ADAMTS13 inhibits oxidative stress and ameliorates progressive chronic kidney disease following ischaemia/reperfusion injury
Abstract
Aims
Reduced A Disintegrin And Metalloproteinase with a ThromboSpondin type 1 motif member 13 (ADAMTS13) levels are observed in kidney disease. We test whether recombinant human ADAMTS13 (rhADAMTS13) mitigates renal injury in chronic kidney disease (CKD) and the potential mechanisms.
Methods
CKD was established 3 months after ischaemia/reperfusion (IR). ADAMTS13 and von Willebrand factor (vWF) levels, renal function and morphological changes were analysed. Afferent arteriolar responses to angiotensin II (Ang II) and acetylcholine (ACh) were measured. Oxidative stress-related molecules were detected.
Results
Higher vWF and lower ADAMTS13 levels were observed in CKD mice, which were markedly attenuated by rhADAMTS13. rhADAMTS13 alleviated renal dysfunction, as documented by decreased blood urea nitrogen (BUN), serum creatinine, kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) levels in CKD mice. Moreover, rhADAMTS13 attenuated transforming growth factor (TGF)-β1/Smad3 activation. Plasma vWF: ADAMTS13 ratio showed positive correlations with malondialdehyde (MDA), hydrogen peroxide (H2O2) and proteinuria, and correlated inversely with superoxide dismutase (SOD) and catalase (CAT). Finally, rhADAMTS13 inhibited reactive oxygen species (ROS) levels and improved microvascular functional disorders, accompanied by the inhibition of glycogen synthase kinase (GSK) 3β hyperactivity and upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2) expression.
Conclusions
Acute kidney injury (AKI) reduces the expression of ADAMTS13 that contributes to progressive CKD, microvascular dysfunction, oxidative stress, inhibition of Nrf2 activity and renal histopathological damage. All of which can be alleviated by administration of rhADAMTS13.
1 INTRODUCTION
Chronic kidney disease (CKD) affects some 8%-16% of the population worldwide and frequently progresses to end-stage renal disease (ESRD) with the associated increased morbidity and mortality,1-3 that can be exacerbated by development of cardiovascular disease.4, 5 Therapeutic options to delay CKD progression remain disappointing.6
The mechanisms that underlie in CKD progression are complex that include renal vascular endothelial dysfunction and thrombosis.7 Since von Willebrand factor (vWF) is released from damaged endothelial cells and recruits platelets, it might aggravate microvascular injury and expedite CKD progression.8 The metalloprotease A Disintegrin And Metalloproteinase with a ThromboSpondin type 1 motif, member 13 (ADAMTS13) cleaves vWF and thereby prevents microthrombosis, microvascular dysfunction and tissue injury.9, 10 Although an elevated plasma ratio of vWF: ADAMTS13 predicts kidney function decline,11 the mechanisms remain unclear. We report that the ratio of vWF: ADAMTS13 predicts acute kidney injury (AKI).12 Since recombinant human (rh) ADAMTS13 cleaves mouse vWF,13 we test the hypothesis that rhADAMTS13 can prevent microvascular dysfunction and averts CKD progression in a mouse model of AKI.
The ischaemia/reperfusion (IR) model of AKI was selected,14 since it shares several features of clinical AKI including glomerular sclerosis, tubulointerstitial fibrosis, tubular atrophy, oxidative stress and inflammation.15 The renal preglomerular afferent arterioles are the major source of renal vascular resistance,16 and are a site of nephron dysfunction in CKD.17 Therefore, their function was studied directly on isolated perfused afferent arterioles and related expression of ADAMTS13, vWF, markers of renal injury, oxidative stress, transforming growth factor (TGF)-β and renal histopathology.
2 RESULTS
2.1 Mice with CKD have reduced expression of ADAMTS13 and enhanced expression of vWF that can be reversed by rhADAMTS13
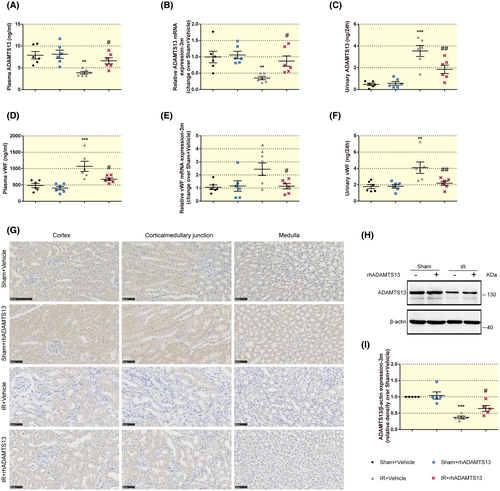
CKD mouse models were established by 3 months after 18 minutes of bilateral ischaemia or sham procedures. Mice received rhADAMTS13 (0.03 nmol/kg body weight, per day) or vehicle intravenously via the tail vein for 3 days after surgery. Mice subjected to IR 3 months previously had reduced plasma ADAMTS13 and renal ADAMTS13 messenger RNA (mRNA) (Figure 1A,B), but increased renal excretion of ADAMTS13 levels (Figure 1C), accompanied by increased plasma vWF, renal vWF mRNA and renal vWF excretion (Figure 1D-F). Renal protein expression of ADAMTS13 was also reduced after IR (Figure 1H), and immunohistochemistry demonstrated reduced ADAMTS13 in the tubulointerstitium, notably at the corticomedullary junction and medulla (Figure 1G). All these alterations were attenuated significantly by rhADAMTS13 administration.
2.2 rhADAMTS13 ameliorates renal dysfunction in mice with CKD
After surgery, mice with IR or sham were randomly allocated to receive 3 days of rhADAMTS13 or vehicle. The body weight of these groups was similar at baseline (Figure 2A).
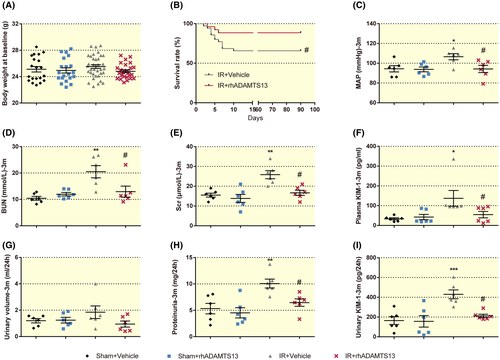
Mouse survival is shown in Figure S1A and the Kaplan-Meier plot in Figure 2B. The administration of rhADAMTS13 to mice subjected to IR improved their survival up to 3 months, and prevented the development of hypertension (Figure 2C) and maintained renal function (Figure 2D,E).
Kidney injury molecule-1 (KIM-1) serves as a urinary and blood biomarker of acute and chronic kidney injury.18 Renal injury in mice with CKD was identicated by increased plasma KIM-1 (Figure 2F), proteinuria (Figure 2H) and renal KIM-1 excretion (Figure 2I). However, despite similar urinary volume (Figure 2G), rhADAMTS13 decreased renal protein and KIM-1 excretion (Figure 2H,i).
2.3 rhADAMTS13 alleviates renal histological damage in mice with CKD
Tubulointerstitial fibrosis, tubular atrophy and collagen I and III and α-smooth muscle actin (α-SMA) deposition are hallmarks of CKD progression.19 Masson trichrome and Sirius red staining assess tubulointerstitial fibrosis.20 CKD mice treated with rhADAMTS13 had less intense staining for Masson trichrome and Sirius red (Figure 3A), lesser renal collagen I mRNA expression and protein (Figure 3A,C,D), accompanied by less expression of α-SMA (Figure 3A,C,E) and less expression of the renal damage markers,21 KIM-1 and neutrophil gelatinase-associated lipocalin (NGAL) (Figure S1D,E).
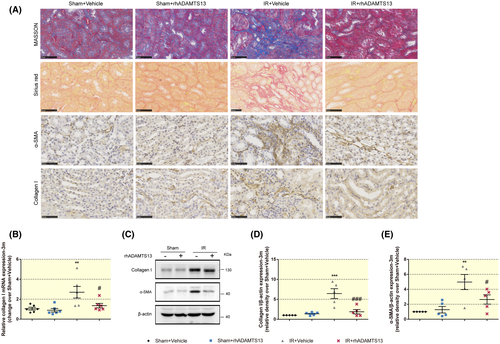
Histopathological analysis documented glomerulosclerosis, tubular atrophy, and cast formation (Figure S1B), with renal atrophy, which was accompanied by reduced kidney weight (Figure S1C). These effects were alleviated by rhADAMTS13.
2.4 rhADAMTS13 inhibits the activation of the TGF-β1/Smad3 pathway in the kidneys of mice with CKD
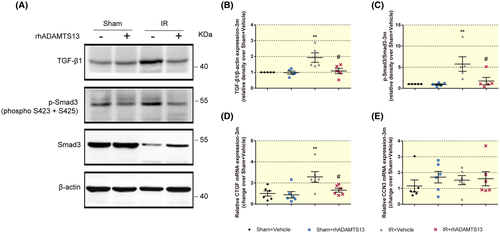
TGF-β1 is the primary factor that drives fibrosis in CKD, and Smad3 is one of the intracellular mediators that transduce signals from TGF-β1. TGF-β1/Smad3 signalling plays a key role in tissue fibrosis.22 rhADAMTS13 prevented the enhanced TGF-β1 expression (Figure 4A,B) and phosphorylation of Smad3 (Figure 4A,C), connective tissue growth factor (CTGF, also as CCN2) (Figure 4D). However, nephroblastoma overexpressed gene (CCN3, also known as NOV) that is a negative regulator of extracellular matrix (ECM) formation,23 was similar among the four groups (Figure 4E).
2.5 rhADAMTS13 prevents renal damage and the increased renal hydrogen peroxide (H2O2) excretion in mice at 1 week and 1 month after ischaemic insult
Figure S2 shows that 1 week after ischaemia, mean arterial pressure (MAP), kidney weight, BUN, Scr and proteinuria were similar among the groups. However, tubular injury was seen after 1 week of IR characterized as tubular dilation, loss of proximal tubule brush border, tubular necrosis and cast formation (Figure S3A), confirmed by increased renal excretion of KIM-1 and mRNA expression of KIM-1 and NGAL (Figure S3B-D), and accompanied by increased renal H2O2 excretion (Figure S3E). All of these alterations were alleviated by rhADAMTS13 treatment. Tubulointerstitial fibrosis was not apparent at this stage (Figure S3A). At 1 month after IR, MAP, BUN, Scr was similar among the groups (Figures S4A-C). However, after ischaemia we noted increased proteinuria (Figures S4D), renal mRNA expression of KIM-1 (Figures S5A), renal KIM-1 excretion (Figures S5B) with cast formation (Figures S4E) and extensive renal Masson trichromatic staining and Sirius red staining (Figures S4E), all of which were attenuated by rhADAMTS13. rhADAMTS13 also reduced TGF-β1 mRNA and α-SMA, collagen I deposition (Figures S5D,E) and the increased renal H2O2 excretion (Figures S5C).
2.6 Oxidative stress is correlated positively with the plasma radio of vWF: ADAMTS13 in mice after IR
Malondialdehyde (MDA) is a peroxidation product of polyunsaturated fatty acids.24 While Superoxide dismutase (SOD) and catalase (CAT) are antioxidant enzymes.25 Evidence of oxidative stress was observed after IR while reduced plasma levels of ADAMTS13 and increased vWF were observed at 1 week, 1 month and 3 months after IR (Figure S6).
Another marker of the progression to CKD is the increase in plasma vWF: ADAMTS13 ratio, compared with the sham group (Figure 5A). Figure 5B-D shows that urinary ADAMTS13 excretion and renal vWF mRNA levels were increased throughout the 3 months after IR, whereas renal ADAMTS13 mRNA levels fluctuated from an early increase of 1 week to a reduction after 3 months.

There was a strong correlation between the plasma ratio of vWF: ADAMTS13 and oxidative stress, that was confirmed by the positive correlation between the plasma ratio of vWF: ADAMTS13 with plasma MDA (Figure 5E) and renal H2O2 excretion (Figure 5G), and the negative correlation with SOD activity (Figure 5F). Proteinuria correlated with the plasma ratio of vWF: ADAMTS13 (Figure 5H), and with renal ADAMTS13 (Figure 5I) and vWF excretion (Figure 5J).
2.7 rhADAMTS13 mitigates oxidative stress and improves microvascular function in mice with CKD
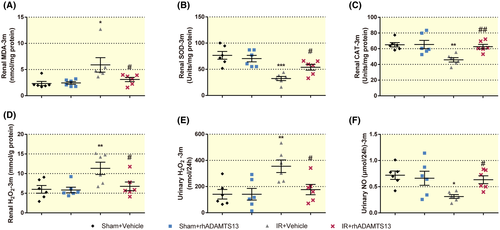
rhADAMTS13 delayed progression from AKI to CKD, decreased renal H2O2 excretion and the plasma ratio of vWF: ADAMTS13 was correlated with oxidative stress. Thus, improved renal outcome after rhADAMTS13 seems linked to diminished oxidative stress. rhADAMTS13 mitigated renal MDA and H2O2 in mice with CKD (Figure 6A,D) and restored the impaired SOD and CAT activity (Figure 6B,C) and the increased excretion of H2O2 and decreased excretion of Nitric oxide (NO) (Figure 6E,F).
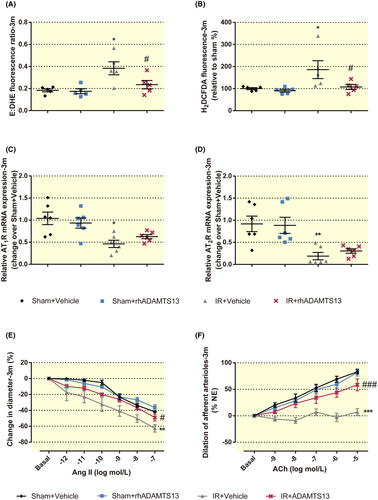
Afferent arteriolar reactive oxygen species (ROS) production, such as superoxide (O2−) and H2O2 play an important role in the microvascular function and affect CKD progression.26 Thus, O2− and H2O2 generation in renal afferent arterioles were detected by ethidium (E): dihydroethidium (DHE) and 2′,7′-Dichlorohydrofluorescein diacetate (H2DCFDA) as previously described.27 rhADAMTS13 mitigated the enhanced O2− and H2O2 generation in afferent arterioles from CKD mice (Figure 7A,B). rhADAMTS13 attenuated angiotensin (Ang) II-induced vasoconstriction and impaired acetylcholine (ACh)-induced vasodilation in renal afferent arterioles (Figure 7E,F), despite maintained mRNA expression for angiotensin II type 1 and 2 receptor (ATR) (Figure 7C,D).
2.8 rhADAMTS13 enhances the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant response to CKD and inhibits of glycogen synthase kinase (GSK) 3β hyperactivity
Nrf2 antioxidant response has been reported to be the primary signal that regulates oxidative stress during transition from AKI to CKD.28 Haem oxygenase (HO)-1 is an intracellular enzyme that has anti-oxidant properties and protects against tissue injury.29 Manganese superoxide dismutase (MnSOD, known as SOD2) is a nuclear-encoded and mitochondria-matrix-localized oxidation-reduction enzyme that regulates cell ROS generation and oxidative stress.30 To explore the mechanisms by which rhADAMTS13 prevents oxidative stress in chronic damaged kidney following IR, Nrf2 and its target antioxidant enzyme, HO-1, SOD2 and CAT were detected. Nrf2 nuclear and cytoplasmic accumulation was diminished in mice with CKD (Figure 8A,B). However, this was restored by rhADAMTS13 and accompanied by the increased mRNA for haem oxygenase (HO)-1 (Figure 8C), manganese superoxide dismutase (MnSOD, known as SOD2) (Figure 8D) and CAT (Figure 8E).
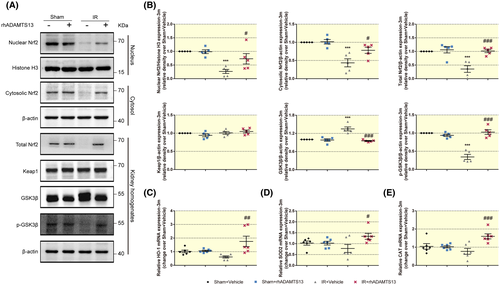
Kelch-like enoyl-CoA hydratase-associated protein 1 (Keap1) regulates the Nrf2 anti-oxidant response,28 and Keap1 protein expression was similar among the groups demonstrated by Western blot analysis in Figure 8A,B. In addition to structural changes of Keap1,31 recently, GSK3β was confirmed to play an important role in both AKI and CKD through regulating Nrf2 expression.28 There was enhanced expression of GSK3β and reduced expression of phosphorylation of GSK3β at the serine 9 residue (p-GSK3β) in mice with CKD, which are considered to inhibit Nrf2 (Figure 8A,B).
2.9 vWF aggravates tubulointerstitial fibrosis in rhADAMTS13-treated CKD mice
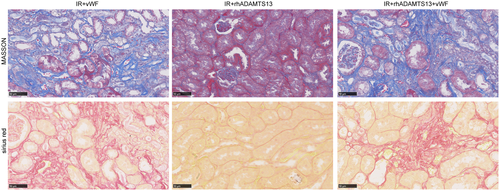
vWF is the activating substrate for ADAMTS13 and ADAMTS13 regulates platelet tethering by vWF.32 Mice with CKD treated with vWF alone or a mixture of vWF and rhADAMTS13 revealed extensive renal fibrosis (Figure 9).
3 DISCUSSION
We confirm that a reduction in ADAMTS13 and an increase in vWF accompany the development of oxidative stress in CKD. Our main new findings are that rhADAMTS13 prevents renal atrophy, glomerulosclerosis, tubulointerstitial fibrosis and renal dysfunction in mice with CKD (Figure 10).
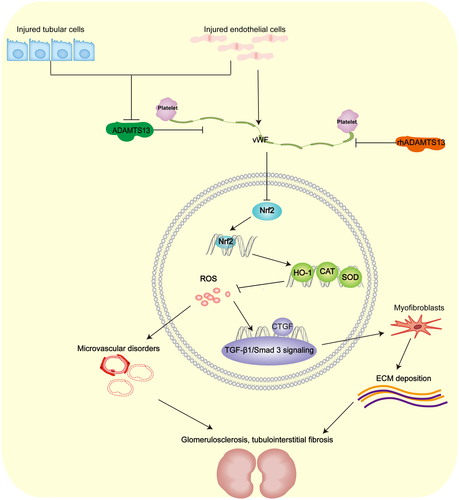
 , inhibitory action;
, inhibitory action;  , activated action; CAT, catalase; HO-1, haem oxygenase-1; CTGF, connective tissue growth factor; SOD, superoxide dismutase
, activated action; CAT, catalase; HO-1, haem oxygenase-1; CTGF, connective tissue growth factor; SOD, superoxide dismutaseIn the present study, mice with CKD are similar to AKI mice, with regard to reduced plasma levels of ADAMTS1312 and decreased renal expression of ADAMTS13. The observed increase in renal excretion of ADAMTS13 in CKD may contribute to its lower plasma level.
ADAMTS13 is expressed in renal tubular epithelial cells, endothelial cells and podocytes,33 that may therefore be sites of its protective effects in the kidney. This protective effect may be dependent on vWF, since vWF aggravated tubulointerstitial fibrosis in mice with CKD given rhADAMTS13, consistent with a previous study.34 Thus, the ADAMTS13-vWF axis may play an important role in the pathogenesis of CKD.
Oxidative stress is primarily involved in the transition to CKD by IR.35 It is linked to podocyte damage, proteinuria and tubulointerstitial fibrosis.36 ROS promotes fibrosis by activating TGF-β signalling that enhances myofibroblast activation and extracellular matrix production.37 Moreover, vascular oxidative stress impairs the regulation of afferent arterioles, which can lead to kidney damage in CKD.16, 26 The present study suggests that oxidative stress is enhanced by vWF, but inhibited by rhADAMTS13. The latter downregulated the TGF-β1/Smad3 pathway, attenuated afferent arteriolar ROS production and restored afferent arteriolar function. Thus, we put forward that rhADAMTS13 may limit renal damage and dysfunction in CKD by reducing ROS.
Nrf2 is a key transcription factor that inhibits oxidative stress.38 It is regulated by GSK3β and extracellular signal-regulated kinase (ERK).28 GSK3β determines the magnitude and duration of Nrf2 expression by facilitating nuclear exclusion and the degradation of Nrf2.28 ERK regulates Nrf2 expression.39 Since rhADAMTS13 regulates ERK expression during AKI in mice,12 we hypothesize that rhADAMTS13 upregulates Nrf2 expression, and thereby inhibits oxidative stress in mice with CKD. We indeed discovered that rhADAMTS13 improves nuclear, cytosolic and total Nrf2 expression that was linked to decreased GSK3β expression and increased p-GSK3β expression. These results suggest that ADAMTS13 activates Nrf2 by the inhibition of GSK3β.
There are important limitations to this study. Clinical CKD is more complex than IR. Whether rhADAMTS13 is effective in preventing other forms of CKD requires further exploration. Moreover, the mechanism by which ADAMTS13 regulates oxidative stress is still not fully clear. Lastly, except for oxidative stress, mechanisms behind the protective role of rhADAMTS13 in CKD remain unknown. Keeping these limitations in mind, there seems to be an important renoprotective role for ADAMTS13/vWF signalling during the transition from AKI to CKD.
4 MATERIAL AND METHODS
4.1 Animal experiments
Kidney ischaemia/reperfusion surgery was performed using male C57Bl/6 mice (8-12 weeks) purchased from the SLAC laboratory animal company (Shanghai, China) as previously reported.12 Both renal pedicles were exposed and cross-clamped by nontraumatic vascular clamps for 18 minutes or sham-operated. Sham-operated or IR mice were treated with vehicle or rhADAMTS13 (Sham + Vehicle; Sham + rhADAMTS13; IR + Vehicle; IR + rhADAMTS13). rhADAMTS13 (4245-AD, R&D Systems, Minneapolis, USA) at 0.03 nmol/kg body weight or vehicle was injected into the tail vein daily for the following 3 days after surgery, since this dose of rhADAMTS13 has been confirmed to protect against AKI in our previous study.12 To examine the interaction between rhADAMTS13 and vWF, in two other sets of experiments, CKD mice were treated with human vWF (Hematologic Technologies, Essex Junction, VT, USA) at 0.3 nmol/kg body weight alone (IR + vWF) or the mixture of rhADAMTS13 and vWF (IR + rhADAMTS13 + vWF) daily for the subsequent 3 days. The mice were killed by isoflurane anaesthesia. Two per cent isoflurane were used to induce anaesthesia and then 5% isoflurane made them lose consciousness at 1 week, 1 month and 3 months. Protocols and procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang University and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
4.2 Measurement of renal function and blood pressure
Mice were placed in metabolic cages (Tecniplast, West Chester, Pennsylvania, USA) for urine collection used for biochemical analysis before sacrificing.40 Mice were exposed to 24 hours of isolation in a metabolic cage with two wire-mesh floors for urine collection. Proteinuria was assessed using commercial kits (03183734190, Roche, Germany). BUN (04460715190, Roche, Germany) and serum creatinine (048106190, Roche, Germany) were used to estimate renal function using commercial kits. Plasma and urine KIM-1 were measured by enzyme-linked immunosorbent assay (ELISA) commercial kits (R&D Systems, Minneapolis, USA). And the mRNA levels of KIM-1 and NGAL were detected by RT-qPCR.
Direct catheterization was used to record blood pressure when mice were anaesthetized with 2% isoflurane as previously described.41 Briefly, the heparinized saline-filled PE-50 catheter was inserted into the left carotid artery and then the signal was detected. Blood pressure was calculated from the recorded signals. The blood pressure recordings were blinded to the observer.
4.3 Detection of NO, MDA, H2O2, enzymatic activity of SOD and CAT
NO was assessed from urinary nitrate (NO3−) + nitrite (NO2−) excretion (Beyotime Biotechnology, Shanghai, China).42 Renal lipid peroxidation was assessed from MDA detected by a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). SOD, CAT activity and H2O2 were assessed using activity assay kits (Beyotime Biotechnology, Shanghai, China).
4.4 RNA extraction and RT-qPCR
Total RNA was extracted from harvested kidneys (Axygen, San Francisco, USA), and cDNA was synthesized with TaKaRa Reverse Transcription System (TaKaRa, Dalian, China). RT-qPCR was tested with SYBR® Premix Ex Taq™ II (TaKaRa, Dalian, China) using the CFX96 Real-Time PCR Detection System (BioRad) and primers (Table 1).
| Primer sequence | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| AT1R | AACAGCTTGGTGGTGATCGTC | CATAGCGGTATAGACAGCCCA |
| AT2R | AACTGGCACCAATGAGTCCG | CCAAAAGGAGTAAGTCAGCCAAG |
| β-Actin | AGAGGGAAATCGTGCGTGAC | CAATAGTGATGACCTGGCCGT |
| ADAMTS13 | GAGGACACAGAACGCTACGTG | TTGGCCGTGATATTCGGAGTA |
| vWF | GGTAGACTTCGGAAACGC | GGAGCAGGAGCAAACATC |
| HO-1 | CTCCCTGTGTTTCCTTTCTCTC | GCTGCTGGTTT-CAAAGTTCAG |
| KIM-1 | AAACCAGAGATTCCCACACG | GTCGTGGGTCTTCCTGTAGC |
| NGAL | CACCACGGACTACAACCAGTTCGC | TCAGTTGTCAATGCATTGGTCGGTG |
| CAT | TCACTGACGAGATGGCACAC | ATCGAACGGCAATAGGGGTC |
| SOD2 | ACACATTAACGCGCAGATCA | AGCCTCCAGCAACTCTCCTT |
| Collagen I | GGGTCTAGACATGTTCAGCTTTGTG | ACCCTTAGGCCATTGTGTATGC |
| TGF-β | GCCACTGCCCATCGTCTACT | CACTTGCAGGAGCGCACAAT |
| CTGF | CTCGGACTGTGATGCCTTAAT | TGGATCCACACCTTGCATTTA |
| CCN3 | GAAGATTCCACGCCAATTCATC | GATCTGCCGGTTTCTCTTAGTC |
- Abbreviations: ADAMTS13, a disintegrin and metalloprotease with thrombospondin motifs 13; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor; CAT, catalase. SOD, superoxide dismutase; CCN3, nephroblastoma overexpressed gene; CTGF, connective tissue growth factor; HO-1, haem oxygenase-1; KIM-1, kidney injury molecule-1; NGAL, neutropil gelatinase-associated lipocalin; TGF-β, transforming growth factor-β; vWF, von Willebrand factor.
4.5 Enzyme-linked immunosorbent assay (ELISA)
Plasma was collected by retro-orbital puncture with 3.8% trisodium citrate. Urine was collected using metabolic cages (Tecniplast, West Chester, Pennsylvania, USA) before sacrifice. Subsequently, levels of ADAMTS13, and vWF in plasma and urine were measured using the commercially available ELISA kits, according to the manufacturer's instructions (ColorfulGene biological technology, Wuhan, China). Plasma and urinary KIM-1 were measured by KIM-1 ELISA commercial kits (R&D Systems, Minneapolis, USA) as recommended by the manufacturer.
4.6 Western blot analysis
Kidneys were homogenized on ice in lysis buffer for protein extraction. Equal amounts of proteins were loaded on 8% to 12% sodium dodecyl sulphate-polyacrylamide (SDS-PAGE) gels and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skim milk and incubated overnight at 4°C with the following primary antibodies: collagen Type I (14695-1-AP, Proteintech), α-SMA (ab5694, Abcam), TGF-β1 (ab179695, Abcam), Smad3 (ab40854, Abcam), phosphorylation of Smad3 at Serine423 and Serine425 (p-Smad3) (ab52903, Abcam), mouse ADAMTS13 (NB110-82382, Novus Biologicals), Nrf2 (ab156883, Abcam, Cambridge, UK), Keap1 (ab227828, Abcam), GSK3β (124456, CST), phosphor (p)-GSK3β (Ser9) (5558, CST), β-actin (4970, CST, Cell Signaling Technology and is from Beverly, MA, USA) and Histone H3 (AF0009, Beyotime). Thereafter, membranes were washed three times with TBST buffer, they were probed with the respective horseradish-peroxidase-conjugated secondary antibodies (Beyotime Biotechnology, Shanghai, China) for 1 hour at room temperature and then visualized by an automated imaging analysis system (Tanon 5200 Multi, Tanon Science & Technology Inc, Shanghai, China). The bands were analysed using Image J software. β-actin or Histone H3 was used as loading control and protein expression was normalized to β-actin or Histone H3. The protein expression of Sham + Vehicle group was used as control to assess the relative density.
4.7 Histopathological staining and immunohistochemistry
Paraffin-embedded formalin-fixed 3µm sections were stained with PAS, Masson's trichrome, Sirius red and immunohistochemistry (IHC) for ADAMTS13, α-SMA, collagen I, markers of myofibroblasts. Tubulointerstitial fibrosis was characterized by collagen deposition in kidneys.19 Masson's trichrome staining is a classical method to detect collagen deposition in kidneys, by which the collagenous fibrosis tissue is stained blue, while the other area is stained red, so renal fibrosis is evaluated by Masson trichromatic staining.20 Collagen deposits are stained red using Sirius Red staining.42 Injured tubules were identified by tubular dilation, loss of brush border, tubular necrosis or cast formation. All the histopathological staining and immunohistochemistry results were assessed by a renal pathologist quantitatively in a blinded fashion.
4.8 ROS measurement of afferent arterioles
ROS generation was quantified via DHE (D23107, Invitrogen, Paisley, UK) and H2DCFDA (HY-D0940, MedChemExpress, Monmouth Junction, USA), as described previously.27 Briefly, the isolated afferent arterioles were incubated with DHE (40 μmol/L) or H2DCFDA (10 μmol/L) staining solution in Dulbecco's modified eagle medium (DMEM) in the dark for 30 minutes at 37°C, then harvested with 0.05% trypsin-EDTA solution, suspended in a fresh DMEM, and immediately analysed with a fluorescent microscope (Olympus, Japan). When reacting with ROS, DHE was oxidized to E and H2DCFDA induced fluorescence. The fluorescent ratio of E to DHE (E: DHE) and the fluorescence of H₂DCFDA were used to detect ROS production in renal afferent arterioles.
4.9 Measurement of afferent arteriolar response to vasoactive agents
A glomerulus with the attached afferent arteriole was isolated and perfused as previously described.12 Increasing doses of ACh (10−9 to 10−5 mol/L) were used to test endothelium-dependent relaxation in renal afferent arterioles under pre-constriction with norepinephrine (NE) 1 × 10−6 mol/L.43 Increasing doses of Ang II (10−12 to 10−7 mol/L) were used to assess the constriction of renal afferent arterioles.27 The arteriolar luminal diameter was calculated as the mean of seven images during the relatively stable oscillation state.
4.10 Statistics
Values are reported as mean ± SEM from data assessed using Prism version 6.0 software (GraphPad Software Inc, La Jolla, CA). Data comparison was performed using unpaired Student's t test, one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test and two-way ANOVA where appropriate. The correlation among the data was evaluated by Pearson's test. Statistical significance was assumed for P < .05.
ACKNOWLEDGEMENTS
This work was supported by grants to Lai EY from National Nature Science Foundation of China (31671193) and the Key Research Development Program of Ningxia (2018BFG0210), Lai EY is a Mercator Fellow supported by the German Research Foundation (CRC 1365). Grants to Patzak A and Persson PB from the German Research Foundation (DFG PA 479/10-2) and (CRC 1365), and to Yali Zheng from National Nature Science Foundation of China (81860136) and the Key research development program of Ningxia (2018BFG0210), to Yixin Liao from National Nature Science Foundation of China (81801450). Thanks to Dr Fei Gao, Durbrain Medical Laboratory, Hangzhou, China, for his support and exam the urine samples using the LC-MS/MS method.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.




