‘Curiouser and curiouser’: the role of vitamin D in the prevention of acute respiratory infection
In this issue of the Journal, Grant et al. report results of an eighth intervention study in children. This is an exploratory analysis of data from a clinical trial of vitamin D supplementation in pregnant mothers and their offspring, conducted to determine the effects of the intervention on incidence of ARI in children aged from birth to 18 months 11. The authors randomised 260 healthy pregnant women in Auckland, New Zealand, to receive daily oral placebo, lower dose vitamin D (1000 IU) or higher dose vitamin D (2000 IU) from 27 weeks’ gestation to birth. Their infants then received corresponding daily oral placebo, lower dose (400 IU) or higher dose (800 IU) vitamin D from birth to 6 months of age. Children were followed up to the age of 18 months, and the proportion of those making at least one primary care visit for ARI was compared between the study arms. In comparison with placebo, higher dose – but not lower dose – vitamin D supplementation was found to be associated with a modestly reduced risk of making at least one primary care visit for ARI, quantified as 87% risk for the higher dose vitamin D versus 99% risk for the placebo (p = 0.004). Intriguingly, this effect was driven by a decrease in ARI visits made by children in the higher dose arm of the study from the age of 6–18 months, that is when the children were no longer taking study medication. No effects of either the higher dose or lower dose vitamin D were seen on time to the first primary care visit for ARI or on the risk of hospitalisation for ARI.
This study is the first trial in children to compare the efficacy of two different dosing regimens for the prevention of ARI. The higher of the two doses is significantly more generous than that currently recommended in pregnancy and infancy by guidelines in Europe, Australasia and the United States. This is an important advance, because observational epidemiological studies tend to report that optimal protection against ARI is associated with 25(OH)D concentrations >75 nmol/L, which are not consistently achievable with the regimens that are currently recommended, particularly in pregnancy. The study is also novel in that both pregnant women and their offspring were randomised. This design feature acknowledges that the major determinant of neonatal vitamin D status is maternal 25(OH)D concentration; it also accommodates the potential for intrauterine vitamin D status to influence outcomes in offspring, as has been shown for other health outcomes such as bone mass. The study also has several noteworthy methodological strengths. Events for the primary analysis were derived from medical record audit, rather than self-report, and outcome data were available for 91% of randomised children. Reported compliance was at least 93% among pregnant mothers and at least 74% among infants at 6 months. A daily dosing regimen was employed: this is significant, because a question mark has recently been raised over the efficacy of intermittent bolus dosing regimens of vitamin D for the prevention of ARI 12.
The study also has some limitations. First, different measures of ARI incidence – parent report, hospitalisation data and primary care consultation data – were utilised for analysis at different follow-up time points. Second, although the majority of pregnant women (66%) had serum 25(OH)D concentrations below the optimum level of 75 nmol/L at baseline, a minority (42%) were vitamin D deficient at the 50 nmol/L threshold. There is some evidence to suggest that individuals with the lowest baseline vitamin D status may have the most to gain from supplementation in terms of protection against ARI 13, 14. Thus, larger and more consistent effects might have been seen if participants in the control arm had had lower baseline vitamin D status. Subgroup analyses restricted to participants with lower baseline vitamin D status were not performed, perhaps because power for such analyses in a trial of this size would have been limited.
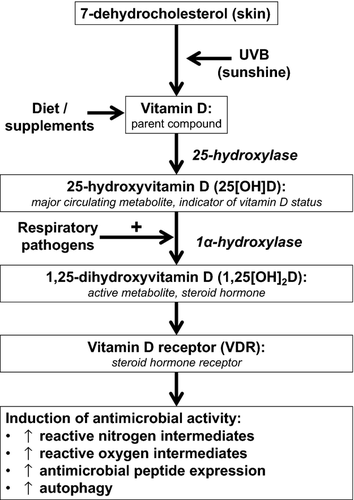
Is the finding of protection against ARI in the high-dose vitamin D arm likely to be real? There are some grounds for scepticism. The lack of consistent effects is the first issue: the positive results of the analysis of the proportion of children with any ARI visit are not mirrored by results of the time-to-event analysis or hospitalisation data. Second is the issue of timing: the effect of the higher dose intervention on ARI was only seen after supplementation was stopped. If this effect is real, its interpretation would require a paradigm shift in our thinking regarding mechanisms by which vitamin D might support antimicrobial responses. In vitro studies suggest that the 25(OH)D concentration at the time of an infectious challenge is likely to be the key determinant of susceptibility: both bacterial and viral pathogens have been shown to induce the 1-alpha hydroxylase enzyme CYP27B1 to drive local synthesis of 1,25-dihydroxyvitamin D, the active metabolite that ligates the vitamin D receptor to upregulate diverse antimicrobial responses (Fig. 1). Given that the half-life of 25(OH)D is approximately 1 month, interarm differences in vitamin D status would not have been maintained for long after discontinuation of trial medication at 6 months. Thus, any real interarm difference in ARI risk observed in the current study would have to have been mediated by a vitamin D-inducible factor other than circulating 25(OH)D concentration at the time of the infectious challenge. Grant et al. 11 raise the possibility that the effect may have been mediated via the influence of intrauterine 25(OH)D concentrations on lung development; an alternative explanation is that supplementation in utero may have resulted in epigenetic changes, which exert a delayed influence on susceptibility to respiratory pathogens. However, both of these potential explanations are rendered less likely by the observation that maternal vitamin D status at 36 weeks was similar in higher versus lower dose arms of the trial 15, yet no protection against ARI was seen in the low-dose arm. Moreover, several related clinical outcomes were explored without correction for multiple analyses: the possibility that the positive result arose from type 1 error must therefore be considered, and the authors acknowledge this.
Despite these caveats, when the findings of this study are taken together with those from other positive trials in the literature, there is still a signal here that is worth exploring. A further primary trial along similar lines, perhaps focusing on pregnant women with lower baseline vitamin D status, administering higher doses of vitamin D in the pregnancy phase and extending the duration of intervention and follow-up, would confirm or refute the findings of this study. In the meantime, inclusion of data from this trial and others in an on-going individual patient data meta-analysis 16 has the potential to increase power to identify factors such as baseline vitamin D status that might modify the efficacy of vitamin D supplementation for prevention of ARI and thus provide insight into the reasons for the striking heterogeneity of results from clinical trials in this field.






