Identical twins affected by congenital cytomegalovirus infections showed different audio-vestibular profiles
The study was funded by Tysta Skolan and the ALF agreement between Stockholm County Council, the Karolinska Institute and Karolinska University Hospital.
Abstract
This study explored whether there were long-term hearing and vestibular outcome differences between five pairs of identical twins who had been infected with the congenital cytomegalovirus (CMV) infection before birth. Data were collected from the medical records at the Audiological Clinic, Karolinska University Hospital, Stockholm. The congenital CMV infection resulted in high variations in vestibular and hearing function within, and between, the genetically identical twin pairs. Clinicians need to be aware that treatment and interventions may need to differ substantially when identical twins have hearing issues related to the congenital CMV infection.
The congenital cytomegalovirus (CMV) infection is caused by primary or recurrent maternal infections. It affects 2-10 per 1000 newborn infants per year and is the leading cause of non-genetic hearing loss in children.1, 2 Recurrent infections are due to the latent virus being reactivated or reinfection with another CMV strain. These account for about 60%-95% of congenital CMV infections, depending on population seroprevalences.3, 4
Around 25% of infected children will have permanent disabilities, such as hearing impairments, cognitive delays, epilepsy, psychomotor and perceptual deficits, visual impairments and feeding problems.5, 6
Studies have described associated vestibular impairment in symptomatic infants, but the prevalence has varied considerably between different reports.7-9 A study at the Karolinska University Hospital found that about 88% of children with congenital CMV infections had vestibular dysfunction, as well as severe hearing impairment. Children with congenital CMV infections may have balance disorders, which are often attributed to psychomotor delays and neglect the possible role of vestibular dysfunction.6
The literature also describes different audio-vestibular manifestations among singletons diagnosed with congenital CMV infections.6, 7 Different maternal factors, such as young maternal age, high risk of viral exposure, seroprevalence and co-existing infections, may play a role in the phenotype of congenital CMV infections. Foetal factors, such as symptomatic neonatal infections, viral loads and delayed intrauterine growth, have been shown to influence the expression of congenital CMV infections.10, 11
We studied five pairs of identical twins affected by congenital CMV infections. The data were collected from the Audiological Clinic, Karolinska University Hospital, Stockholm, Sweden, and the twins were followed up from 9 to 19 years. Our study explored whether the congenital CMV expression was different in the same twin pairs and whether the incidence of infection, hearing impairment and other long-term disabilities were higher than our singleton study 6 and the literature.
The twins had been diagnosed after neonatal blood or urine tests or retrospective analysis of the dried blood samples.12 There was one female twin pair and four male twin pairs. Two sets had one hearing impaired twin and one twin with normal hearing, two had two hearing impaired twins and one had a hearing impaired twin and one who died at 3 years of age (Table 1).
| Case number | Order | Sex | GA | MA | Zygosity | Placenta | Type of inf. | Sympt. | HI | Pat. head movements | Walking age (months) | Vestibular function | ROP | Other diagnoses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | F | v. 34 | 26 | MZ | MC-DA | N/A | No | No | No | 15 | N/A | No | No |
| 2 | F | v.34 | 26 | MZ | MC-DA | N/A | Yes | Unilateral deafness | No | 15 | N/A | No | No | |
| 2 | 1 | M | term | 31 | MZ | N/A | N/A | No | Bilateral profound HI | N/A | 18-19 | Bilateral dysfunction (asymmetry) | No | Asperger |
| 2 | M | term | 31 | MZ | N/A | N/A | Yes | N/A | N/A | N/A | N/A | N/A | Deceased | |
| 3 | 1 | M | v.27 | 29 | MZ | MC-DA | Primary | Yes | Bilateral profound HI | Yes | 25 | Bilateral loss | Yes | ADHD as young |
| 2 | M | v.27 | 29 | MZ | MC-DA | Primary | Yes | Unilateral deafness, Contralateral HI for high frequencies | Yes | 25 | Bilateral loss | Yes | ADHD as young | |
| 4 | 1 | M | v.36 | N/A | MZ | MC-DA | Primary | No | Unilateral deafness, contralateral delayed fluctuating | No | 22 | Bilateral loss | No | No |
| 2 | M | v.36 | N/A | MZ | MC-DA | Primary | No | Bilateral rapid progressive HI | Yes | 22 | Bilateral loss | No | No | |
| 5 | 1 | M | v.37 | 28 | MZ | MC | N/A | Yes | No | Yes | 18 | Unilateral loss | No | OCD |
| 2 | M | v.37 | 28 | MZ | MC | N/A | No | Bilateral profound HI | Yes | 18 | Bilateral dysfunction (asymmetry) | Yes | Asperger |
- Abbreviations: DA, diamniotic; GA, gestational age (weeks); HI, hearing impairment; MA, mother's age at childbirth (years); MC, monochorionic; MZ, monozygotic; OCD, obsessive compulsive disorder; Pat. head mov., pathological head movements; ROP, retinopathy; Sympt, symptoms at birth.
We gathered the background data from the medical records and interviews with the parents: mother's age and seroprevalence, children's gestational age, type of infection, symptoms at birth and placenta chorionicity. We specifically asked about pathological head movements and positions, like pseudo-opistotonus or backwards thrusting.6, 13 The nine surviving twins had been assessed for hearing impairment, motor proficiency delays, neurodevelopmental disabilities, language delay and visual impairment. Hearing impairment was mostly identified through the universal newborn hearing-screening test and confirmed with the auditory brainstem response test. The two children who did not have profound hearing impairment at birth were followed up with regular pure tone audiometry. Vestibular function was assessed in four twin pairs with caloric stimulation, through video head impulse tests and vestibular-evoked myogenic potentials.14 All children underwent an ophthalmological examination.
The clinical presentation varied both between, and within, the twin pairs (Table 1). Hearing impairment varied from profound bilateral deafness to unilateral hearing impairment, with no, partial or late onset on the other side. Of the seven children tested for vestibular dysfunction, six had bilateral dysfunction and one had unilateral loss. All had normal or high cognitive function and had spoken Swedish as their first language.
A short description of each twin pair follows.
Two females were born by Caesarean section at 34 weeks, after one showed intrauterine growth retardation. One twin, born weighing 1500 g, failed the newborn screening test. She had unilateral deafness, with typical hearing on the other side. Her sister showed typical hearing and no other clinical symptoms. No vestibular testing was carried out, but both started walking at around 15 months.
Two male twins were born outside Sweden, and one died at 3 years of age, after severe sequelae at birth. The surviving twin had bilateral profound hearing impairment diagnosed at 3.5 years. He could not talk and could only use basic gestures. He received bilateral cochlear implants at the age of four and now speaks Swedish as his first language. He started walking at around 18-19 months. Vestibular testing showed bilateral dysfunction with asymmetry. He also has high-functioning Asperger's syndrome.
Two male twins with twin-to-twin transfusion syndrome were delivered by caesarean section at 27 weeks. The donor twin had severe to profound hearing impairment and was fitted with hearing aids. At 4 years of age, he received a cochlear implant for his worst ear. His brother was fitted with hearing aids for severe hearing loss in one ear and sensorineural high-frequency hearing impairment in the other. Both had unilateral retinopathy and bilateral vestibular loss. They were initially diagnosed with attention deficit hyperactivity disorder, which was later ruled out (Figure 1) .
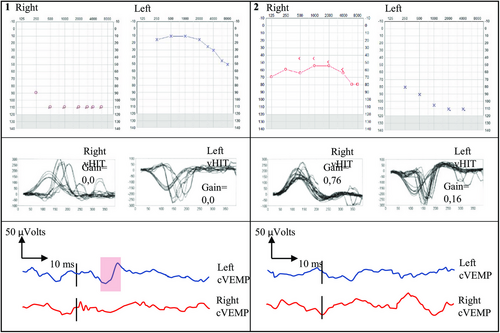
The male twins born before newborn screening was established were reviewed. One was fitted with hearing aids at 1 year, but his hearing rapidly deteriorated. He had his first cochlear implant at 3 years and the other at 6 years. He developed exceptional hearing and age-equivalent spoken language ability. His brother had presumed congenital unilateral hearing impairment from birth in one ear and typical hearing in the other ear. Vestibular testing showed bilateral loss in both boys. The second twin's hearing started to fluctuate when he was 15 years old, and he then received a cochlear implant for his congenitally deaf ear.
The final male twins were born naturally at 37 weeks after an uncomplicated pregnancy and the first twin had typical hearing. The second twin failed neonatal screening test, was diagnosed with bilateral profound hearing impairment and received bilateral cochlear implants at 9 months. He also had unilateral retinopathy, bilateral vestibular dysfunction with asymmetry and high-functioning Asperger's. His apparently asymptomatic, hearing brother had unilateral vestibular loss of function without any apparent motor proficiency disorders (Figure 2).
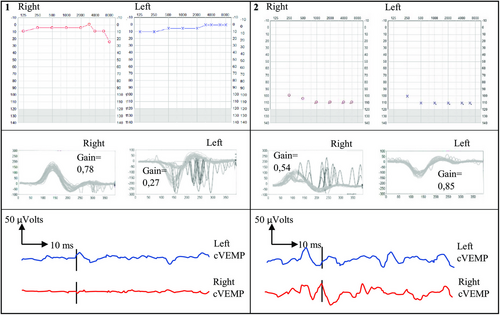
A systematic literature search was conducted using MEDLINE, and 28 reports covering 54 twin pregnancies were identified. The search strategy, table of findings and full reference list are detailed in Appendix S1.
To our knowledge, only five pairs of identical twins affected by congenital CMV infections have been described in the last 65 years. These studies only provided information on the hearing function in one pair and no information about their vestibular function.
The evident heterogeneity in audio-vestibular presentation in these twins could have been motivated by the pathogenesis of the congenital CMV infection. We have known for decades that twins react differently to congenital CMV infections, but the physiopathology remains unclear.3 Animal models found the virus in the inner ear bone marrow and the stria vascularis was the first structure to be identified. However, the diverse inner ear involvement may be due to different inflammatory responses, secondary to the infection.15, 16 One human study found high viral loads in endolymphatic structures, especially in the stria vascularis, Reissner's membrane and dark cells of the labyrinth. Another found the virus in the perilymphatic fluid of a deaf patient up to 17 years after infection.11, 17 This could explain the unilateral, late-onset or progressive hearing impairment presentation of congenital CMV infections.
Of the nine surviving twins in our study, two had typical hearing, two had unilateral hearing impairment at birth and five had bilateral sensorineural hearing impairment. The twin who died was not tested. In one case, the congenital hearing impairment progressed rapidly, leading to profound impairment by the age of three.
Prospective longitudinal studies of children with asymptomatic congenital CMV infections showed that approximately half who developed hearing impairment had bilateral deficits, varying from mild high-frequency loss to profound hearing impairment.18, 19
Only five pairs of identical twins were described in the 15 reports that considered zygosity. In contrast, we studied identical twins, which provides important insights into the diversity of congenital CMV clinical findings when the same congenital traits exist.
The virus enters through the placenta and uses it as a major reservoir where it replicates before affecting the foetus. Human leucocyte antigen G and interferons that limit viral replication protect foetal cells from CMV.20 In our study, four twin pairs shared the same placenta, confirming the singularity of the maternofoetal environment with different manifestation in the foetuses. In the reviewed literature, identical twins that shared the same placenta and amniotic sac were equally infected and symptomatic. In contrast, monochorionic-diamniotic twins presented with the infection, but did not have the same clinical outcomes. This was confirmed by the three monochorionic-diamniotic twin pairs in our series—cases one, three and four—where different audio-vestibular patterns were observed.
In our study, four of the five twin pairs were male. Of the 28 papers we reviewed, 11 mentioned gender and 60% were male.
Vestibular disorders linked to congenital CMV associated hearing impairment have rarely been reported.6, 7, 9 Our findings showed that the cochlea and vestibular tissues were both vulnerable to congenital CMV infections and this could sometimes be isolated. In the fifth twin pair, one sibling had unilateral isolated vestibular loss, but no hearing impairment, while the other twin had deafness and bilateral partial vestibular loss. Unilaterally affected children walked earlier, confirming previous findings on walking age and vestibular loss.21, 22 This highlights the importance of having at least unilateral vestibular residual to maintain typical motor development.
Larger reports of twins diagnosed with congenital CMV infections showed high numbers of stillborn births and terminations due to severe damage.23, 24 Only one of our twins had severe sequelae and died, and the others had high functioning, despite cases with Asperger's, balance disorders and hearing impairment. Our twin results were in line our singleton study.6
In conclusion, congenital CMV infections among genetically identical twins can still lead to high variations in hearing disabilities, but our study provided more consistent material about heterogeneous audio-vestibular impairment. The maternofoetal environmental seemed to influence the hearing impairment pattern within the twin pairs. Vestibular function was generally affected, with possible differences within the same pair. The impact on motor development, related to the degree of vestibular loss, was as expected. Given the long follow-up period, it was possible to observe late-onset hearing impairment, which was most probably due to congenital CMV infections.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
Ethical approval was granted by the regional ethical committee (Nr 2012/128-31/1), and informed consent was obtained from the parents.




