Incidence of childhood type 1 diabetes mellitus in Ireland remains high but no longer rising
Funding information
We are grateful to the National Children's Hospital Foundation for financially supporting this study.
Abstract
Aim
The global incidence of type 1 diabetes mellitus (T1DM) varies considerably geographically. Ireland has a high incidence of T1DM. Incidence accelerated between 1997 and 2008, although more recent data (2008–2013) suggested stabilisation in the incidence rate (IR). This study sought to determine IRs for 2014 to 2018.
Methods
Incident cases were prospectively recorded through the established Irish Childhood Diabetes National Register (ICDNR). Cases were verified, and IRs were calculated. Capture-recapture methodology was identical to previous studies. Age and seasonality data were compared.
Results
A total of 1429 cases were reported (age range 0.45–14.98 years), with significantly more males (772, 54%) and male-to-female ratio of 1.17 (95% CI 1.05, 1.29). Standardised IRs for T1DM in the period were 28.0; 29.6; 30.9; 27.0; and 27.1/100,000/year, respectively. There was a slight reduction in standardised IR, more marked in females than males (9.9% v 1.6%). The highest IR remains in the 10- to 14-year-old age group (44% of total cases). Seasonality of diagnosis is persistently higher in autumn and winter.
Conclusion
Ireland remains a high incidence country, despite a minor reduction in incidence rates. Ongoing incidence monitoring through national registers is vital to inform healthcare services, research relating to aetiology and paediatric diabetes management.
Abbreviations
-
- GDP
-
- Gross Domestic Product
-
- GDPR
-
- General Data Protection Regulation 2018
-
- GNI
-
- Gross National Income
-
- ICDNR
-
- Irish Childhood Diabetes National Register
-
- IR
-
- Incidence Rate
-
- PCRS
-
- Primary Care Reimbursement Service
-
- SPSS
-
- Statistical Package for Social Sciences
-
- T1DM
-
- Type 1 Diabetes Mellitus
Key Notes
- Ireland remains a high incidence country for T1DM in children under 15 years but the rate is no longer rising.
- Incidence rates remain highest in 10- to 14-year-olds.
- Winter and autumn continue as peak seasons of diagnosis.
1 INTRODUCTION
The incidence of type 1 diabetes mellitus (T1DM) in children aged under 15 years varies widely internationally and over time. The management of children with T1DM is complex and varies with age. T1DM is associated with long-term morbidity. Knowledge of the incidence of diabetes is critical in order to provide appropriate healthcare services in order to achieve optimal metabolic control.
A number of high incidence countries, notably Spain, Norway, Sweden, Ireland and Finland, have reported a stabilisation or slowing of their incidence rates (IR),1-5 while other countries particularly in central and eastern Europe who had lower IR6, 7 reported continued increases. The IR in Finnish children decreased from 57.9/100,000/year to 52.2/100,000/year between 2003/6 and 2015/2018.5 Patterson et al.8 noted a reduction or a levelling-off in the rate of increase for some high-risk countries, although the overall rate of increase in incidence rates was calculated to be 3.4% annually so rates and trends continue to be variable. This recent Eurodiab collaborative study looking at data for 26 centres representing 22 countries from 1989 to 2013 also found the rates of increase were similar in boys and girls in the younger age groups but higher in boys than girls in the 10- to 14-year-old age group (3.3% and 2.6% per annum, respectively).8
In Ireland, the highest rates of T1DM have been predominately in the older 10–14 year age category to date.4, 9 This trend was seen in a number of other high incidence countries, such as Norway,2 Australia,10 Spain1 and Canada,11 while others report the highest increases in children aged under 5 years, such as Poland,6 Hungary12 and Romania.13 Recent research has shown that countries with a lower overall incidence rates of T1DM have had a faster rise in T1DM incidence particularly in children aged under 5 years.12, 13 Poland's rate rose markedly in their study period 1989 to 2012 from 5.36 to 22.74/100,000/year.6
Berhan et al and the Swedish Childhood Diabetes study group 20113 report data from 1978 to 2007 which found that a higher rate of increase was seen in younger children in the first two decades of their study, but interestingly, this trend switched to older age at onset in the study group born from 2000 onwards, along with more stable rates documented from 2005 to 2007. The authors postulate that this is due to changes in ‘non-genetic’ environmental risk factors affecting young children, particularly suggesting an effect of declining rates of overweight and obesity in the Swedish young childhood population. A study from the Netherlands by Spaans et al14 mirrors this stabilisation of rates in children under five years, approximately two decades ago. Parviainen et al in 2020 confirmed that incidence rates have decreased in young children in Finland with a plateau seen between 2006 and 2011.5 No change was noted in the IR in the older 10–14 category, and the authors report a slight increase in the median age at diagnosis.5
The Irish Childhood Diabetes National Register (ICDNR) was established in 2008, for the purpose of closely monitoring the national annual incidence rate of type 1 diabetes mellitus (T1DM). Ireland has a high incidence rate of T1DM4, 9, 15 but the marked increase in incidence seen between 1997 and 2008 was not maintained and T1DM incidence had effectively stabilised in the Irish population between 2008 and 2013,4 with an average per annum change in the period of 0.9% overall, −0.01% for males and +1.9% for females and a peak incidence rate of 28.8/100,000/year. It was uncertain whether this was a true stabilisation of rates or merely a fluctuation.
The aims of this study were to determine whether the apparent stabilisation in incidence rates had continued, whether the ages of children at diagnosis and proportion of males and females or season of diagnosis had changed.
2 METHODS
The ICDNR established in 2008 undertakes prospective registration of all physician-diagnosed incident cases of T1DM in those aged under 15 years resident in the Republic of Ireland (ROI) who are cared for in the 20 centres nationally. The case definition, inclusion and exclusion criteria have been published previously and are consistent with the WHO Diabetes Mondiale-DiaMond study16 and remain unchanged.4, 9 The methodology was previously described4, 9 and briefly included initial continuous case reporting from all paediatric centres nationally, with each centre contacted by ICDNR a minimum of four times per year. Following the initial case report, case verification was undertaken with detailed data provided on clinical and sociodemographic characteristics including age, sex, symptoms, family history and date of commencement of insulin therapy. Anonymous data regarding age category and gender were provided for verified incident cases notified by centres who did not complete full registration with the ICDNR (classified as non-participants) to permit calculation of incidence rates. Data were extracted from the ICDNR on 23 July 2020. The ICDNR was the main or primary source of case identification. Capture-recapture methodology was applied to estimate the completeness of ascertainment16, 17 using data from the Primary Care Reimbursement Service (PCRS), a central government agency as a second source of case identification as previously described.4, 9 The PCRS was chosen as the secondary source of case identification as it is the only national computerised data set for patients with diabetes and is the only possible source currently available to provide a measure of ascertainment. Cases identified by the ICDNR were crosschecked with the PCRS data set using personal identifiers to identify those cases identified by each source independently and those common to both sources. Capture-recapture methodology was applied to provide an estimate of ascertainment by the primary source (ICDNR), the secondary source (PCRS) and both sources combined.
In Ireland, treatment for patients with T1DM is provided by the State free of charge and independent of means. It is administered by the PCRS under two different healthcare schemes, namely the Long-term Illness (LTI) or Medical Card schemes. Applications for these schemes are made from local hospitals to the local healthcare office and forwarded to the PCRS.4, 9 Multiple rounds of cross-ascertainment checks are held in collaboration with the PCRS to ‘clean’ the data and ensure cases identified in this source meet the case definition. In the study period, there have been changes in practice and legislative changes within our Primary Care Reimbursement Service (PCRS) resulting in many children and adults with diabetes moving between healthcare reimbursement schemes from the end of 2014 (ie Long-term illness and Medical Card).
Statistical analysis was undertaken using IBM Statistical Package for Social Sciences (SPSS) version 26. Exact confidence intervals for category-specific and standardised rates were obtained using the statistical software R. The direct method of standardisation was employed which allows comparison of incidence rates internationally and over time.18 The 2018 base population with equal numbers in each age category was used as the standard population. Population data were based on census data and intercensal estimates of population.19 A Poisson regression was run on the category-specific rates as the dependent variable with the log (population) included as an offset to examine the relationship between age category, year and sex. Each variable was treated as a categorical variable.
In relation to seasonality, meteorological seasons were used which consist of splitting the year into four three-month seasons. Using this system, the seasons are defined as follows: spring (March, April and May), summer (June, July and August), autumn (September, October and November) and winter (December, January and February).
3 RESULTS
In the four-year period (2014–2018), a total of 1429 children (772 male) aged under 15 years were verified as incident cases to the ICDNR for calculation of incidence rates. Participation rate was 97.8%. All 20 centres nationally providing care to children with T1DM participated and returned data.
In the period 2014 to 2018, the estimated ascertainment of the primary source (ICDNR) was 93.5%; 91.5%; 82.9%; 85.1%; and 76.1%, and 89.5%; 87.8%; 84.8%; 87.6%; and 87.6% for the secondary source (PCRS). Estimated ascertainment for both sources combined was 99.3%; 99%; 97.4%; 98.2%; and 97%.
The mean age at diagnosis was 8.94 years (SD 3.62). The youngest child was diagnosed at 0.45 years and the oldest at 14.98 years. There were significantly more males than females (male-to-female ratio was 1.17 (95% CI 1.05, 1.29)).
Children were divided into three age categories for analysis, namely: 0–4.99 years (17%), 5–9.99 years (38%) and 10–14.99 years (44%). In the period 2014 to 2018, there was no significant difference in the age category at diagnosis over the years (χ2 = 8.07, df = 8, p = 0.43) (Table 1).
| Age category by year of diagnosis | ||||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | All | |
| % | % | % | % | % | % | |
| 0–4 | 17 | 19 | 20 | 16 | 14 | 17 |
| 5–9 | 36 | 38 | 39 | 42 | 36 | 38 |
| 10–14 | 47 | 43 | 41 | 42 | 50 | 44 |
| All | 275 | 294 | 310 | 274 | 276 | 1,429 |
Note
- (χ2 = 8.07, df = 8, p = 0.43).
Standardised incidence rates are presented which compensate for underlying changes in population structure and permit comparison between populations and over time. The standardised IRs for T1DM in the period (2014–2018) were 28.0; 29.6; 30.9; 27.0; 27.1/100,000/year, respectively, with minor fluctuations in both sexes. Overall, there was a fall in the standardised incidence rate at −3.35%, more marked in females (at −9.9%) compared with a slight increase in males (+1.57%) (Figure 1). The average annual percentage change was −0.85% overall, +0.39% in males and −2.57% in females.
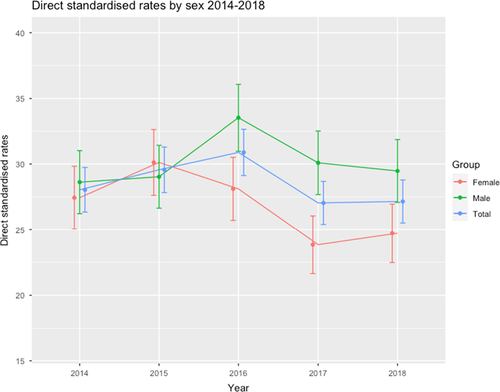
3.1 Incidence rates by age and sex
Category-specific incidence rates denote the incidence relative to the demographic experience of the population during the study period. Age and sex category-specific incidence rates for the five-year period were calculated (Table 2) (Figure 2).
| Category-specific incidence rates of type 1 diabetes mellitus per 100,000 per year (incident cases) | |||||
|---|---|---|---|---|---|
|
Year Sex Age category |
2014 | 2015 | 2016 | 2017 | 2018 |
| Male 0–4 years | |||||
| Incidence rate | 13.07 | 14.50 | 21.80 | 13.25 | 12.86 |
| 95% CI | (8.28,19.60) | (9.39, 21.41) | (15.35,30.05) | (8.30,20.06) | (7.96,19.66) |
| Cases (n) | 23 | 25 | 37 | 22 | 21 |
| Male 5–9 years | |||||
| Incidence rate | 27.98 | 35.29 | 35.84 | 37.15 | 27.45 |
| 95% CI | (20.70,37.00) | (27.12,45.16) | (27.66,45.68) | (28.84,47.09) | (20.38,36.19) |
| Cases (n) | 49 | 63 | 65 | 68 | 50 |
| Male 10–14 years | |||||
| Incidence rate | 44.81 | 37.29 | 42.91 | 39.88 | 48.11 |
| 95% CI | (35.00,56.53) | (28.46,48.00) | (33.45,54.22) | (30.84,50.74) | (38.26,59.71) |
| Cases (n) | 71 | 60 | 70 | 66 | 82 |
| Female 0–4 years | |||||
| Incidence rate | 14.17 | 18.12 | 15.46 | 13.25 | 12.18 |
| 95% CI | (9,08,21.09) | (12.23,25.87) | (10.10,22.83) | (8.20,20.25) | (7.33,19.02) |
| Cases (n) | 24 | 30 | 25 | 21 | 19 |
| Female 5–9 years | |||||
| Incidence rate | 30.54 | 29.27 | 31.63 | 27.27 | 28.05 |
| 95% CI | (22.74,40.15) | (21.72,38.59) | (23.83,41.17) | (20.10,36.15) | (20.75,37.08) |
| Cases (n) | 51 | 50 | 55 | 48 | 49 |
| Female 10–14 years | |||||
| Incidence rate | 37.59 | 42.98 | 37.24 | 31.05 | 33.92 |
| 95% CI | (28.47,48.71) | (33.24,54.68) | (28.28,48.14) | (22.97,41.05) | (25.56,44.16) |
| Cases (n) | 57 | 66 | 58 | 49 | 55 |
| Male & Female 0–4 years | |||||
| Incidence rate | 13.61 | 16.28 | 18.71 | 13.25 | 12.53 |
| 95% CI | (10.00,18.10) | (12.26,21.18) | (14.34,23.99) | (9.59,17.84) | (8.95,17.06) |
| Cases (n) | 47 | 55 | 62 | 43 | 40 |
| Male and Female 5–9 years | |||||
| Incidence rate | 29.23 | 32.35 | 33.78 | 32.20 | 27.74 |
| 95% CI | (23.78,35.55) | (26.66,38.89) | (28.01,40.39) | (26.69,38.74) | (22.55,33.78) |
| Cases (n) | 100 | 113 | 120 | 116 | 99 |
| Male and Female 10–14 years | |||||
| Incidence rate | 41.28 | 40.07 | 40.14 | 35.57 | 41.19 |
| 95% CI | (34.44,49.09) | (33.38,47.71) | (33.49,47.73) | (29.37,42.70) | (34.58,48.70) |
| Cases (n) | 128 | 126 | 128 | 115 | 137 |

The results of the Poisson regression on the category-specific rates indicated there was no significant change in the incidence of T1DM over years (χ2 = 3.85, df = 3, NS). Overall, there was a significant difference between the rates for males and females (χ2 = 4.83, df = 1, p = 0.028) and a significant difference in the age rates (χ2 = 197.2, df = 2, p < 0.001). However, there was no significant evidence of an age × sex interaction (χ2 = 0.72, df = 2, NS).
The results of the analysis suggest that the overall rate for males was between 1.01 and 1.25 times that for females. Incidence rates differ by age category at T1DM diagnosis (Figure 2). The results of the Poisson regression show that the rate for 5–9 age group is between 1.80 and 2.43 times higher than the rate for 0–4 age group and the rate for the 10–14 age group is between 2.30 and 3.09 higher than the rate for the 0–4 age group.
3.2 Seasonality
Aggregating the data over the years and assuming a uniform distribution, we would expect 25% of cases in each season. The analysis showed a significant difference from this pattern (χ2 = 50.63, df = 3, p < 0.001) with a higher percentage presenting in autumn (28%) and winter (31%) months. There was no evidence of a difference in seasonal pattern over the five years (χ2 = 7.33, df = 12, NS) (Table 3).
| Season of diagnosis by year | ||||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | All | |
| % | % | % | % | % | % | |
| Spring | 23 | 24 | 21 | 22 | 18 | 22 |
| Summer | 18 | 18 | 20 | 20 | 21 | 19 |
| Autumn | 29 | 26 | 26 | 29 | 29 | 28 |
| Winter | 30 | 32 | 33 | 29 | 32 | 31 |
| N | 275 | 294 | 310 | 274 | 276 | 1,429 |
Note
- χ2 = 50.63, df = 3, p < 0.001.
- a Denotes meterological seasons defined as spring (March, April, May), summer (June, July, August), autumn (September, October, November) and winter (December, January, February).
4 DISCUSSION
The National Register (ICDNR) continues to be acceptable to clinicians and families with all centres and 97.8% of children participating. The period 2014–2018 confirms that the high incidence rate of T1DM in the Irish population aged under 15 years has been maintained. However, the standardised incidence rate in the period fell by 3.35% overall. The standardised incidence rates in the period showed mild annual variation ranging from 27.0 to 30.9 cases/100,000/year with minor fluctuations in both genders. There was a slight increase of 1.57% in males and reduction of 9.9% in females. Finland, which has the highest incidence of T1DM in the world, similarly reported an overall stabilisation of rates with increased IR in boys and decreased in girls.20 The Eurodiab study of the period 1989–2013 found the rates of increase were similar in boys and girls in the younger age groups but higher in boys than girls in the 10- to 14-year-old age. The apparent separation in the trends of the incidence rate in males and females between 2015 and 2016 is difficult to understand and requires further research. There was no reported change in the population general health during 2016. Poisson regression was undertaken on the category-specific rates which showed that overall there is a male and female difference. There was no evidence of a year by sex interaction. However, the numbers may be too small to show this effect.
This stabilisation and reduction of the incidence of T1DM in Ireland is similar to that of other high incidence countries.1-3, 8 Perhaps, the underlying environmental precipitants of T1DM in our population have passed or reached saturation and the incidence has reached its peak. It could also be postulated that cases are presenting later in the young adult and adult groups, although we believe this is unlikely. Limited data are available regarding T1DM incidence in adulthood as no adult diabetes register currently exists in Ireland. A global systematic review published in 2015 found that in 23 of 35 countries (66%), the IR of 0- to 14-year-olds was higher than that of the 15–19 age group with less cases in adulthood than childhood overall.21
A high incidence of T1DM has been associated with economic prosperity in Ireland and elsewhere, and it is notable that the stablisation or reduction in our IR has mirrored a decline in economic prosperity and a period of austerity.4, 22 Irish Gross Domestic Product (GDP) fell steadily from 2007 only exceeding 2007 levels in 2015, and Modified Gross National Income (GNI) fell steadily from 2007 only exceeding base levels in 2016.23
The highest number of cases continues to be diagnosed in the older 10–14-year age category in our population, and a number of other populations who have a similar incidence of T1DM to Ireland also show this trend, particularly Norway and Australia.2, 10, 24 Some centres have shown the number of cases and incidence rates in the younger age categories are increasing suggesting a shift to an earlier age at diagnosis.6, 12, 13 We have not found this. An earlier age at diagnosis provides additional challenges for families and healthcare staff resulting in increased duration of diabetes and potential for diabetes-related complications. However, a later age at diagnosis in adolescence is also extremely challenging for individuals, their families and diabetes services due to the potential of delayed diagnosis with increasing independence and the challenges in management of chronic conditions in the often turbulent adolescent years.
Most cases were diagnosed in the autumn (28%) and winter months (31%).25 This supports our findings from the initial six years of the ICDNR.4 This finding is usual for countries with an Oceanic climate.26 This study by Chen et al looking at global data from 1965 to 2012 also found higher incidence in centres with higher latitude and less sunshine duration.26 A low mean temperature was shown to affect an increase in incidence,26 and low temperature was independent of hours of sunshine.27
Overall Ireland's summers within this small timeframe (2014–2018) were variable, and it was not possible to conclude with any certainty at this point the effect of temperature or sunshine over each mid-summer on numbers diagnosed. 2014–2018 data did demonstrate that most cases were diagnosed in the cooler winter and autumn seasons.
A limitation of this study is the small number of years to investigate trends in the data. Further research is needed to confirm and better understand differences in incidence rates between males and females. Measuring ascertainment in this period has been particularly challenging due to changes within the PCRS system; legislative changes, namely the introduction of the General Data Protection Regulation 2018 (GDPR) and Irish Health Research Regulations (2018); and the requirement for data anonymisation. Due to these legislative changes, cross-ascertainment for 2018 had to be undertaken before all incident cases were notified to the Register. Further identification of common cases from earlier years in later rounds of ascertainment was precluded due to anonymisation. While some inaccuracies have been identified within the PCRS data set resulting in the inclusion of cases not meeting the case definition, there were additional cases within the PCRS data set which could not be further verified. Due to ethical and data protection constraints, the ICDNR are not permitted direct access to cases identified by the PCRS alone to verify their accuracy as a result the PCRS data may include additional cases not meeting the case definition resulting in a falsely low ascertainment rate for the ICDNR, as previously demonstrated.9
In future, the process of notification to the PCRS will change with direct reporting from local centres to the PCRS avoiding multiple data entries and enabling data verification. This will improve its utility for capture-recapture application.
In summary, we know that the incidence of type 1 diabetes mellitus in the Irish population is no longer rising consistent with earlier reports from the ICDNR. However, Ireland remains a high incidence country with the majority of new diagnoses occurring in the older 10–14-year-old age category. The peak diagnostic seasons with 59% overall being diagnosed are winter and autumn compared to 41% during spring and summer. This is consistent with the Irish (Oceanic) climate.
5 CONCLUSIONS
Continued monitoring of annual incidence rates and epidemiology of this important disease with a national register is vital in order to inform national healthcare planning, support audit and optimise clinical care. Monitoring of standardised national incidence rates permits international monitoring of T1DM epidemiology to identify trends and variations in incidence, thereby aiding the search for aetiology and the provision of optimum clinical care.
ACKNOWLEDGEMENTS
We would like to express our gratitude to the paediatric endocrinologists, diabetes nurse specialists, paediatricians nationally, children and young people with diabetes and their caregivers, and the Primary Care Reimbursement Service for their support of this study.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
STATEMENT OF ETHICS
Ethical approval was obtained for this study from the Tallaght University Hospital (TUH)/St James Hospital (SJH) Joint Research Ethics Committee.




