Adult vascular dysfunction in foetal growth-restricted guinea-pigs is associated with a neonate-adult switching in Nos3 DNA methylation
Bernardo J. Krause and Emilio A. Herrera equally contributed to the design of this study.
Abstract
Aim
Foetal growth restriction (FGR) is associated with endothelial dysfunction and cardiovascular diseases in adult subjects. Early vascular remodelling and epigenetic changes occurring on key endothelial genes might precede this altered vascular function. Further, it has been proposed that oxidative stress during development may determine some of these epigenetic modifications. To address this issue, we studied the in vivo and ex vivo vascular function and Nos3 promoter DNA methylation in arteries from eight-month-old guinea-pig born from control, FGR-treated and FGR-NAC-treated pregnancies.
Methods
Femoral and carotid arteries in vivo vascular function were determined by Doppler, whilst ex vivo vascular function and biomechanical properties were assessed by wire myography. Levels of eNOS mRNA and site-specific DNA methylation in Nos3 promoter in aorta endothelial cells (AEC) were determined by qPCR and pyrosequencing respectively.
Results
FGR adult showed an increased femoral vascular resistance (P < .05), stiffness (P < .05) and arterial remodelling (P < .01), along with an impaired NO-mediated relaxation (P < .001). These effects were prevented by maternal treatment with NAC. Endothelial-NOS mRNA levels were decreased in FGR adult compared with control and FGR-NAC (P < .05), associated with increased DNA methylation levels (P < .01). Comparison of Nos3 DNA methylation in AEC showed a differential methylation pattern between foetal and adult guinea-pigs (P < .05).
Conclusion
Altogether, these data suggest that adult vascular dysfunction in the FGR does not result from early changes in Nos3 promoter DNA methylation, but from an altered vessel structure established during foetal development.
1 INTRODUCTION
Foetal growth restriction (FGR) represents any condition that constrains or negatively alters foetal growth trajectory and is clinically defined as foetuses that show impaired intrauterine growth (estimated foetal weight below the 10th percentile for its gestational age), along with vascular compromise (eg, altered placental or foetal artery Doppler indexes).1 Babies born with FGR have increased perinatal morbidity and mortality and in the long-term greater risk of developing cardiometabolic diseases at adulthood.2, 3 The later would be partially as consequence of epigenetic changes in key vascular genes, such as eNOS, that will be set during foetal life, but also influenced by vascular morphological and functional alterations that result from the chronic blood flow redistribution occurring in response to the restricted foetal nutrient and oxygen delivery.3, 4 In this context, hypoxia and oxidative stress have been postulated as driving forces of the adult cardiovascular risk resulting from FGR.4, 5 However, the contribution of early vascular remodelling and epigenetic mechanisms in the long-lasting programming of FGR-associated cardiovascular dysfunction remains elusive.
The long-lasting effects of an impaired foetal growth on vascular function are expressed since stages of life.6 For instance, a decreased endothelial function is observed in small for gestational age neonates,7 as well as in umbilical and chorionic arteries derived from FGR placentas.8, 9 These traits of FGR-associated endothelial dysfunction persist in vitro,9-11 characterized by altered expression of proteins involved in NO-dependent vasodilation (ie, eNOS and arginase). Specifically, altered expression of eNOS in FGR-derived endothelium results from diverse epigenetic modifications in the NOS3 gene promoter,10, 11 an effect that can be reprogrammed to a “normal type” by knocking-down DNMT1.10 Additionally, we recently reported that FGR results in an altered eNOS expression and Nos3 gene promoter DNA methylation in a guinea-pig model of FGR, which is comparable between foetal systemic and umbilical arteries.12 Notably, maternal antioxidant treatment with N-acetylcysteine (NAC) restored foetal growth by enhancing placental efficiency and reverting the functional and epigenetic programming of eNOS in arterial endothelium.12 However, there are no studies addressing whether this epigenetic alteration in Nos3 or other endothelial-related genes is maintained until adulthood as an early origin of the endothelial dysfunction.
Here, we proposed that adult Nos3 promoter methylation in arterial endothelial cells is established during foetal development and this is affected by FGR that primes epigenetic programming on adult vascular dysfunction. To address this hypothesis, we studied the endothelial function in carotid and femoral arteries, as well as the eNOS mRNA levels and Nos3 promoter DNA methylation pattern in aortic endothelial cells from foetuses and adult offspring from an FGR guinea-pig model. To rule out the contribution of an early vascular remodelling, biomechanical properties of carotid and femoral arteries were determined. Furthermore, the effects of an antenatal antioxidant treatment were assessed on the endothelial function and vascular properties.
2 RESULTS
2.1 Neonatal and post-natal growth
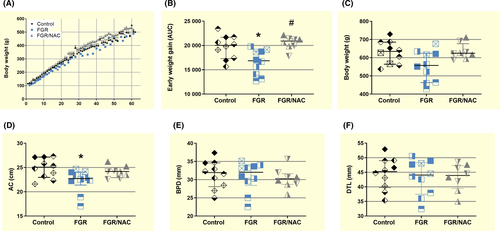
Gestational age at birth (ie, the length of gestation) was higher in the FGR group compared with control and NAC-treated FGR neonates, without significant changes in the birthweight (BW) (Table 1). Conversely, adjusted birthweight by the length of gestation (ABW) was lower in FGR compared with controls. Furthermore, biparietal diameter (BPD), digito-talsal length (DTL) and abdominal circumference (AC) were lower in FGR neonates. The decrease in these parameters occurs along with a reduced AC-to-BW and DTL-to-BW ratios, but an increased BPD-to-DTL ratio, in FGR neonates, suggesting an impaired foetal growth. Conversely, FGR/NAC neonates showed a decreased BPD but an increased BPD-to-DTL ratio compared with control (Table 1).
| Control (n = 10) | FGR (n = 6) | FGR/NAC (n = 6) | |
|---|---|---|---|
| Length of gestation (days) | 67.3 ± 0.3 | 69.6 ± 0.2* | 66.6 ± 0.2# |
| BW (g) | 110.6 ± 3.8 | 99.5 ± 5.1 | 93.0 ± 6.0 |
| ABW (g) | 110.6 ± 3.8 | 85.6 ± 4.4* | 96.8 ± 6.2 |
| BPD (mm) | 20.8 ± 0.4 | 18.3 ± 0.4* | 18.7 ± 1.0# |
| DTL (mm) | 35.3 ± 0.9 | 31.3 ± 0.4* | 32.0 ± 1.3 |
| AC (cm) | 11.9 ± 0.6 | 9.9 ± 0.4* | 10.1 ± 0.4 |
| BPD/BW | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.21 ± 0.01# |
| AC/BW | 0.12 ± 0.01 | 0.09 ± 0.01* | 0.11 ± 0.01 |
| DTL/BW | 0.37 ± 0.02 | 0.30 ± 0.01* | 0.37 ± 0.02# |
| BPD/DTL | 0.51 ± 0.02 | 0.61 ± 0.01* | 0.58 ± 0.01* |
Notes
- Birthweight (BW), gestational age-adjusted birthweight (ABW), biparietal diameter (BPD), digito-talsal length (DTL), abdominal circumference (AC). Values expressed as mean ± SEM. Statistical differences (one-way ANOVA, Bonferroni post-test):
- * P < .05 vs control
- # P < .05 vs IUGR.
Post-natal growth during the first 60 days was lower in FGR compared with control and FGR/NAC offspring (Figure 1A,B). This effect in FGR offspring was also observed at 8 months of age resulting in a reduced AC (Figure 1D), without changes in body weight (Figure 1C), BPD (Figure 1E) or DTL (Figure 1F).
2.2 Vascular function in adult FGR offspring
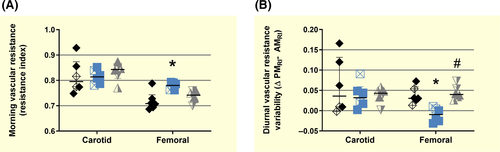
In order to identify in vivo markers of vascular dysfunction, carotid and femoral artery resistance index were followed at morning and evening times during 30 days in 7-month-old guinea-pig offspring. Morning-carotid artery resistance index was not different among control, FGR and FGR/NAC guinea-pigs (Figure 2A). Conversely, FGR showed a higher morning-femoral artery resistance index compared with control and FGR/NAC. Carotid artery resistance index was higher in the evening compared with morning, without differences among groups (Figure 2B). Similarly, femoral artery resistance index was higher in the evening compared with morning in control and FGR/NAC guinea-pigs; however, FGR offspring showed no diurnal variation in femoral artery resistance index (Figure 2B).
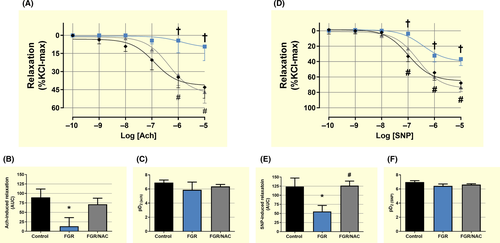
Ex vivo vascular reactivity showed no changes in Ach- and SNP-induced responses in foetal carotid arteries among FGR, control and FGR/NAC groups (Figure S2) In contrast, carotid arteries from FGR adult guinea-pigs showed a decreased L-NAME-inhibited relaxing response to acetylcholine (Ach) compared with control and FGR/NAC guinea-pigs (Figure 3A,B), without differences in the sensitivity among groups (Figure 3C). Similarly, SNP-dependent relaxation was reduced (Figure 3D,E), without changes in the sensitivity (Figure 3F), in FGR carotid arteries compared with control and FGR/NAC offspring.
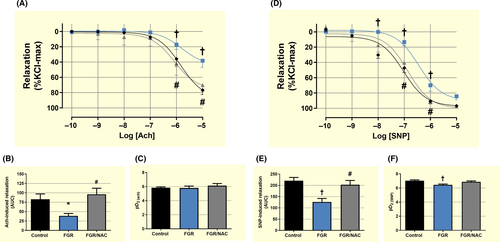
Conversely, no changes in maximal responses to Ach and SNP were observed in foetal femoral arteries; however, sensitivity to both agents was decreased in FGR vessels compared with control (Figure S3). Relaxing response to Ach was reduced in femoral arteries from FGR adult guinea-pigs compared with control and FGR/NAC guinea-pigs (Figure 4A,B), without differences in the sensitivity among groups (Figure 4C). However, maximal SNP-dependent relaxation (Figure 4D,E) and sensitivity (Figure 4F) were reduced in FGR femoral arteries compared with control and FGR/NAC offspring.
2.3 Biomechanical and morphological properties of carotid and femoral arteries in adult FGR offspring
Histological analysis of carotid arteries showed no differences in the relative area of the intima, media and adventitia among adult guinea-pig groups (Figure 5A,B). This effect was also observed in the biomechanical response in stretch-strain curves (Figure 5C and Table 3), with no differences in the initial (E1) or the final (E2) slopes, as well as the elbow point (λc) of the experimental curves among groups. In contrast, adult FGR carotid arteries showed an increased opening angle compared with control and FGR treated with NAC (Figure 5D).
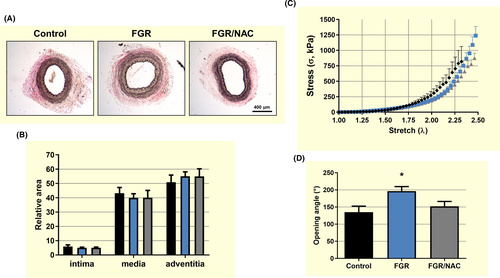
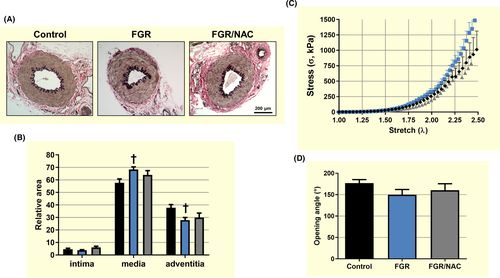
Adult FGR femoral arteries showed an increased media area and a decreased adventitia area compared with control (Figure 6A,B) that was associated with an increased biomechanical stiffness in the stretch-strain curve (Figure 6C) determined by a lower elbow point (λc) (Table 2). Conversely, there were no differences in other biomechanical properties (Table 2) including the opening angle among femoral arteries from adult guinea-pig groups (Figure 6D).
| Control (n = 6) | FGR (n = 6) | FGR/NAC (n = 6) | |
|---|---|---|---|
| Carotid artery | |||
| E1 [kPa] | 34.5 ± 4.4 | 35.9 ± 2.0 | 44.6 ± 4.8 |
| E2 [kPa] | 4317.1 ± 455.2 | 3704.9 ± 292.6 | 3998.5 ± 234.3 |
| λc | 1.99 ± 0.06 | 2.14 ± 0.03 | 2.10 ± 0.02 |
| σc | 274.0 ± 26.6 | 279.3 ± 17.2 | 286.1 ± 27.4 |
| Femoral | |||
| E1 [kPa] | 30.2 ± 5.9 | 26.0 ± 2.0 | 29.9 ± 4.1 |
| E2 [kPa] | 3912.4 ± 448.5 | 4091.2 ± 310.5 | 3802.0 ± 184.5 |
| λc | 1.99 ± 0.04 | 1.85 ± 0.04* | 2.08 ± 0.06# |
| σc | 289.7 ± 40.0 | 251.9 ± 22.7 | 198.3 ± 16.9 |
Notes
- E1, initial slope; E2, final slope; λc, elbow/transition point; σc, Cauchy stress at the transition point. Values expressed as mean ± SEM. Statistical differences (one-way ANOVA, Bonferroni post-test):
- * P < .05 vs. control;
- # P < .05 vs. FGR.
2.4 Aorta endothelial cell eNOS expression and adult/foetal Nos3 DNA methylation
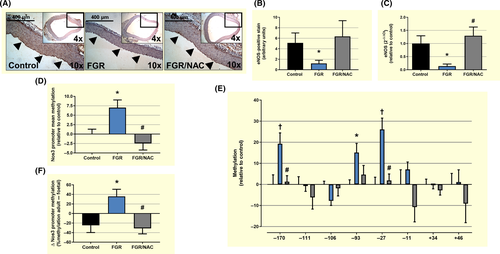
Relative in situ eNOS protein levels, determined by IHC in paraffin-fixed aorta samples, showed a decrease in eNOS at the endothelial layer in FGR adult subjects, but comparable levels between control and FGR/NAC groups (Figure 7A,B). Similarly, levels of eNOS mRNA in cultured aorta endothelial cells were decreased in FGR adult guinea-pigs compared with control and FGR/NAC adults (Figure 7C). Conversely, comparison of global DNA methylation of Nos3 promoter among adult subjects showed higher levels of DNA methylation in FGR endothelial cells compared with control (Figure 7D). This increase in Nos3 promoter DNA methylation was associated with a substantial increase in the methylation levels of 3 specific sites (CpGs −170, −93 and −27) (Figure 7E). Furthermore, comparison of global DNA methylation of Nos3 promoter between adult and foetal (Figure S4A and B) subjects from the same group showed a life-course increase in Nos3 promoter DNA methylation in FGR adults, an effect absent in control and FGR/NAC groups (Figure 7F). This switch in FGR adults was mainly due to an increased DNA methylation CpG −170 and CpG −27 (Figure S4C).
3 DISCUSSION
This study aimed to demonstrate whether adult endothelial dysfunction resulting from being foetal growth-restricted is mediated by in utero-oxidative stress-mediated epigenetic programming of eNOS expression. To address this issue, we studied the in vivo and ex vivo vascular function and Nos3 promoter DNA methylation in arteries from eight-month-old guinea-pig born from control, FGR-treated and FGR-NAC-treated pregnancies. Adult FGR guinea-pigs showed an increased peripheral resistance index and signs of vascular remodelling in femoral arteries, along with an impaired endothelial function in carotid and femoral arteries. Additionally, in adult FGR aorta endothelial cells there was a decreased eNOS expression associated with an increased Nos3 promoter methylation. Notably, these alterations in vascular and endothelial function were prevented by maternal treatment with NAC. Life-course follow-up showed that Nos3 promoter was differentially methylated between FGR foetuses and adults; however, the impairment in endothelial function and signs of vascular remodelling were comparable within this group. Altogether, these data suggest that long-term endothelial dysfunction in the FGR is mainly due to an early vascular remodelling rather than an intrauterine DNA methylation-dependent programming of key endothelial genes.
Compelling evidence shows that foetal growth restriction is associated with a lower adult height13, 14 and long-term endothelial and cardiovascular dysfunction.2, 3 Here, we determined whether FGR resulting from a progressive occlusion of uteroplacental blood flow15, 16 impairs post-natal growth and long-term endothelial function, as well as the preventive effect of the maternal treatment with NAC on this intrauterine programming. In this model, we observed a delay in the delivery with no changes in neonatal weight in the FGR group, whilst the NAC treatment induced the inverse effect. However, the correction of birthweights to the length of gestation reported for the control group suggests that neonates from dams in which the uterine arteries were occluded have a lower weight, confirming previous results, from our group and others,15, 16 when term foetuses is delivered by C-section at a comparable gestational age. Nonetheless, neonates from the FGR group showed markers of an impaired intrauterine growth at birth, as suggested by shorter hindlimb bones and the asymmetry between the head circumference and abdominal circumference. Similar results have been reported elsewhere in young adult FGR guinea-pigs,17, 18 effects that were partially reverted by the NAC treatment. This impaired foetal growth was projected at short-term by a reduced post-natal weight gain, as well as a reduced abdominal circumference as adults (8-month-old). Here, we provide further evidence that NAC, a dietary supplement with very limited adverse effects in pregnancy,19 prevents the immediate and long-term effects on post-natal growth trajectory in a model of suboptimal uteroplacental blood flow. In this context, these data show that the present guinea model of FGR shows comparable alterations in post-natal growth and risk of stunting as has been observed in humans and support the relevance of guinea-pig models for studying long-term consequences of an altered foetal development,20 as well as the potential use of NAC as therapeutic agents during pregnancy.19
An impaired foetal growth is associated with long-term endothelial dysfunction and vascular remodelling that can be observed as early as birth. In fact, low birthweight neonates show a reduced peripheral endothelial-dependent relaxation,7 an effect that can be also observed in placental and umbilical arteries from FGR pregnancies.8, 21 This impaired endothelial function is also observed in low birthweight children.22, 23 Additionally, an impaired foetal growth in humans is associated with vascular remodelling in the aorta at birth,24 as well as an increased aortic stiffness at childhood.25 Recent reports show that growth-restricted guinea-pig foetuses have an impaired NOS-dependent relaxation in systemic and umbilical arteries12 which occurs in parallel with pro-constrictive vascular remodelling in the aorta and femoral arteries, but not in the carotid artery.26 In this study, FGR adult guinea-pigs showed an increased in vivo femoral vascular resistance with no diurnal variability, but a normal resistance in the carotid artery. These in vivo parameters from carotid and femoral arteries were paralleled by biomechanical properties and structural characteristics. Similar markers of increased arterial stiffness have been reported in young,17 adult18 and aged27 FGR guinea-pigs. These data reinforce that arterial remodelling during late gestation is a driving force for long-term vascular function28 and contribute to the cardiovascular risk resulting from an impaired foetal growth.
Conversely, NOS-dependent vascular relaxation and NO-dependent vascular relaxation were substantially impaired in carotid and femoral arteries in adult FGR guinea-pigs, but not in FGR/NAC, in terms of sensitivity and maximal response. It worth to mention that FGR prevention by the maternal treatment with NAC, an effect that could result from an increased placental efficiency,12 contributes to revert the long-term vascular effects of a decreased uteroplacental blood flow. Notably, a mild impairment in the endothelial-dependent relaxation was present at the end of gestation in femoral but not carotid arteries from FGR subjects, suggesting a bed-specific dysfunction and remodelling that worsens during the life course, as has been observed in other models of FGR.18, 29, 30 Altogether, these findings support the notion that an early vascular remodelling and progressive impairment in the endothelial function are in the basis of the long-term vascular dysfunction of the FGR.
It has been proposed that early activation of epigenetic mechanisms during foetal development would prime the endothelial and vascular dysfunction observed in adults born with FGR, establishing an altered gene expression at long-term.3 It is worth to note that, gene promoter DNA methylation levels are not always associated with gene expression. Genome-wide and gene-specific DNA methylation analysis in endothelial cells from different sources show that transcript levels of certain genes (ie, arginase-2) are independent of their gene promoter DNA methylation, whilst others (ie, eNOS) show an inverse relationship between the promoter DNA methylation and the mRNA levels.10, 31 This relationship between NOS3 promoter DNA methylation and eNOS mRNA levels has been reported as an important mechanism regulating the endothelial-specific expression of eNOS.32 Conversely, studies in animal models show that foetal exposure to hypoxia33 or a suboptimal uteroplacental perfusion12 induces a DNA methylation-mediated eNOS upregulation, but impaired endothelial function in foetal systemic and umbilical arteries. Similarly, human umbilical artery endothelial cells from pregnancies affected by FGR show an increased eNOS expression that results from permissive histone post-translational modifications,11 as well as DNMT1-mediated decreased NOS3 promoter DNA methylation.10 However, no studies have determined whether this altered epigenetic programming of eNOS expression is preserved until adulthood. At our best knowledge, this study reports for the first time the life-course DNA methylation status of Nos3 gene promoter, and its association with eNOS mRNA levels, in endothelial cells from animals affected by FGR. Adult FGR guinea-pigs showed lower eNOS transcript levels, paralleled by a global and site-specific increased Nos3 promoter DNA methylation, compared with control and FGR-treated adults. Site-specific changes occurred in the context of consensus sequences for transcriptional factors related to basal eNOS expression (Figure S5).32 Furthermore, aorta endothelial cells from FGR guinea-pigs showed an increased Nos3 promoter DNA methylation compared with FGR foetuses, confirming that DNA methylation in this gene is strongly associated with the actual eNOS gene expression,34 and suggesting that this trait is not defined at long-term during foetal development. Previous studies in FGR animal models show that eNOS expression and activity are dynamically regulated during the life course, suggesting an early upregulation12, 33 that is maintained in young adult subjects,35 as well as a downregulation at long-term.29, 30 A comparable pattern in life-course eNOS expression has been reported in the genetic model of spontaneously hypertensive rats.36
Intrauterine oxidative stress has been implicated in the cardiovascular programming resulting from an altered, either increased or decreased, foetal growth.5 Glutathione metabolism plays a key role in controlling the activation of redox signalling pathways, reactive oxygen species reduction and cellular redox status, under physiological and pathological conditions.37, 38 In this context, low birthweight in humans is associated with oxidative stress and an impaired glutathione metabolism in the mother and the foetus.39, 40 In this study, antenatal maternal treatment with a glutathione precursor (NAC) was tested, in order to prevent the long-lasting vascular effects of FGR. Previous studies using diverse models of FGR in guinea-pigs have demonstrated that maternal treatment with NAC reduces markers of oxidative stress in different cardiovascular tissues.12, 41-43 The present results showed that antenatal treatment with NAC was associated with a normal perinatal and post-natal growth, and endothelial function. Furthermore, NAC treatment prevented the FGR-induced vascular remodelling and changes in Nos3 promoter DNA methylation in both foetal and adult subjects. Altogether, these results, along with the FDA-approved use of NAC for intrauterine interventions,19 support the potential use of glutathione precursor for preventing the short- and long-term effects of FGR. However, it remains to be determined whether the post-natal period could be used as another therapeutic window as has been suggested elsewhere,44 especially considering the life-course changes in DNA methylation.
In conclusion, these data suggest that conditions that restrain foetal growth result in premature and permanent impaired vascular function. In this regard, life-course regulation of vasodilator pathways in the FGR endothelium may result from multiple factors that lead to a pro-hypertensive environment (eg, early vascular remodelling) rather than an early and persistent epigenetic programming. In this context, antenatal maternal treatment with NAC was able to prevent most of the effects, suggesting that foetal oxidative stress might have an important role in vascular remodelling and programming. Further studies are required to confirm whether these life-course changes in epigenetic markers occur on other key vascular genes and elucidating the mechanisms underlying the epigenetic modifications, as well as the post-natal intervention opportunities associated with those mechanisms.
4 MATERIALS AND METHODS
4.1 Ethics statement
All animal care, procedures and experimentation were approved by the Ethics Committee of the Faculty of Medicine, from the Pontificia Universidad Católica de Chile (1130801) and Universidad de Chile (protocol CBA# 0694 FMUCH), and conducted in accordance with the ARRIVE guidelines and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
4.2 Animals and experimental design
Twenty-nine adult female Pirbright White guinea-pigs (Cavia porcellus) were used for this study. All animals were housed in individual cages under standard conditions (30%-35% humidity, 21-22°C and a 12-: 12-hour light-dark cycle), with controlled food-by-body weight intake with a commercial diet (LabDiet 5025, guinea-pigs, 20-30 g/day). After confirming pregnancy, the pregnant guinea-pigs were randomly assigned to the control (n = 10) or FGR (n = 16) group. Additionally, starting on day 34 of pregnancy half of the sows from the FGR group were treated with 500 mg/kg/day of N-acetyl cysteine 12 (Sigma-Aldrich, cat #A7250) in the drinking water, adjusted daily to maternal body mass. All pregnant sows were subjected to aseptic surgery on gestation day 35, either sham-operated (control), or to progressive uterine artery occlusion (FGR).12, 15 Briefly, under general anaesthesia (ketamine 60 mg/kg, xylazine 4 mg/kg and atropine 0.1 mg/kg IM) an infra-umbilical midline laparotomy was performed, exposing the gravid uterus. In the FGR group, ameroid constrictors (COR-2.00-SS; NW Research Instruments, Inc, USA) were placed bilaterally around the base of each uterine artery. The abdominal wall and skin were then sutured in layers with absorbable sutures (Vicryl 4/0; Ethicon, USA). Finally, surgical staples (AutoSuture; Covidien, Dominican Republic) were installed in the skin. As part of this procedure, animals received analgesia (carprofen 4 mg/kg SC) and prophylactic antibiotic (20 mg oxytetracycline/kg SC) treatments. The skin staples were removed 7-8 days after surgery. The control group underwent the same surgical procedure, but without placement of the ameroid constrictors. After the surgical intervention, foetal viability was confirmed and sows only carrying 2 foetuses were considered for further studies, and this resulted in the exclusion of two dams that had only one viable foetus after surgery, and one litter in which a neonate showed impaired limb mobility after birth.
4.3 Euthanasia at near term
At 60-62 days of gestation (~90% of pregnancy), part of the control (n = 4), FGR (n = 4) and FGR/NAC (n = 4) sows and their foetuses were sacrificed with a maternal anaesthetic overdose (sodium thiopental 150 mg/kg IP, Opet, Laboratorio Chile). Remaining sows [control (n = 5), FGR (n = 5) and FGR/NAC (n = 4)] were allowed to deliver and their offspring [control (n = 10), FGR (n = 10) and FGR/NAC (n = 8)] followed up until adult age.
4.4 Post-natal follow-up and adult euthanasia
Post-natal body weight (BW) follow-up was determined using a digital scale (CTA SCALE, CTA 30E). Further, biparietal diameter (BPD) and digito-talsal length (DTL) were measured weekly with a 6″ Digital Vernier Caliper (Harbor Freight Tools, Pittsburgh, CA), and the abdominal circumference (AC) using a metric tape. Further, at 7 months old, we assayed daily, until the euthanasia, carotid and femoral Doppler flow analysis by ultrasound (SonoVet R3, Samsung Medison), establishing the resistance index (RI). Ultrasound measurements were performed on every adult, in awake condition, at 8-10 am and 17-19 pm. At 8 months old, animals were sacrificed with an anaesthetic overdose (sodium thiopentone 200 mg/kg IP, Opet, Laboratorio Chile). Once confirmed the cardio-respiratory arrest, femoral, carotid and aortic arteries were carefully obtained and clean.
4.5 Carotid and femoral artery vascular function
Carotid and femoral arteries were excised, and 2-mm proximal segments were mounted on a wire myograph (610M System Myograph Multiwire, DMT) to determine vasoactive responses as previously reported.12 In order to determine the NOS-dependent vasodilation, vessels were pre-constricted with half-maximal KCl concentration (40.8 mmol/L) and the isometric force in response to cumulative concentrations of acetylcholine (10−8-10−5 mol/L) was determined as the difference between the responses in the presence and absence of a NOS inhibitor (L-NAME, 100 µmol/L). The NOS-independent response to NO was determined with sodium nitroprusside (SNP, 10−9-10−5 mol/L) in pre-constricted vessels.12
4.6 Passive response
Vessel segments of 2 mm of carotid and femoral arteries were mounted in a wire myograph for stretch-stress analysis as previously reported.26 The passive response measurements consists in to hold by hooks a vessel ring, and subject the sample to a radial elongation. During the test, the load (F) and the displacement of the hooks (Δ) were recorded. Five parameters were derived to represent the passive mechanical responses of the arterial wall: the magnitude of the slope at the beginning (E1) and at the end (E2) of the stress-stretch curve, the stress at the elbow of the stress-stretch curve (σc) and the stretch and stress at the breaking point (λR, σR).
4.7 Ring opening test
For the determination of residual stress in the different arteries studied, opening angle measurements in vessels rings were performed as reported.26 The opening angle was used to measure the angle (α) subtended by the ends after making a radial cut of a circular segment of the artery. Briefly, the ring was immersed in calcium-free Krebs at 37°C ± 1°C for about 5 minutes, and subsequently cut radially and photographed after 20 minutes. With this, the internal diameter is obtained from the circumference that best fit the open ring and the mean thickness was considered as the mean of 5 measurements of each sample.
4.8 Histology and immunohistochemistry
Arterial intima, media and adventitia areas were determined using Verhoeff-Van Gieson stains. Elastic tissue stain was freshly prepared by mixing 30 ml of 5% alcoholic haematoxylin, 12 ml of 10% ferric chloride and 12 ml of iodine lugol. Tissue sections were submitted to Verhoeff stain for 20 minutes and then rinsed in distilled water. Then, sections were differentiated with 2% ferric chloride until the elastic fibbers were distinguished and the background was colourless to light grey. Slides were rinsed in distilled water, placed in sodium thiosulphate for 1 minutes and washed in running tap water. Sections were counterstained in Van Gieson stain for 2 minutes and differentiated in 95% alcohol.
For immunohistochemistry, deparaffinized and rehydrated histological sections of 4 mm were subjected to heat-induced antigen retrieval using 100mmol/l citrate buffer (pH 6.0). Samples were treated with 3% H2O2 in PBS for 30 minutes to quench endogenous peroxidase activity. After rinsing in PBS for 5 minutes, all slides were incubated for 1 hour with protein block solution (Cas-Block; Zymed Laboratories, South San Francisco, CA, USA). Sections were incubated for 18 hour at 4°C with primary anti-eNOS (BD Transduction Laboratories, #610296). Immunostaining was performed using HRP-conjugated secondary antibody and binding was determined with NovaRED Kit (Vector, Burlingame, CA, USA), treating for 4 minutes. Slides were counterstained with Harris haematoxylin and permanently mounted. The specificity of the staining was determined by incubation of sections in the absence of the primary antibody.
Microphotographs were obtained at 10x and 40x magnifications with an Olympus CX31 microscope (Olympus Corporation, Tokyo, Japan). Intima, media and adventitia absolute areas (mm2) were determined using the Image J with a calibrator image for each magnification. Relative area was calculated as [(specific area)/(intima + media + adventitia)].
4.9 Primary cultures of aorta endothelial cells
Primary cultures of endothelial cells from foetal and adult aortae were obtained according to the method proposed by Chen and colleagues45 with some modifications.12 Briefly, vessel samples (1-2 cm long) were cut into pieces of ~1 mm2 which were seeded and maintained at 37°C in a 25-cm2 plate with Medium 131 and 20% MVGS. After 60 hours, the tissue was gently removed and the plate was washed with phosphate-buffered saline and fresh culture medium was added. The endothelial phenotype was confirmed by immunocytochemistry, using vWF (SAB1402960, Sigma, USA) and CD31 (P8590, Sigma, USA) antibodies (Figure S1). Previous to RNA and DNA extraction, cells were starved overnight in Medium 131 and 2% MVGS. All these determinations were carried out in cells at third passage.
4.10 Quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen), and polymerase chain reactions were performed as described.12 Aliquots of 1 μg of total RNA were reverse-transcribed using ImPROM-II RT Kit (Promega). Three different RNAs, the ribosomal RNA 18S (sense, 5′-TGC ATG GCC GTT CTT AGT TG; antisense, 5′-AGT TAG CAT GCC AGA GTC TCG TT-3′), the messenger RNA for β-actin (sense, 5′-AAC GAT GCC GTG CTC AAT G-3′; antisense ATA TCG CTG CGC TCG TTG TC) and RPLP2 (ribosomal protein lateral stalk subunit P2) (sense, 5′-GCG CCA AGG ACA TCA AGA AG-3′; antisense, 5′-CCA GCA GGT ACA CTG GCA A-3′), were used as reference genes for eNOS (sense, 5′-AGC CAA CGC GGT GAA GAT C-3′; antisense, 5′-TTA GCC ATC ACC GTG CCC-3′) mRNA quantification by SYBR® Green Real-time PCR. Quantification was carried out using the geometrical average of 2-ΔΔCT for eNOS relative to the three different reference genes.
4.11 Analysis of DNA methylation
Methylation status of the promoter region of Nos3 guinea-pig gene was determined using DNA bisulphite modification coupled with DNA sequencing.10, 12 DNA was isolated from ~10−6 EC using DNeasy Blood & Tissue Kit (Qiagen), and 500 ng of total DNA extracts was treated with sodium bisulphite using EpiTect Bisulfite Kit (Qiagen). Promoter regions were amplified by PCR using specific primers for Nos3 (Table 3). Average core promoter and site-specific CpG methylations were determined as percentage using a PyroMark Q96 MD (Qiagen).
| CpGs −220 to −170a | |
| Forward | 5′-GTT TTT TATA TAA TGGG ATA GGA ATA AGG T-3′ |
| Reverse | biotin5′-CCC AAC CAA CCT TAT TCC TAT-3′ |
| Sequencing | 5′-GTT GGG AGG TTT TGA A-3′ |
| CpGs −111 to −93a | |
| Forward | 5′-AAG TAG GGA GGG GGT TGA G-3′ |
| Reverse | biotin5′-CCC AAC CAA CCT TAT TCC TAT-3′ |
| Sequencing | 5′-GAG GGG AGG GGT ATT-3′ |
| CpGs −27 to −11 | |
| Forward | 5′-AAG GAA AAG GTT AGG GTT TTG T-3′ |
| Reverse | biotin5′-TCC TAA CCC ACA CTC TTA AAA TTA C-3′ |
| Sequencing | 5′-GTT AGG GTT TTG TTG GA-3′ |
| CpGs +34 to+46 | |
| Forward | 5′-AGA GTT GAA GGG AGG TTG ATA TGG-3′ |
| Reverse | biotin5′-AAC CCT ACT TAC CAC ACA ATC C-3′ |
| Sequencing | 5′-TTA AGA GTG TGG GTT AG-3′ |
- a This set of primers was designed on the complementary strand.
4.12 Data and statistical analyses
For biometry and in vivo vascular reactivity analyses, data from siblings of the same sow were considered as replicates, and values were expressed as median [interquartile range]. For ex vivo vascular reactivity, biomechanical characterization and molecular biology analyses, animals were randomly assigned considering at least one offspring from each sow from each group and data were expressed as mean ± SEM. Concentration-response curves were analysed using an agonist-response best-fit line, where the maximal vasomotor response was expressed as percentage of the contraction induced by 40.8 mM K+ (%Kmax for relaxation) and the vascular sensitivity was expressed as pD2 (-logEC50).8, 12, 21 Differences were considered significant when P ≤ .05 (Prism 5.0; GraphPad Software). The specific statistical analysis for each result is indicated in figure footnotes.
5 PHYSIOLOGICAL RELEVANCE
- Adult FGR guinea-pigs show a bed-specific vascular remodelling and dysfunction that can be traced back to foetal life.
- Life-course endothelial function impairment in FGR guinea-pigs is associated with a switch in Nos3 promoter DNA methylation between foetuses and adults.
- A persistent vascular remodelling, instead of an early epigenetic programming of Nos3, would represent the main driving force for the endothelial dysfunction resulting from FGR.
ACKNOWLEDGMENTS
This work was funded by Fondecyt 1151119, 1170608 and 1181341; Proyecto Puente P1714/2017 VRI-PUC; Proyecto FONDEQUIP EQM-120205; and Proyectos Basales USA1899 Vridei 051916GH-PAP of the Universidad de Santiago de Chile.
Special thanks to Mrs Mireya Delgado for her excellent assistance in histology determinations.
CONFLICT OF INTEREST
None.




