalgesia in rats.104 Transdermal administration of a CGRP-antagonist peptide through a microneedle patch demonstrated a potent anti-hyperalgesic effect in a sterile inflammatory pain model where UVB radiation was applied to rat cheeks.105 Withdrawal latency to thermal stimuli was extended in rat that received the microneedle patch.
Antioxidants appear to significantly blunt the UVR-induced increase in CGRP expression in vitro. Application of phytoglobin, a plant-based neuroglobin mimic and NO scavenger, to dorsal root ganglion in vitro decreased CGRP expression and increased nerve cell survival after UVB radiation.106 A compound of L-carnosine and Rhodiola rosea (RCAC) applied to normal human epidermal keratinocytes exposed to UVR for 5 days showed restoration of pro-inflammatory cytokines, IL-1α and TNF-α, to control levels, decrease of CGRP and SP by 50%, and increase of β-endorphin (β-END) and enkephalin to threefold of control.107
There is evidence that CGRP plays a role in dermatologic conditions, such as psoriasis.108 Evidence supporting the modulation of neuropeptide levels by phototherapy for treatment of skin conditions is inconclusive and sparse. One study compared baseline serum concentrations of CGRP and SP for 59 patients with psoriasis who received 20 narrowband UVB exposures (NB-UVB) to 50 healthy subjects, matched for age and sex.109 Although baseline serum concentrations of CGRP and SP were significantly higher among patients with psoriasis compared to controlsα, there was no significant change after 20 NB-UVB treatments. Another study looking at the effect of phototherapy on itch examined punch biopsies from the non-inflamed gluteal skin of 10 patients before and after 20 treatments.110 Biopsies showed significantly reduced cutaneous nerve fibre density and decreased CGRP-immunoreactive nerve fibres in the dermis, which may explain the success of phototherapy in ameliorating itch. Also, CGRP was found on ECs and on LCs in psoriatic plaques.111 The effect of phototherapy on CGRP expression in dermatologic conditions is an area for future research.
4 SUBSTANCE P
SP is an 11 amino acid peptide that is part of the tachykinin family, along with neurokinin A and neurokinin B.112, 113 Alternative splicing of the preprotachykinin-A gene leads to synthesis of SP and related peptides.114 SP is distributed throughout the central and peripheral nervous systems, with a preference for peripheral branches of the PNS compared to the dorsal root115 and often colocalized with CGRP. 75 SP is also synthesized and released by mast cells, monocytes and eosinophils.27 SP can bind to three receptors, NK1, NK2 and NK3, with the highest affinity for NK1.116 SP is degraded in peripheral tissues by neutral endopeptidase (NEP).117
SP exhibits pro-inflammatory characteristics within the local and systemic immune system. SP enhances T-cell proliferation, immunoglobulin production, IL-1, IL-6 and TNF-α production by monocytes and histamine and TNF-α release from mast cells.118 SP also plays a role in leucocyte extravasation as shown by induction of vascular cell adhesion molecule 1 (VCAM-1) and P-selectin in dermal microvascular ECs.119, 120 Similarly to CGRP, SP stimulates ECs to proliferate121 and to synthesize nitric oxide, resulting in angiogenesis and potent vasodilation.82 SP has been shown to accelerate wound healing via enhanced angiogenesis in diabetic mice.122 SP may play a role in the pathogenesis of psoriasis and atopic dermatitis. 123, 124 Patients with psoriasis were noted to have increased SP+ and NK1R+ peripheral blood eosinophils compared to healthy controls.123 Patients with atopic dermatitis were noted to have higher plasma SP and greater SP+ and NK1R+ expression in populations of immune cells compared to healthy controls.124 This increased expression of SP and its receptor may mediate pathologic conditions through its ability to degranulate mast cells, enhance leucocyte extravasation and stimulate persistent angiogenesis leading to chronic inflammation.
SP is upregulated after UVR exposure as demonstrated by increased immunoreactivity in the rat dorsal horn and lateral spinal nucleus (involved in nociception).125, 126 This upregulation is characterized by an increase in neuropeptide content rather than an increase in overall nerve fibre density.88 SP plays a pro-inflammatory and vasodilatory role in the late phase (after 12 hours) of UVR inflammation.126 SP or NK1 antagonists were shown to reduce late phase oedema and erythema,126 attenuate blood flow up to 27% after 24 hours127 and delay the reduction of necrotic area after UVR.128 SP is also important in the local immune response after UVR exposure. Mice were exposed to UVB radiation and were treated with an SP agonist at the same site.129 The SP agonist was able to restore a normal CHS response. In the absence of UVR, an SP agonist was shown to be able to significantly exaggerate ear swelling response to a high-dose hapten if applied at the same site, but not a different site, indicating a local but not systemic role. SP agonist alone did not alter epidermal LC density or morphology.
UVR has also been shown to alter the expression of SP content, receptors and function in local effector cells. Evidence points to an increase in intracellular [Ca2+] as a mechanism of SP induction of mast cell degranulation.130, 131 Although SP is a potent inducer of histamine release from mast cells, UVB radiation inhibits this SP-induced release in rat mast cells in vitro partially through suppression of an increase in intracellular [Ca2+].132 In a human study comparing sun-exposed to sun-protected skin biopsies of 20 elderly patients, a correlation between severity of photodamage and increases in epidermal innervation and neuropeptidergic sensory nerve fibres was noted.133 In addition, mast cells in photodamaged skin possessed a larger amount of SP within their granules and keratinocytes, and vascular ECs exhibited reduced expression of NK1R. A study analysing the effect of UVA therapy for patients with atopic dermatitis found that SP receptors on dermal ECs were strongly decreased after light therapy.134 Another study on patients with psoriasis receiving NB-UVB therapy showed that while both full length and truncated forms of serum SP are higher in patients with psoriasis compared to control, only serum truncated SP is significantly decreased after irradiation.135 As the role of angiogenesis in chronic inflammation has become evident,136 perhaps targeted phototherapy may disrupt the inflammatory effects of SP, in part, by downregulation of receptors for SP on ECs and decrease in serum SP levels.
CGRP and SP appear to have some opposing immunologic functions, and although both neuropeptides are released after UVR, the effects observed in vivo are primarily those of CGRP. Several reasons have been proposed as explanations for this. CGRP and SP are both degraded by NEP, but SP is cleaved 88-fold more rapidly than CGRP, perhaps leading to a significantly decreased concentration of SP and, thus, weaker physiologic effects.137 Also, relevant effector cells may express a higher density of CGRP versus SP receptors, as shown by evidence above that UVR can modulate the number of SP receptors on effector cells. Although SP is capable of degranulating mast cells to release TNF-α, the concentration needed is quite high and would allow SP to also bind the lower affinity NK2 receptor, which may have alternate or dampened physiologic effects.138 The interplay between CGRP and SP is a topic of research that is relevant for both the pathologic and therapeutic effects of UVR.
5 NERVE GROWTH FACTOR
NGF is a member of a family of neuropeptides, termed neurotrophins, that promote neuronal growth and survival. Neurotrophins also include brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin 4/5.139 NGF is important in regulation of neurotransmitters and neuropeptide synthesis in sympathetic and sensory nerve cells140 with both CGRP and SP gene expression regulated by NGF.141 Indeed, binding of NGF to its receptor on sensory neurons leads to increased mRNA for CGRP.142 An NGF precursor, proNGF, is the initial product of Ngf gene expression but may have opposite downstream effects to those of mature neurotrophins depending on receptor density. The most likely candidate for cleavage of proNGF to NGF is the intracellular convertase furin.143 Interestingly, furin expression at the mRNA level has been shown to be increased in HaCaT cells by both UVA radiation (2 kJ/m2) and UVB radiation (0.2 kJ/m2).144 However, in a later study looking at furin protein expression by Western blot, UVA radiation decreased furin levels in HaCaT cells at a high dose with no effect at a low dose, similar to that given in the earlier study, while low- (0.2 kJ/m2) and high-dose UVA radiation decreased furin expression.145 However, high-dose UVB radiation (2 kJ/cm2) enhanced furin protein levels in Colo16 cells.145 The role of UVR in regulating furin expression seems to require further investigation. Proneurotrophins, such as proNGF, bind with high affinity to the p75 neurotrophin receptor (p75NTR), while mature neurotrophins, such as NGF, bind preferentially to the Trk family of receptor tyrosine kinases (TrkA, TrkB and TrkC). NGF selectively binds TrkA. Depending on receptor ratios, interaction of proneurotrophins with p75NTR may promote cell survival or apoptosis while binding of mature neurotrophins to Trk receptors always promotes cell survival.146 NGF regulates not only neurons but also mast cells, lymphocytes, granulocytes, monocytes, keratinocytes and ECs,147 and can be synthesized by keratinocytes,148 fibroblasts149 and ECs.150 NGF has been shown to be increased in the nerves supplying inflamed skin,151 and NGF levels are upregulated in psoriasis,152, 153 atopic dermatitis154, 155 and asthma.156, 157
UVB radiation leads to synthesis and release of NGF from epidermal keratinocytes peaking 8 hours after treatment.158-160 UVC radiation, which affects the uppermost skin layers, was also shown to increase NGF expression in a human study in vivo.161 NGF alone is capable of inducing systemic suppression of CHS response in mice; however, this effect is not seen in neuropeptide-depleted or mast cell-depleted mice.158 Anti-NGF antibodies also abrogate UVB-induced systemic suppression of CHS responses in mice and mast cell degranulation in vitro.158 NGF most likely mediates its immunomodulating effects through the stimulation of synthesis and/or release of neuropeptides. NGF has been shown to produce a dose-dependent increase in CGRP mRNA dependent on the TrkA receptor in rat sensory neurons.142 In a rat paw inflammatory model, anti-NGF serum was able to prevent increases in SP and CGRP content in the sciatic nerve. Local injections of NGF into a non-inflamed paw or intrathecal infusion after sciatic nerve transection induced an increase or counteracted a decrease in SP and CGRP content respectively.151, 162, 163 Evidence points to a series of events, as shown in Figure 1, where UVR induces NGF production and release from epidermal keratinocytes which bind to receptors on cutaneous sensory nerves that, after engagement with NGF, induce transcription of neuropeptides such as CGRP and SP.
NGF protects cutaneous cells from UVB-induced apoptosis. Human keratinocytes experienced increased apoptosis in vitro after UVB exposure when pretreated with a NGF receptor inhibitor, while keratinocytes overexpressing NGF were found to be partially resistant to apoptosis induced by increased doses of UVB.164 Resistant keratinocytes were found to have decreased Bcl-XL, which may in turn block caspase activation. Additionally, supplementation of medium with NGF protected melanocytes from UV-induced apoptosis in vitro by upregulating Bcl-2.165 A similar in vivo experiment showed that rat eyes and skin pretreated with topical NGF and exposed to UVB experienced decreased apoptosis along with increased Bcl-2 and decreased Bax expression in exposed tissues.166 Although human keratinocytes also synthesize neurotrophin-3, BDNF and neurotrophin-4/5, only NGF exhibits anti-apoptotic effects.167 UVB appears to downregulate this protective effect as evidenced by decreased expression of TrkA in basal keratinocytes and p75NTR in nerve fibres after UVB exposure to normal human skin in vivo.168 Another study showed that pretreatment of human dermal fibroblasts with propolis extract increased their viability after UVR via increased expression of NGF.169 Propolis is a resinous material collected by bees from the buds and exudates of the plants that is mixed with bee enzymes, pollen and wax.170 The exact composition, actions and possible uses of propolis remain speculative and are under study.
NGF is partially responsible for UVR-induced hyperalgesia and allodynia. NGF and proinflammatory cytokines (TNF, IL-1β, and IL-6) were increased in a biphasic temporal pattern corresponding to the pattern of thermal hyperalgesia and cold and tactile allodynia after UVR exposure in rats.171 Chemical depletion of sensory afferents via capsaicin or guanethidine injection produced significant alteration of hyperalgesia and allodynia, suggesting a role for products of sensory nerves in these effects. An earlier study by the same group showed that UVB-induced hyperalgesia and upregulation of NGF and proinflammatory cytokines could be prevented in a dose-dependent manner by IL-10 or IL-13 administration.172 Another study showed that anti-NGF treatment in rats exposed to UVB was able to reverse 45% of thermal hyperalgesia and 30% of mechanical allodynia.173 NGF’s role in hyperalgesia may be related to sensitization of nociceptors. NGF has been shown to increase the expression and insertion of TRPV1 receptors in sensory neurons.174 A TRPV1 antagonist increased heat pain tolerance at the site of UVB-induced inflammation in a randomized cross-over trial in humans.175 TRPV4 knockout mice are shown to have decreased sensitivity to noxious and mechanical stimuli after UVR.103 New therapeutic approaches centred around NGF may be useful in treating inflammatory pain, including UVR hyperalgesia.
Modulation of NGF plays a role in the efficacy of phototherapy for atopic dermatitis. Psoralen + UVA (PUVA) and NB-UVB both decrease intraepidermal nerve growth and normalize abnormal expression of NGF in an acetone-treated, inducible-dry skin model in mice.176 In a human study, skin biopsies taken from patients with chronic atopic dermatitis before and after PUVA showed decreased epidermal nerve density and decreased fluorescence intensity of NGF in the epidermis.177 Because NGF has been implicated in several other inflammatory skin conditions, further research aimed towards understanding the mechanism of the therapeutic effects of phototherapy is warranted.
6 NITRIC OXIDE
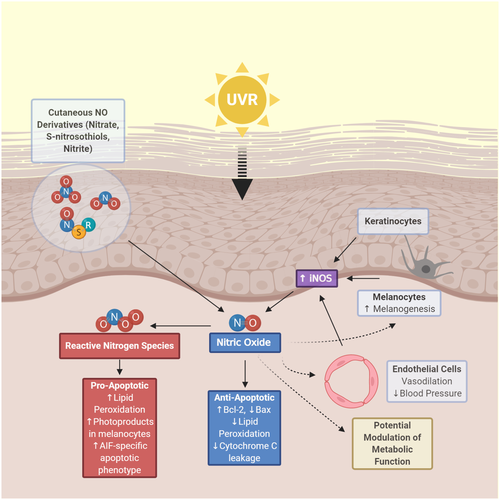
Functioning as a vasodilator, neurotransmitter and immunomodulator, NO is a gaseous signalling molecule that, despite a half-life of a few seconds in blood, performs a wide range of biologic functions. The phenomenon of ‘photorelaxation’, whereby vascular smooth muscle dilates when irradiated with UVR or visible light below 450 nm, was discovered over 60 years ago.178 An unstable humoral substance termed endothelial-derived relaxing factor (EDRF) was deemed responsible for these vasodilatory effects, and EDRF was later identified as NO.179 NO is produced enzymatically by three main isoforms of NOS: endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). While eNOS and nNOS constitutively express low levels of NO in a calcium-dependent manner, iNOS can be activated by UVB irradiation,180 proinflammatory cytokines181 or LPS182 in a calcium-independent manner leading to the production large amounts of NO which can result in pathologic consequences.183 NO is also produced non-enzymatically via reduction of NO stores such as nitrites, nitrates and S-nitrosothiols (RSNOs).184 NO release via enzymatic and non-enzymatic pathways after UVR has been shown to have immunomodulatory properties, and recent studies have shown beneficial health effects of decreased blood pressure and improved metabolic markers that will surely comprise future areas of research on NO.
NO production is increased after UVR. In vitro studies showed that NO production and expression of iNOS mRNA are significantly increased at various time points after UVB irradiation of normal human keratinocytes, HaCaT cells, PAM212 cells,185 murine macrophages186 and human dermal ECs.187 A study isolating keratinocytes, microvascular ECs, melanocytes and fibroblasts from human skin showed that keratinocytes and microvascular ECs release the greatest amount of NO after UVA radiation exposure.188 A keratinocyte supernatant isolated from mice exposed to UVB radiation showed that iNOS expression began increasing at 8 hours with a maximum at 72 hours.189 Bridging the gap between iNOS expression beginning at 8 hours is the non-enzymatic release of NO. Normal human skin contains nitrite and RSNO concentrations at 25- or 360-fold higher concentrations than plasma, and UVA irradiation leads to the formation of NO from these molecular sources.190 Another study showed that within 30 minutes of UVA exposure, NO-related products, especially nitrate, in the human epidermis, superficial vascular dermis and human skin sweat are capable of releasing NO.191 Early release of NO from cutaneous stores followed by later expression of iNOS account for the increase in NO seen after UVR.
NO is a mediator of UVR-induced immunosuppression. An NOS inhibitor applied to skin of mice abrogated loss of CHS response from UVA and UVB.66, 86 Pretreatment with NOS inhibitor also prevented reduction of LC from epidermis.66 A human study also demonstrated that pretreatment with a NOS inhibitor, but not a ROS inhibitor, could prevent immunosuppression. This was measured by loss of CHS response to nickel after ssUVR exposure, designed to mimic sunlight and including both UVA and UVB radiation.192 However, systemic administration of antioxidants does inhibit UVB-induced suppression of the CHS response in both the low- and high-dose mouse models.193 An in vitro study showed that NO application induced the expression of Treg cells, and phototherapy in patients with atopic dermatitis led to an increased ratio of Treg to Teffector cells.194 This increased ratio corresponded with a clinical improvement in the patients with atopic dermatitis. Further research should be carried out to characterize the immunomodulatory benefits of NO release stemming from UVR exposure or targeted phototherapy.
NO plays an important role in melanogenesis after UVR exposure. NO donors activate human melanogenesis in vitro, and conditioned media from UVA- and UVB-exposed keratinocytes stimulate tyrosinase activity in melanocytes, with the effect reversed by NO scavengers.195, 196 UVB radiation also induces an increase in intracellular cGMP in melanocytes in an NO-dependent mechanism that can be reversed by NO antagonists.196 Additionally, preincubation with α-MSH stimulates increased NO release in vitro from B16 murine melanoma cells and human melanocytes after UVB radiation.197 This modulatory effect of α-MSH is mediated by stimulation of iNOS as shown by reversal of findings with application of a selective iNOS inhibitor. NO released from keratinocytes and melanocytes after UVR exposure appears to act in a paracrine and autocrine fashion, respectively, to stimulate melanogenesis.195, 197
NO production after UVR exposure is both anti- and pro-apoptotic. Rat aortic ECs were pretreated with proinflammatory cytokines so that they expressed iNOS and produced NO. The ECs were found to be protected from UVA-induced apoptosis with an associated increase in Bcl-2 expression and decrease in UVA-induced overexpression of Bax.198 Similarly, an NO donor added during or after UVA1 radiation significantly protected rat ECs from apoptosis and necrosis. This protection strongly correlated with both inhibition of lipid peroxidation and cytochrome c leakage.199 Another study showed that cytosolic iNOS expression inversely correlated with lipid peroxidation decrease after UVB exposure in murine skin.200 However, other studies have shown that NO does play a role in lipid peroxidation. UVB irradiation of hairless mice led to an increase in skin lipid peroxidation and iNOS after 24 hours, which actually decreased under treatment with a iNOS inhibitor.201 Further experiments showed that expression of catalase and glutathione is decreased in epidermal layers after UVB irradiation which allows H2O2 to accumulate and react with NO to form peroxynitrite.202 These conflicting results likely result from differences in the ratio of concentration of NO to RNS including peroxynitrite. While an antioxidative cell milieu promotes formation of NO that scavenges initiators of lipid peroxidation and leads to cell protection, an oxidative milieu promotes ROS and RNS leading to cell death.184 Different cell types have differing antioxidative capacity. In contrast to ECs, the presence of exogenous nitrite enhances UVA-induced lipid peroxidation in dermal fibroblasts in a concentration-dependent manner.203 NO was also shown to inhibit DNA-adduct excision in human fibroblasts damaged by UVC, indicating a potentially carcinogenic role.204 More recent research has shown that UVR-induced peroxynitrite can excite fragments of melanin that can go on to induce CPDs in melanocytes even hours after UV exposure has ended.205 Additionally, UVA irradiation of HaCaT cells in the presence of nitrite inhibited caspase activity but induced an apoptosis-inducing factor (AIF)-specific apoptotic phenotype, an alternate form of cell death to discard of damaged keratinocytes.206 Ultimately, the balance between NO and RNS production after UVR appears to dictate whether a cell will survive or undergo apoptosis.
Recent research has built on the foundation of NO as a vasodilator, and uncovered evidence that release of NO after UVR results in a decrease in blood pressure. Two separate groups conducted experiments examining the effect of whole-body UVA irradiation on blood pressure in humans. Both showed a significant decrease in blood pressure within 30 minutes. Although the effect could not be modified by acute dietary intervention with nitrate, it was independent of iNOS leading to the conclusion that the change in blood pressure was attributable to the release of NO from cutaneous stores in the skin.207, 208 Another group asked whether whole-body UVA irradiation and/or nitrate gel ingestion could affect athletic performance. Results of a cycling time trial showed that performance was significantly improved in a group receiving UVA treatment and ingesting nitrate gel, but not in the groups receiving either intervention alone.209 Building on these findings, an analysis of records of a 342,000 North American cohort of chronic haemodialysis patients showed evidence that incident solar UVR is associated with a lower systolic blood pressure even after adjusting for environmental temperature.16 Interestingly, another study showed that blue light (γ = 450 ± 5 nm) also decreased systolic blood pressure while increasing NO species and nitroso compounds in healthy male volunteers.210 The implications of these findings may come to reshape our current public health and clinical guidelines.
NO release after UVR modulates metabolic function in murine models. In mice fed high-fat diets, suberythemal exposure to UVR (65% of output in UVB range) led to reductions in fasting glucose levels and development of liver steatosis compared to mice not receiving UVR. 19 These results were dependent on cutaneous release of NO as evidenced by suppression of effects if mice were pretreated with a topical NO scavenger. In a subsequent low-dose UVR experiment, mice on high-fat diets exhibited significantly reduced weight, weight gain, fasting insulin, and liver steatosis compared to mice not receiving UVR and the UVR effect on liver steatosis was reversed by a topical NO scavenger.18 Most recently, interscapular brown adipose tissue of mice was analysed in a similar experimental set-up using high-fat diets and repeated low-dose UVR exposure.17 Results showed no shift in heat production, but the typical increase in white adipocyte phenotype in brown adipose tissue found after a high-fat diet was significantly reduced. Once again, these results were reversed by pretreatment with a topical NO scavenger, and were unable to be reproduced with increased dietary nitrate. Cutaneous release of NO appears to be the most effective way in producing these metabolic benefits, and further research to characterize pathways involved is required. A summary of the findings regarding NO described in the previous paragraphs is shown in Figure 2.
7 PROOPIOMELANOCORTIN (POMC), ALPHA-MELANOCYTE STIMULATING HORMONE (α-MSH), BETA-ENDORPHIN (β-END) AND ADRENOCORTICOTROPIC HORMONE (ACTH)
POMC is a precursor protein that is classically expressed by the pituitary gland. Proteolytic cleavage of POMC by proprotein convertases (PCs) results in a diverse group of biologically important neuroendocrine peptides. These include the melanotropic peptides, such as α-, β- and γ-MSH; opioid peptides, such as β-END and β-lipotropin (β-LPH), and the stress peptide adrenocorticotropic hormone (ACTH).211 These peptides bind to five melanocortin receptors (MC-Rs) which have overlapping specificities.212 In the past several decades, evidence has arisen detailing the role of skin as a peripheral neuroendocrine organ capable of transcribing and splicing the POMC gene.213 Melanocytes, keratinocytes, microvascular ECs, LCs, mast cells, fibroblasts, monocytes and macrophages have all been reported to express POMC peptides.27 In addition to well-known effects on pigment, α-MSH exhibits an anti-inflammatory role in skin and cytoprotective function against UVR damage.212 The effects of UVR exposure in relation to α-MSH, as well as ACTH, β-END and other POMC derivatives will be explored in the following section.
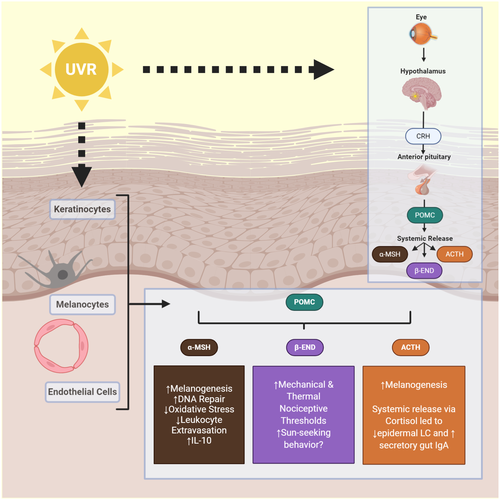
POMC and its derivatives are upregulated after UVR. The POMC gene has increased expression in normal and malignant human melanocytes and keratinocytes after UVB in vitro.214 This effect is abolished by pretreatment with a free radical scavenger. Human skin exposed to ssUVR in vivo showed increased POMC mRNA at 3 hours and increased α-MSH proteins by 24 hours. This effect is abolished by prior application of sunscreen.215 Human keratinocytes have been shown to produce significantly more α-MSH, ACTH, β-END and β-LPH after UVB irradiation.216-218 Furthermore, human dermal microvascular cells can also produce α-MSH and ACTH after UVB.219 In addition to upregulation of the peptides themselves after UVR, MC-1R mRNA is increased in normal human melanocytes in vitro and in human skin in vivo,215, 220 along with PC1 in keratinocytes in vitro.51 This UVB induction of POMC and MC-1R promoters in mice has been shown to be mediated by a p38 stress-activated kinase signalling to the transcription factor, upstream stimulating factor-1 (USF-1).221 USF-1 KO mice failed to activate POMC and MC-1R expression in response to UVR indicating its important role in UV protection. Of note, UVB radiation has been shown to activate the hypothalamic-pituitary-adrenal axis in mice leading to increased plasma levels of CRH, CTH and corticosterone, and increased mRNA for steroidogenic acute regulatory protein (StAR) and steroid 11β-hydroxylase in the adrenals.222 UVR to just the murine eye has also been shown to have downstream systemic effects via the hypothalamopituitary-POMC pathway. Local UVB irradiation of murine eyes resulted in increased plasma α-MSH and stimulation of epidermal melanocytes.223 The authors showed that this system was activated via an iNOS-dependent neuronal network involving the first branch of the trigeminal nerve passing through the ciliary ganglia. They later demonstrated that the Gp91 phox subunit of NADPH in the CNS contributes to this central pigmentation pathway.224 The ability of UVR to systemically modulate the neuroendocrine system is explored in several studies presented throughout the following paragraphs.
Induction of melanogenesis after UVR is mediated through expression of POMC and release of α-MSH, ACTH and possibly adrenaline. Tumour-suppressor protein p53 promotes cutaneous pigmentation following UVR by direct transcriptional activation of POMC in the skin, and the absence of p53 ablates the tanning response.225 POMC mRNA and protein were significantly induced in response to p53 overexpression in the murine PAM212 keratinocyte line, and introduction of a dominant-negative p53 allele abrogated induction of POMC following UVR in PAM212 and human primary foreskin keratinocytes. Binding of p53 to the POMC promoter was detected following UVR. Moreover, p53 null mice showed no measurable POMC mRNA upregulation, although basal expression was not affected, and a deficient tanning response following UVR. ACTH and α-MSH release after UVR bind to MC-1R and stimulate cAMP formation allowing melanocytes to overcome G1 arrest.226 Treatment of human melanocytes with α-MSH in vitro increases eumelanin synthesis.226 Treatment of human melanocytes with ACTH increased tyrosinase activity227 and stimulated melanogenesis.228 Mice housed in stressful high population density conditions or restrained for 4 hours per day were shown to have increased tanning and number of melanocytes compared to controls and with this effect abrogated by corticostatin.228 Neprilysin, a NEP expressed at the surface of human melanocytes, is downregulated by UVB radiation which may permit increased melanogenesis.229 UVB irradiation of the murine eye can increase melanocytes in the epidermis indicating that there is a central axis for pigmentary response.230 Adrenaline is released from keratinocytes in vitro after UVB radiation exposure, and primary human melanocytes incubated in adrenaline solution or conditioned media from UVB-irradiated keratinocytes were noted to produce melanin.231 This effect was abrogated by the pretreatment of cultures with timolol.
Genetic variants in the MC-1R gene may be responsible for increased risk of developing skin cancer. Some MC-1R variants have been associated with the red hair colour, fair skin and poor tanning ability phenotype (RHC trait) along with increased risk for developing melanoma232 and non-melanoma skin cancer.233 The ability of different human melanocyte cultures to respond to α-MSH with stimulation of cAMP formation, increased tyrosinase activity and proliferation was determined and followed by sequencing of the MC-1R gene. Several amino acid substitutions created loss of function mutations that sensitized melanocytes to damaging effects of UVR to DNA.234 Melanocytes with a variant human allele of MC-1R that reduces the ability to produce eumelanin were found to have decreased phosphorylation of p38 and p53 leading to deficient DNA repair after UVR and α-MSH stimulation.235 Further research showed that RHC trait-associated MC-1R variants lack interaction with phosphatase and tensin homolog (PTEN) after UVB radiation which results in failure to inactivate AKT. Subsequent hyperactivation of PI3K/AKT signalling leads to premature senescence or oncogenic transformation in the presence of the BRAF (V600E) mutation.236 Restoration of variant MC-1R function was explored in mice. Pharmacologic MC-1R palmitoylation rescued defects in loss-of-function MC-1R variants by triggering increased pigmentation, stimulating UVB-induced G1 cell cycle arrest and restoring control of senescence and melanomagenesis.237
The neuroendocrine hormone α-MSH appears to protect cells from apoptosis, repair DNA damage and reduce oxidative stress after UVR. Normal human melanocytes exposed to UVB radiation and stimulated with α-MSH in vitro exhibited decreased apoptosis, reduced CPDs as a result of promotion of nucleotide excision repair,238 reduced levels of hydrogen peroxide,239 reduced generation of 8-oxoguanine and increased protein levels of catalase and ferritin.240 Similar research showed that α-MSH facilitated formation of DNA repair complexes and repair of DNA adducts in human melanocytes exposed to UVR via activation of MC-1R.241 Normal human foreskins in vivo and primary human keratinocytes in vitro exposed to UVB radiation and stimulated with α-MSH exhibited significantly reduced CPDs and 6-4 pyrimidine-pyrimidone photoproducts, along with fewer sunburn cells.242 This effect was found to be dependent on a functional MC-1R and xeroderma pigmentosum group A (XPA) DNA repair protein. UVB radiation has been shown to reduce Nrf2- and Nrf-dependent gene expression, important in the regulation of antioxidant proteins, in normal human keratinocytes and melanocytes, but α-MSH treatment was able to prevent this suppression in both cell types in vitro.243 Evidence of a connection between α-MSH and peroxisome proliferator-activated receptor gamma (PPARγ) has been demonstrated in melanocytes,244 and exogenous PPARγ activation was shown to have similar protective effects against UVR damage to those of α-MSH.245 In addition, tumour suppressor gene p16 is upregulated normally after UVR, and a greater increase is found in pretreatment with α-MSH.246 Addition of a competitive antagonist for MC-1R blocks the observed increase in p16 expression in the control and α-MSH groups. Building on the association between p53 and α-MSH, interference in p53 function led to a sustained increase in hydrogen peroxide in UV-treated human melanocytes.247
ACTH, α-MSH and β-END reduce inflammation and exhibit immunomodulatory properties. Human dermal microvascular ECs treated with α-MSH showed reduced expression of VCAM-1 and E-selectin via downregulation of nuclear factor-kβ indicating that α-MSH has the ability to suppress leucocyte extravasation.248 Monocytes249 and normal human keratinocytes250 treated with α-MSH show upregulation of anti-inflammatory IL-10. Injection of α-MSH prior to sensitization with antigen resulted in a hapten-specific tolerance in mice51 which could be abrogated by pretreatment with anti-IL-10 antibody.251 Separate research has shown that IL-10 expression is enhanced in murine keratinocytes exposed to UVR in vitro, and anti-IL-10 antibody neutralized the ability of supernatants from UV-irradiated keratinocytes injected into mice to suppress DTH reactions in vivo.252 One study has suggested that MC-1R is not necessary for induction of the immunosuppressive effect of α-MSH after UVR exposure.253 Mice lacking MC-1R were found to not have an altered inflammatory or immunosuppressive response to UVR. The authors propose that the role of α-MSH is not excluded, but MC-1R is not required because MC-3R may be the predominant anti-inflammatory receptor for melanocortins as evidenced by findings in previous studies.254, 255 UVA radiation exposure of the murine eye was shown to increase ACTH and cortisol levels in plasma, more than observed in animals irradiated on the ear,256 although an increase, albeit of smaller magnitude, was observed with ear irradiation. This experiment also showed a downregulation of the number of epidermal LCs in epidermal sheets derived from the ear and upregulation of secretory IgA in the gut, which indicates that systemic modulation of downstream organs may be plausible after UVA exposure to the eye. In a mouse model of colon carcinoma induced by azoxymethane and dextran sodium sulphate, UVA radiation exposure of the eye resulted in elevated β-END and methionine-enkephalin, upregulation of their respective receptors, decreased IL-6 and TNF-α ( tumor promoters during early colitis-associated carcinogenesis), increased apoptosis markers and decreased cell proliferation markers compared to control.257 The authors concluded that UVA irradiation of the murine eye was able to ameliorate symptoms of colon carcinoma through upregulation of β-END and methionine-enkephalin. Another experiment exposed the back skin of mice to UVB radiation and found that levels of CRH, β-END, ACTH and corticosterone were enhanced.258 Furthermore, splenocytes taken at varying time points from 30 minutes to 24 hours after exposure had suppressed IFN-γ production after mitogen stimulation, indicating a role of the HPA axis in immunomodulation after UV exposure. The authors speculate in a later review article that this rapid release of systemic neuroendocrine hormones and induction of immunosuppressive changes in splenocytes may be independent of the central HPA axis.259 The prospect of local UVR exposure, especially to the eye, leading to systemic modulation of end organs warrants further attention and research.
Research suggests that the addictive quality of UVR exposure is associated with the release of β-END.21 Although plasma levels of β-END in humans do not increase after UVR exposure,260-262 skin biopsies of healthy volunteers showed increased epidermal β-END 24 hours after exposure to NB-UVB.263 Interestingly, blue light irradiation (γ = 453 nm) significantly enhanced both keratinocyte culture β-END production and systemic β-END concentrations, along with bioactive NO-derivatives, in healthy individuals.264 The topic is further complicated by the observation that administration of an opioid antagonist, naltrexone, to a small group of frequent users of tanning beds induced opioid withdrawal-like symptoms of jitteriness, nausea and shaking.265 A mouse study showed systemic β-END elevation after chronic UVR exposure that was accompanied by increased mechanical and thermal nociceptive thresholds, increased opioid-mediated behaviours (ie Straub tail) and withdrawal symptoms after administration of opioid antagonist (naloxone).266 These effects were absent in β-END knockout mice and dependent on keratinocyte expression of p53. These data suggest that excessive sun-seeking behaviour may have a physiologic basis, and the potential exists to harness the release of β-END after UVR or blue light for therapeutic interventions. A summary of the findings regarding POMC and its derivates described in the previous paragraphs is shown in Figure 3.
8 CONCLUSION
Research illuminating the mechanisms by which UVR exposure leads to inflammation, carcinogenesis, pigmentation, immunomodulation and other effects has progressed substantially in the past several decades. In the preceding paragraphs, the effect of UVR on the skin has been viewed through the narrow lens of the neuropeptides and neuroendocrine hormones involved. Moving forward, a framework must be constructed that accounts for the important molecular responses to UVR, such as formation of DNA photoproducts, isomerization of trans-UCA, activation of aryl hydrocarbon receptor, secretion of PAF and synthesis of vitamin D, as well as the release of neuropeptides and neuroendocrine hormones. In continuing to uncover the complicated pathways involved in the effects of UVA, UVB and ssUVR, the scientific community will be better posed to utilize UVR as a therapeutic tool in not just cutaneous diseases, but also, perhaps, for graft versus host disease affecting internal organs,267 as well as in other areas. Furthermore, studies that show the benefits of solar radiation exposure, including vitamin D synthesis, decreased blood pressure,16 regulation of metabolic dysfunction19 and even prevention of atherosclerosis,268 mark important contributions in the ongoing debate269 regarding the ideal amount of sunlight. The future remains bright for understanding the role of UVR in human health and disease.
ACKNOWLEDGEMENT
We thank Wanhong Ding, Lori Stohl, Zakir Bulmer, Yee Jung Kim and John A. Wagner for their ongoing support. All figures were created with BioRender.com.
CONFLICT OF INTEREST
RDG is on the scientific advisory boards of Elysium Health and Hoth Therapeutics. He has research agreements with Leo Pharma, the Leo Foundation, Galderma, Pfizer and Elysium Health.
AUTHOR CONTRIBUTIONS
CRM wrote the first draft, and RDG edited, proofread the paper and rewrote sections.




