Hypothermic treatment for neonatal asphyxia in low-resource settings using phase-changing material—An easy to use and low-cost method
Abstract
Aim
To evaluate whether phase-changing material can be used for therapeutic hypothermia of asphyxiated newborns in low-resource settings.
Methods
Prospective interventional study of asphyxiated term infants fulfilling criteria for hypothermia treatment at Vietnam National Children's Hospital from September 2014 to September 2016. Hypothermia was induced within 6 hours after birth and maintained for 72 hours by a phase-changing material mattress with melting point of 32°C. Rectal temperature was continuously measured, and deviations from target temperature range 33.5-34.5°C were recorded.
Results
In total 52 infants (mean gestational age 39.3 ± 1.1 weeks) included and cooled, the median temperature at initiation of cooling was 35.3 (IQR 34.5-35.9)°C. The median time to reach target temperature was 2.5 (IQR 2-3) hours. The mean temperature during the cooling phase was 33.95 ± 0.2°C. Throughout the cooling phase, the target temperature range (33.5-34.5°C) was maintained more than 80% of the time. Rate of rewarming was 0.5 ± 0.14°C/hour.
Conclusion
Phase-changing material can be used as an effective cooling method. Though not a servo-controlled system, it is easy to induce hypothermia, maintain target temperature and rewarm infants in a slow and controlled manner without need for frequent changes and minimum risk of skin injury.
Abbreviations
-
- aEEG
-
- amplitude-integrated electroencephalography
-
- CFM
-
- cerebral function monitoring
-
- HIE
-
- hypoxic-ischemic encephalopathy
-
- IQR
-
- Interquartile range
-
- MRI
-
- magnetic resonance imaging
-
- NICU
-
- neonatal intensive care unit
-
- OBGY
-
- obstetrics and gynaecology
-
- PCM
-
- phase-changing material
-
- PLIC
-
- posterior limb of the internal capsule
-
- SD
-
- standard deviation
-
- TOBY trial
-
- Total Body Hypothermia
-
- TPN
-
- total parenteral nutrition
-
- VNCH
-
- Vietnam national children's hospital
Keynotes
- Cooling is standard treatment for neonatal hypoxic-ischemic encephalopathy; however, there are limited resources for cooling equipment in low-middle income countries.
- We show that phase-changing material (PCM) can induce hypothermia effectively and maintain temperature at target range during 80% of the treatment period.
- Future studies are needed to determine the feasibility of PCM during transportation.
1 INTRODUCTION
Neonatal hypoxic-ischemic encephalopathy (HIE) in term infants constitutes a serious public health problem which often has life-long neurological consequences. An estimated 3-5 out of every 1000 live term births are affected,1 a quarter of which have severe symptoms; 10%-30% of affected children do not survive; 25-30% of HIE survivors will have long-term neurodevelopmental disabilities that include cerebral palsy, seizure disorder and mental retardation.2 The incidence may be 10-fold higher in low-income countries.3
Clinical trials in term newborns in high-income countries have shown that therapeutic hypothermia is effective and safe as a treatment for neonatal encephalopathy caused by birth asphyxia.4, 5 Three systematic reviews6-8 concluded that therapeutic hypothermia can significantly reduce death and medium-term disability after neonatal encephalopathy and that it is safe in an intensive care setting. A prerequisite is that the treatment is started within 6 hours after birth and, ideally, as soon as possible.9, 10
There is still limited experience with hypothermia treatment in low- and middle-income countries, and a great heterogeneity in study protocols limits the evaluation.11 Current methods of cooling are expensive, need continuous power supply and a range of expensive consumables, and are very difficult to use during transportation,12 a major issue because it is hard to reach a neonatal unit with adequate resources for cooling treatment in time. Phase-changing materials (PCMs) appear to be ideal for such purposes. PCMs do not require electricity; they are biologically safe for humans, are low-cost and can be reused almost infinitely, and they are likely to provide a more stable cooling temperature than other ‘low tech’ methods such as ice.13
A PCM is a substance (usually a salt hydride, fatty acid, and ester or paraffin, such as octadecane) with a high heat of fusion, which is capable of storing or releasing large amounts of energy per unit weight (or volume) while maintaining a given temperature.14, 15 Like water, a PCM has three phases: solid, liquid and steam, although only the solid-liquid change is used. A PCM substance with a set ‘melting point’ of, for example 33.5°C will be solid at room temperatures of ~25°C.
When the human body (37°C) is in contact with a PCM, the PCM will absorb body heat, and the temperature of the PCM itself will rise. When the PCM reaches its melting point, a great deal of additional energy is absorbed from the body in contact with the PCM, which remains at its specific set temperature until the phase change eventually occurs. It is this natural property of PCMs that effectively cools contacting bodies for extended periods of time. A PCM works as a heat sink and thus stabilises the temperature of whatever it is in contact with.
So far, there have been several animal and human trials worldwide 14, 16, 17 that have used PCMs as a mode of cooling neonates. Results from the multicentre randomised controlled trial in India concluded that therapeutic hypothermia of neonates with HIE using a PCM-based cooling device was feasible and safe when practiced in level 3 NICUs. A PCM-based device was comparable to standard servo-controlled equipment in maintaining the target temperature.16, 17
Based on results from previous studies, we proposed PCMs as a cooling technique in order to achieve hypothermia without the need for electricity or water, and without risk of overcooling in order to treat more patients who otherwise would be at risk for HIE damages of the brain, and lower the amount of injuries due to asphyxia or HIE. The aim of this study was to determine the feasibility of whole body cooling to 33.5-34.5°C for 72 hours by use of a PCM mattress in a low-resource setting, such as at Vietnam National Children's Hospital (VNCH).
2 METHODS
2.1 Study participants
This non-randomised intervention study took place in Hanoi between September 2014 and September 2016. Asphyxiated term newborn infants from 8 provincial hospitals with an average 2-hour transport distance from Vietnam National Children's Hospital (Hanoi Obstetrics and Gynecology Hospital, National Hospital of Obstetrics and Gynecology, Ha Dong General Hospital, Thai Binh Provincial Hospital of Obstetrics and Pediatrics, Hai Duong Provincial Hospital of Pediatrics, Bac Giang Provincial Hospital of Obstetrics and Pediatrics, Vinh Phuc Provincial Hospital of Obstetrics and Pediatrics, Hung Yen Provincial Hospital) who fulfilled criteria for hypothermia treatment after birth asphyxia and referred to VNCH were included in the study, as there was no inborn delivery at VNCH.
2.2 Study procedures
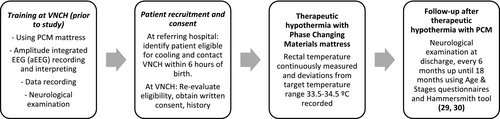
The study process is summarised in Figure 1.18, 19
2.3 Patient recruitment and consent
2.3.1 Referring hospitals
Doctors at the obstetric hospitals identified patients potentially eligible for cooling using eligibility criteria, as in the TOBY study20 (Table 1): upon being granted verbal consent (yes/no) in which the parents were explained about the newborn's condition and therapeutic hypothermia treatment, and once contact was made with the on-call doctor at VNCH NICU for a transfer. During transportation, passive cooling was used (removing clothes, no heater).21 Basic data regarding the mother (pregnancy complications, mother having any sign of infection, type of delivery, time of ruptures of membranes, amniotic fluid check and contact information) and the child (place of birth, gestational age, weight, resuscitation Y/N, Apgar score, first gasp in min, whether the infant was heated) was collected.
| Infants ≥ 36 wk gestational age and ≤6 h after birth, and at least one A criterion and one B criterion |
| Criteria A (perinatal factors) |
| Apgar score ≤ 5 at 10 min |
| Continued need for resuscitation, 10 min |
| pH < 7.0 and/or base deficit >16 mmol/L (Blood gas within 60 min) |
| Criteria B (encephalopathy) |
| Altered consciousness |
| Abnormal tone (hypertonia or hypotonia) |
| Abnormal primitive reflexes |
| Exclusion |
| >6 h after birth at time of referral/evaluation; coagulopathy with active bleeding; prenatally diagnosed syndromes, malformations or metabolic disorders not compatible with survival. |
2.3.2 At VNCH
On arrival at VNCH, all patients were evaluated again for eligibility by the NICU doctor. Written consent form was acquired from any one of the parents who was transferred with the infant; in all cases, it was the father because the mother was still at the hospital. Once a patient was enrolled, the complete obstetric history was obtained, and the degree of HIE was determined using Sarnat's clinical staging system (Appendix 1). Basic patient data were recorded, including respiratory symptoms, oxygen dependence, oxygen saturation, capillary refill time, convulsions (clinical and/or seizures on aEEG), vitality (scored as spontaneous activity, alertness, reaction to stimulation and muscular tonus), need for immediate shock therapy and time from birth until hypothermia treatment was started, seizures before hypothermia treatment and highest registered rectal temperature before treatment.
The infant was put on the PCM mattress (Medical Cooling Sweden AB; by TST AB) and nursed in a cot naked or lightly covered with a cotton sheet. The PCM mattress that was used contained 8 PCM packs with a specific melting point of 32°C, built into two layers of 4 × 2 PCM sheets (illustrated in Figure 2). The mattress was covered with a fabric with possibilities for providing temperature changes between the PCM and the patient through thermal conductance. A rectal temperature probe was inserted 2-3 cm into the rectum and was connected to a multiparameter monitor to monitor core temperature. The target rectal temperature was 33.5°C-34.5°C. The temperature was continuously monitored and recorded every hour for 72 hours. If the temperature decreased below 33.5°C, a folded cloth sheet was inserted between the infant and the PCM mattress, and the infant was covered with a sheet. If the temperature reached <33.0°C (lower alarm limit), the steps taken to increase the core temperature were to place a sheet between the PCM bed and the infant, to cover the infant with another sheet or to switch on the radiant warmer. The radiant warmer was used in manual mode with an output of 10% to begin, and was then adjusted in increments or decrements of 5%, depending on the infant's temperature.
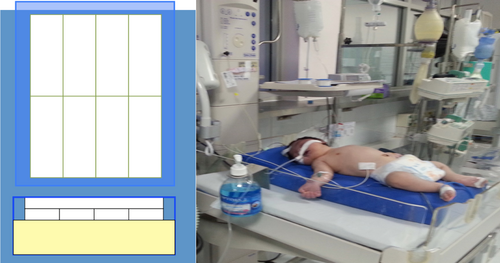
After 72 hours of cooling, the infant was rewarmed by removing the mattress and then naturally rewarming in room temperature to a normal temperature by no more than 0.5°C/h. The radiant warmer was added for rewarming if the targeted temperature of 36°C was not reached.
2.3.3 Treatment and monitoring
During the hypothermia treatment, standard medical care and treatment of the NICU were given to all patients. During cooling, infants were sedated with an intravenous infusion of low-dose morphine 10 μg/kg/hour as soon as cooling started. Phenobarbital was administered if clinical and/or an aEEG seizure were detected (loading dose 20 mg/kg and maintenance dose 10 mg/kg/day). aEEG was used to monitor patient during active cooling and for 24 hours after rewarming was completed. All patients were given total parenteral nutrition (TPN) 40 mL/kg/day and nil by mouth for 72 hours. Adjustments for TPN on successive days were made individually based on patients’ kidney functions, and serum sodium and fluid balances. Kidney and liver function, serum sodium, potassium, calcium and magnesium were tested daily during treatment. The severity of the infant's encephalopathy was assessed and scored daily during hospitalisation and at discharge using encephalopathy scores.
2.4 Outcomes
2.4.1 Primary outcome
Stability of rectal temperature during treatment as determined by time out of target temperature range 33.5-34.5 C (%).
2.4.2 Secondary outcomes
Number of temperature corrections needed, time to reach target temperature (hour), rate of rise in temperature during rewarming (°C/h), seizure episodes during cooling and during rewarming, brain MRI (normal/abnormal), mortality during and after hypothermia treatment (%).
2.5 Data analysis
Demographic factors and clinical characteristics were summarised with counts (percentages) for categorical variables, mean (standard deviation [SD]) for normally distributed continuous variables or median (interquartile or entire range) for other continuous variables. Demographic and clinical variables were compared using unpaired comparisons obtained from chi-square for categorical variables and t tests for continuous variables.
2.6 Ethics
This study followed its procedures in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983, and was approved by the Ethical Review Board of National Hospital of Pediatrics Research Institute for Child Health (RICH) (NHP - RICH- 13-002).
Verbal consent was elicited at the local hospital by the referring doctor regarding the transfer for possible cooling therapy. Official written consent was obtained from the parents when they came to the neonatal ward of VNCH by the admitting doctor.
3 RESULTS
In this study, a total of 52 newborns were cooled using PCMs between September 2014 and September 2016. The mean gestational age was 39.3 ± 1.1 weeks. Of the 52 infants, 12 cases (23%) were delivered in a emergency cesarean section due to complications of labour such as foetal distress, cord prolapse or shoulder dystocia. Regarding Apgar scores, only 53.8% infants had scores registered. In many other cases, the referring hospitals did not give Apgar score so we have to use other A criterion to assess whether the infant fulfilled cooling criteria. The baseline characteristics of the mothers and the infants prior to therapeutic hypothermia treatment are, respectively, shown in Tables 2 and 3. We only have information on complications at delivery for 14 mothers; others were missing from the referring hospitals.
| Baseline characteristic | No | % |
|---|---|---|
| Age – y | 27 ± 6 | |
| Intrapartum complications | ||
| Foetal heart rate deceleration | 10 | 19 |
| Cord prolapse | 2 | 4 |
| Shoulder dystocia | 2 | 4 |
| Emergency ± cesarean delivery | 12 | 23 |
| Baseline characteristic | No | % |
|---|---|---|
| Age when cooling started – h | 3.25 ± 1.1 | |
| Male sex – No | 35 | 67 |
| Birth weight – grams | 3360 ± 620 | |
| Apgar score ≤ 5 at 10 mina | 28 | 54 |
| Lowest blood gas pHa | 7.12 (6.8-7.25) | |
| Status on admission to VNCH | ||
| Seizure (clinical and/or aEEG) | 30 | 58 |
| Use of anticonvulsant agent | 24 | 46 |
| Hypotension | 8 | 15 |
| Sepsis | 5 | 10 |
| Coagulation disorder | 2 | 6 |
| Mechanical ventilation | 50 | 96 |
| Total duration of hospital care (d) | ||
| Median | 12 | |
| IQR | 8-18 | |
- a Even though in the protocol Apgar score and blood gas at 60 min were asked for, some referring hospitals had not been capable of sending this information so we had to use other A criterion to assess whether the infant fulfilled cooling criteria. Blood gas was only available for 32/52 infants, in 3 out of 6 hospitals possibility for analysing blood gas not available.
Regarding infants’ levels of encephalopathy according to Sarnat scoring, 3(6%) infants had mild, 38 (73%) had moderate and 11 (21%) had severe encephalopathy; 30 (57.7%) infants had convulsions (clinical and/or aEEG) on admission. Of those 30 infants with convulsion, 24 (80%) were treated with an anticonvulsant agent, either phenobarbital or midazolam, or both, at referring hospitals.
A total of 3744 temperature readings were recorded for 52 patients during 72 hours of therapeutic hypothermia treatment. Median temperature at initiation of cooling was 35.3 (IQR 34.5-35.9)°C, which was also the temperature on arrival at VNCH as the patients were immediately placed on PCM. Median time for reaching the target temperature of 33.5°C was 2.5 (IQR 2-3) hours.
The mean ± SD temperature during the cooling phase was 33.95 ± 0.2°C. Throughout the cooling phase, the target temperature range (33.5-34.5°C) was maintained 80% of the time. The occurrence of temperatures reading below 33.5°C, above 34.5° and below 32°C was 10.21%, 9.23% and 0.58%, respectively. No reading above 35.5° was recorded within 72 hours for all infants.
As for the rewarming phase, the rate of rewarming was 0.5 ± 0.14°C/hour, and the average time for infants to reach 36.5°C was 5 ± 1.23 hours. The temperature profiles of all 52 infants are shown in Figures 3 and 4.
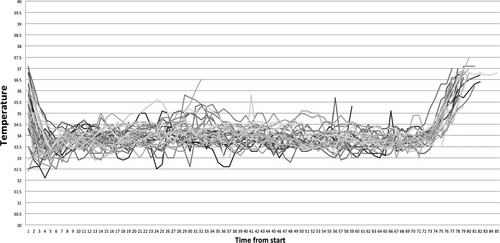
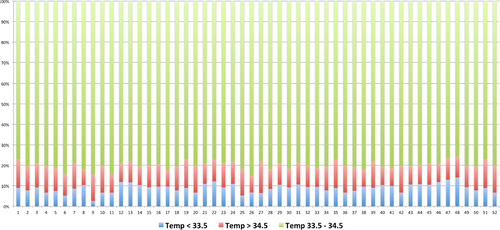
Among the 30 infants that presented with seizures on admission, seizure was controlled with phenobarbital in 25 cases and 5 remained with seizures until rewarming phase. Cooling had to be discontinued for 4 (7.7%) infants because parents decided to withdraw treatment due to poor neurological prognosis based on cerebral function monitoring (aEEG) and seizure control. Forty-eight infants who completed cooling therapy received brain MRI at 7 days of age. Twenty-seven (56.2%) had normal MRI, 8 had abnormal signal intensity within the posterior limb of the internal capsule (PLIC), and 13 had diffuse abnormalities.
The median hospitalisation time was 12 days (IQR 8-18).
4 DISCUSSION
In this study, we have shown that infants with HIE can be cooled effectively using a PCM mattress.
All patients were born at local hospitals with travel distances of approximately 2-3 hours or more during rush hours by ambulance without proper transport incubators, and temperature and vitals monitoring during transportation. Therefore, most of them presented with low temperatures at initiation of cooling, median 35.3 (IQR 34.5-35.9)°C. This is lower than in the NICHD trial (36.6 ± 1.0°C),5 where all patients were born at the hospital where cooling was performed. However, in comparison with Thayyil S et al trial in India (35.2 ± 1.3°C),17 our mean temperature was slightly higher. In the Indian trial, no information was provided on whether patients were inborn or transported. The study also pointed out about that PCM cooling was effective only when the ambient temperature was <28°C; in this study, we always set the room temperature inside the nursery at 26-27°C and continuously measured and regulated during treatment. In the current study, hypothermia treatment was initiated in all infants within 6 hours, although it would have been even better if we could have started within 3 hours, due to reasons mentioned by Thoresen et al.10
Though not a servo-controlled system, it was possible to induce hypothermia to reach the target temperature using the PCM mattress within 2.5 (IQR 2-3) hours. Compared to Thayyil et al trial,17 the time taken to reach the target temperature in our study was longer, but their temperature at initiation was also lower than ours, and the PCM used in their study had lower melting points compared to ours (29°C vs 32°C),22 which could have caused the differences in effectivity. Both studies required more time to reach the target temperature compared to the trial using servo-controlled equipment, as in that conducted by the NICHD (within 90 minutes).5 On the other hand, we feel that our way of cooling offers more stability and less of fluctuation than some servo-controlled systems. This is because our PCM mattress is in very close contact with the skin of the patient and PCMs do not have a swing in phase as a servo-controlled system does, where the temperature is fed back to the processor of the servo-control system, which adjusts the temperature of the cooling system to reach the intended temperature of the patient, who will then try to compensate while getting a lower temperature then that which is fed back to the servo-control system. Normally, the adjustment at the servo-control system is performed every minute.12
In terms of the ability to maintain the target temperature of 33.5°C-34.5°C, our results show that the PCM mattress provided stable body temperatures during cooling and that infants had temperatures below 32°C only 1% of the time. However, since the PCM mattress is a low-technology cooling device, it requires trained nurses and pre-specified interventions in order to maintain the target temperature. Interventions mentioned in the study procedures such as inserting a folded cloth sheet between the infant and the PCM mattress, covering the infant with another sheet or switching on the radiant warmer, were required in all cases. In the study by Thomas et al,22 different types of PCM blocks with different melting points (29°C and 21°C) were used for interventions similar to those of our study, but with PCM blocks added or removed to adjust temperatures. Many other ‘low-tech’ cooling methods, such as ice or frozen gel packs, are also labour intensive, and may result in marked temperature fluctuations and shivering, with a potential loss of neuroprotective efficacy.23-25
The rewarm of cooling infants was initiated in a slow and controlled manner (0.5°C/h) using PCMs. The advantage of PCMs over cool gel packs is that there is no need for frequent changes, there are fewer fluctuations in temperature and the risk of skin injury is minimised, which in this study resulted in no patients developing any skin ailment during treatment. An even more stable temperature might be possible using a blanket with PCMs integrated into the fabric; some non-medical fabrics are now on the market for other temperature spans; however, their use in a study such as this would require a medically approved version with our target temperature 26
Working in a low- to middle-income setting in a country on a fast track towards modern health care in the most advanced hospitals is challenging. Vietnam is one of the fastest growing economies, and technology and knowledge are rapidly changing. The staff size at hospitals is always limited, and the staff members need to learn how to operate new equipment and to increase general medical knowledge at the same time as they are busy handling the ward. Also, what was needed and recorded in medical journals at the beginning of the study is not the same as in the later phases. This is even more noticeable in referral hospitals. The theoretic knowledge at the referral hospitals and the receiving hospital, and the amount of patient history obtained at admission, improved during the study. Examples of other challenges are that the ward where the study was performed had a severe shortage of doctors and nurses who were caring for 150-200 neonatal patients in a small and crowded space designed for 80 patients. The ward also had various types of medical equipment from different donors that were often being used without appropriate training, which created handling problems, a problem frequently observed in NICUs around the globe. The situation improved when a part of one room was reserved for hypothermia that could be isolated from air conditioning units and fans. The standard practice of care in Vietnam is that severely ill neonates are isolated from their parents due to practical reasons, mainly limited space and crowded wards. The goal is to have caretakers more involved and closer to their newborns, as many studies have shown that the first connection between mother and child is of critical importance 27, 28
The study has some limitations. The nursing interventions during which it was discovered that the temperature readings were out of target range were not objectively measured. Our study was not designed to incorporate variations in endogenous thermogenesis or environmental temperatures. A different ambient temperature, especially in cases of diurnal temperature swing, may also affect the efficacy, stability and life expectancy of PCMs. These factors have to be carefully considered when interpreting the findings. Further, the effective cooling duration of the PCM mattress and its optimal melting point for newborn infants needs to be addressed more thoroughly; shielding the patients with a transparent cover that keeps the infants on the mattress and also reflects outside temperature swings better will be done during follow-up studies.
We could not use the eEEG/CFM as an inclusion criteria, since it was not available at the local hospitals and could not be used during transportation. The evidence of eEEG/CFM from other studies shows it is valuable if used correctly with the right timing.29, 30 However, it should be used during the whole treatment to be comparable with other studies.
5 CONCLUSION
Use of a PCM mattress is an effective and low-cost method of cooling infants in low- to middle-income countries, and probably during transportation anywhere. However, as this is not a servo-controlled system, careful monitoring and good nursing care are needed, especially during the induction and rewarming phases. However, this caveat applies to conventional servo-controlled systems as well. Further studies are needed to look at the influence of the environmental temperature on the performance of PCM mattresses and to determine their feasibility during transportation. Larger studies in other clinical settings are also needed to detect more unusual complications and handling problems with the method. Further, the optimal usage of PCM mattresses (if used for other brief terms, such as for short-distance transportation, which is a potential follow-up study to this one) and the optimal melting point for newborn infants need to be addressed in depth.
ACKNOWLEDGEMENTS
This work was undertaken at VNCH, which received a proportion of funding from the Swedish Research Council (VR), the Swedish Foundation for International Cooperation in Research and Higher Education, STINT (through the TRAC Collaboration Sweden-Vietnam). The authors would like to thank Medical Cooling Sweden for supplying the PCM mattress used in this study and all the clinical staff, from the director of the hospital to the nurses and cleaning staff of the Neonatal and Neurology departments at VNCH, for all their hard work. We would also like to acknowledge the support of Karolinska Institutet, the Research Institute for Child Health and Vietnam National Children's Hospital for giving us time to do important research that might save lives or help newborns with HIE to enjoy a better long-term outcome.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to present.
Appendix 1: Level of encephalopathy (Sarnat staging)
| Severity | Stage 1 (mild) | Stage 2 (moderate) | Stage 3 (severe) |
|---|---|---|---|
| Level of consciousness | Hyperalert | Lethargic or obtunded | Stupor or coma |
| Activity | Normal | Decreased | Absent |
| Neuromuscular control | |||
| Muscle tone | Normal | Mild hypotonia | Flaccid |
| Posture | Mild distal flexion | Strong distal flexion | Intermittent decerebration |
| Stretch reflexes | Overactive | Overactive | Decreased or absent |
| Complex or primitive reflexes | |||
| Suck | Weak | Weak or absent | Absent |
| Moro (startle) | Strong | Weak | Absent |
| Tonic neck | Slight | Strong | Absent |
| Autonomic function | |||
| Pupils | Mydriasis | Miosis | Variable |
| Heart rate | Tachycardia | Bradycardia | Variable |
| Seizures | None | Common | Uncommon |




