Imaging diabetic retinal disease: clinical imaging requirements
TP, EKdJ, LS, ARB and AIdH conceived the original ideas. VS and MBL performed the review of literature, and wrote the manuscript, in consultation with TP, EKdJ, LS, ARB, AIdH, BJK, JG and CBH.
Abstract
Diabetic retinopathy (DR) is a sight-threatening complication of diabetes mellitus (DM) and it contributes substantially to the burden of disease globally. During the last decades, the development of multiple imaging modalities to evaluate DR, combined with emerging treatment possibilities, has led to the implementation of large-scale screening programmes resulting in improved prevention of vision loss. However, not all patients are able to participate in such programmes and not all are at equal risk of DR development and progression. In this review, we discuss the relevance of the currently available imaging modalities for the evaluation of DR: colour fundus photography (CFP), ultrawide-field photography (UWFP), fundus fluorescein angiography (FFA), optical coherence tomography (OCT), OCT angiography (OCTA) and functional testing. Furthermore, we suggest where a particular imaging technique of DR may aid the evaluation of the disease in different clinical settings. Combining information from various imaging modalities may enable the design of more personalized care including the initiation of treatment and understanding the progression of disease more adequately.
Introduction
Diabetic retinopathy (DR) is a sight-threatening complication of diabetes mellitus (DM) with a global prevalence of 34.6% (Yau et al. 2012). The development of high-quality DR screening programmes (DRSP) coupled with improved DM management already had a beneficial effect on DR incidence and prevalence in some countries (Liew et al. 2014). However, in most countries equity of service provision has yet to be achieved, and further improvements are required to prevent vision loss in people with DM (PwDM) globally (Lee et al. 2015). Appropriate imaging technologies followed by timely, detailed image analysis are imperative to reduce the DR burden on patients and society.
Diabetic retinopathy-related vision loss can occur as a result of vessel hyperpermeability and/or ischaemic changes, both of which can be visualized using appropriate imaging techniques. Today, most referral and treatment decisions are predominantly based on dilated retinal examination combined with fundus photography and optical coherence tomography (OCT). This is the case even when other imaging modalities, such as fluorescein angiography (FA), OCT Angiography (OCTA) and functional testing are available, offering more detailed insight into certain aspects of DR.
In addition, the DR severity scale, originally developed by the Early Treatment of Diabetic Retinopathy Study (ETDRS) research group, is widely recognized as the basis for categorizing DR severity and predicting the risk of visual loss (ETDRS 1991a). Although such categorical classification is useful for clinical decision making, it offers limited insight into individual patient's risk of progression or response to treatment.Furthermore, the heterogeneous clinical manifestation of DR demands a multimodal imaging approach to permit more granular understanding of DR and to pave the way towards personalized health care.
There is no substitute for good clinical patient history and where appropriate, subsequent clinical examination. Slit-lamp biomicroscopy (SLB) provides the basis of good clinical decision making as it allows for a 3-dimensional view of both the anterior and posterior segment of the eye; a distinct advantage especially in evaluating diabetic macular oedema (DME) and neovascularization. Where imaging is not available, SLB remains a valid way of assessing DR/DME patients. With the advent of new imaging modalities, it is worth working towards photography aided service as colour fundus photography (CFP) has been shown to have a higher sensitivity in detecting DR (Scanlon et al. 2003).
This review discusses recent advances of the relevant available imaging modalities for DR/DME and how various modalities contribute to effective decision making. Furthermore, this review aims to deliver a structured thinking process for developing a minimal set of imaging requirements in various healthcare settings.
Standard Colour Fundus Photography
Colour fundus photography (CFP) is the cornerstone of DR imaging. The ETDRS research group set the gold standard for CFP use in clinical trials, capturing 7-field 30–degree stereoscopic images in mydriatic eyes (Fig. 1; ETDRS 1991a). Clinically, CFP is most useful in the detection of DR features detailed in Table 1 (Table 1), Scanlon et al. (2003) showed the 2-field 45-degree macula plus optic disc-centred images used currently in most DRSPs to be equally effective in detecting disease as 7-field 30-degree images, but requires less time and less light exposure. The ability to use red-free image setting is essential for analysing CFP, allowing for better detection of certain retinal lesions, such as microaneurysms and retinal vascular changes, intraretinal microvascular anomalies (IRMA) and neovascularization elsewhere (Venkatesh et al. 2015).
| Feature | Appearance | Aetiology | Visible on modality |
|---|---|---|---|
| Increased capillary permeability | |||
| Microaneurysms | Focal dilatations of retinal capillaries. With SLB and CFP they appear as round, well-circumscribed red dots. On early phase FFA they appear as hyperfluorescent pinpoint dots, and can show focal leakage on late phase FFA. | As a result of pericyte and endothelial cell and smooth muscle cell loss, retinal arteriolar dilatation may increase capillary pressure and lead to focal dilatation of capillaries. | SLB, CFP, UWFP, FFA, OCTA |
| Hard exudates | Yellow/white, waxy deposits with sharp margins. Can be seen on OCT as hyperreflective material in the inner retinal layers. | Extravasation of lipids and proteins as a result of blood-retina barrier breakdown. | SLB, CFP, UWFP, OCT |
| Haemorrhages | Dot-, blot- or flame-shaped. Haemorrhages can be distinguished from microaneurysms, as they are larger, have less distinct margins, are less perfectly round, and not visible on FFA. | Small ruptures of vulnerable retinal structures: microaneurysms, dilated capillaries. Dot and blot haemorrhages are located in the inner retina, while flame haemorrhages can be present in the nerve fibre layer. | SLB, CFP, UWFP |
| Macular oedema | Increased macular volume on SLB and OCT. Often presents with fluid-filled cystoid spaces visible on OCT, but can also appear as diffuse oedema. Hyperfluorescent areas on FFA. | Accumulation of fluid with protein and lipids in the retina, caused by blood-retina barrier breakdown. | SLB, FFA, OCT, stereoscopic CFP |
| Ischaemic changes | |||
| Cotton wool spots (‘soft exudates’) | Greyish white fluffy lesions, not clearly delineated. On FFA, cotton wool spots are hypofluorescent in the early phase but can stain in late phase. | Infarction of nerve fibre layer from occlusion caused by arteriolar occlusion. | SLB, CFP, UWFP, FFA |
| Venous beading | Focal dilated segments of retinal venules. | Thickening of retinal venules, adjacent to areas of capillary non-perfusion. | SLB, CFP, UWFP, FFA |
| Intraretinal microvascular abnormality (IRMA) | Remodelling of retinal capillaries characterized by clusters of irregular tortuous vessels or dilation of existing capillaries, without proliferative changes. Can be distinguished from neovascularization by no or less profuse leakage on FFA. These vessels do not extend over vascular branches. Can be seen on OCTA with intraretinal flow below ILM. | Remodelling of retinal capillaries occurs as a result of retinal hypoxia. | SLB, CFP, UWFP, FFA, OCTA |
| Neovascularization | Abnormal new blood vessels, extension across both arterial and venous branches. Mostly located within 10–15 mm of the optic disc: neovascularization of optic disc, but can be located anywhere: neovascularization elsewhere. Show profuse leakage on FFA. On OCTA through the lesion show retinal new vessels breaching the ILM. | Proliferation of fibroblasts as a result of retinal hypoxia secondary to regional closure of the retinal capillary bed. | SLB, CFP, UWFP, OCT, FFA, OCTA |
- Abbreviations: CFP = colour fundus photography; FFA = fundus fluorescein angiography; ILM = Inner limiting membrane; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; SLB = split lamp biomicroscopy; UWFP = ultra-widefield photography.
For assessment of retinal thickness, stereoscopic CFPs have been shown to have moderate correlation with OCT findings (Davis et al. 2008). While stereoscopic images were widely used in the era of non-digital imaging, their use is diminishing predominantly due to the successful implementation of OCT pathways.
Development of portable fundus cameras opens up new avenues for service provision. In telemedicine ophthalmology and DR screening settings, these cameras assist healthcare provision in communities currently without access to optimal eye care, for example, in rural areas or developing countries. Studies that compare portable cameras, including smartphone-based retinal photography with standard CFP had promising results, although these cameras require further evaluation in different healthcare settings and in various populations (Rajalakshmi et al. 2015; Ryan et al. 2015). Although studies have shown good agreement between handheld devices and ETDRS 7-standard field photography, there are still an ungradable rate that varies greatly and need to be brought down for better implementation in clinical screening (Aquino et al. 2021; Salongcay et al. 2021).
Artificial intelligence (AI) is part of modern medicine and is being used in multiple settings. Deep learning (DL) is a class of machine learning (LeCun et al. 2015; Ting et al. 2019). Deep learning in medicine has been tested on image analysis and diagnostic performance in chest radiographs diagnosing tuberculosis (TB) (Lakhani & Sundaram 2017) and in differentiating skin cancer between malignant melanomas versus benign nevi with success (Esteva et al. 2017). In ophthalmology, these new technologies have also been introduced. Automated DR detection software takes advantage of the digital nature of CFPs and aims to deliver a safe and efficient system for identifying certain DR features on a timely manner (Abramoff et al. 2010). Deep learning algorithms show high sensitivity and specificity rates for detecting referable DR on CFP (Abramoff et al. 2013; Gulshan et al. 2016). These automated detection tools are subject to improved efficacy and safety, leading to a feasible screening approach that is now FDA-approved (Krause et al. 2018; van der Heijden et al. 2018; Olvera-Barrios et al. 2021). This DL system achieved an area under curve (AUC) of 0.980 and a sensitivity of 96.8% and specificity of 87.0% (Abramoff et al. 2016) The limitations are still the scalability and gathering more knowledge on it might perform in a different populations and ethnicities. Ting et al. (2017) evaluated the diagnostic performance of DL system for DR using 76 370 retinal images from the Singapore National Diabetic Retinopathy Screening Program and 10 multi-ethnic DM cohorts, achieving an AUC of 0.936 for referable DR and AUC of 0.958 for visually threatening DR (VTDR). Companies such as Google AI Healthcare is also showing strong interest having developed their own using 128 175 retinal images, subsequently, tested it on the publicly available databases EyePACS-1 and Messidor-2. The results of AUC was 0.991 for EyePACS-1 and 0.990 for Meesidor-2 are encouraging (Gulshan et al. 2016).
Even though multiple systems are available and have been tested against ophthalmic specialists, these DL systems still need further approval and testing in clinical settings before full implementation becomes a reality in everyday clinical setting. Many systems are also working on implementing detection of Age-related macular degeneration (AMD; Burlina et al. 2017; Ting et al. 2017; Grassmann et al. 2018) and glaucoma (Li et al. 2018; Thompson et al. 2020). Both of these diseases are common in the elderly population and would provide additional information on the imaging already taken for DR screening.
Widefield and Ultra-Widefield Photography
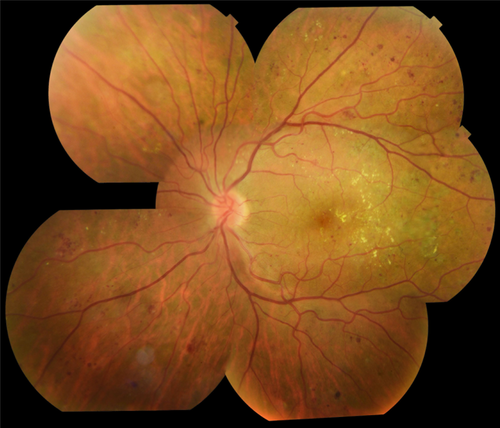
Widefield and ultra-widefield photography (UWFP) provide imaging of a larger retinal area in a single image compared to conventional CFP. New ultra-widefield cameras are able to capture up to a 200 degree field of the retina (Fig. 2) covering up to 80% of the retina, compared to 30% with ETDRS 7-field 30 degrees (Byberg et al. 2019) some UWFP even do so without mydriasis (Witmer & Kiss 2013). In addition, several of these cameras employ scanning laser ophthalmoscopy technology, using red and green reflectance imaging, instead of a white-light, multi-wavelength flash photography. This technique eliminates the reflection of light from interfaces in the ocular media but leads to an artificial colour rendition (Webb & Hughes 1981).
Comparison between ETDRS 7-field CFP and UWFP showed good agreement for DR detection and staging severity (Silva et al. 2013). The same agreement for central and macula DR staging was shown in a sample of patients with type 2 DM, where 5-field 30-degree photos were merged and compared to Optos UWFP, but with more peripheral lesions identified by the Optos (Byberg et al. 2019). Another study comparing ETDRS 7-field CFP to UWFP masked to cover the same area of the retina showed moderate to substantial agreement, but peripheral lesions on UWFP associated with an increased DR severity by 2 or more steps (ETDRS grading) in approximately 11.0% (Aiello et al. 2019). Likewise, peripheral lesions not detected by CFP correlated with an increased risk of DR progression and 4.7-fold increase risk for progression to proliferative DR over a period of 4 years (Silva et al. 2015a). For the detection of peripheral neovascularizations, UWFP performed better than standard 2-field 45-degree images (Talks et al. 2015). The clinical utility of this imaging modality must be explored further in order to understand how these data might alter risk assessment for DR detection and progression and how to communicate such differences to the patient and healthcare providers.
To the best of our knowledge, the standard 2-field CFP is still the most used in DR screening (Squirrell & Talbot 2003; Fenner et al. 2018). It is widely available and has well-documented grading schemes. Ultrawide-field photography captures more of the fundus and therefore might gain more popularity as it becomes more cost-effective to buy these machines. If the increased risk for progression to PDR in eyes with predominantly peripheral lesions (PPL) is confirmed, there might be a need to update DR classification in relation to risk stratification. This is explored in the prospective DRCR Protocol AA (Aiello et al. 2019).
Fundus Fluorescein Angiography
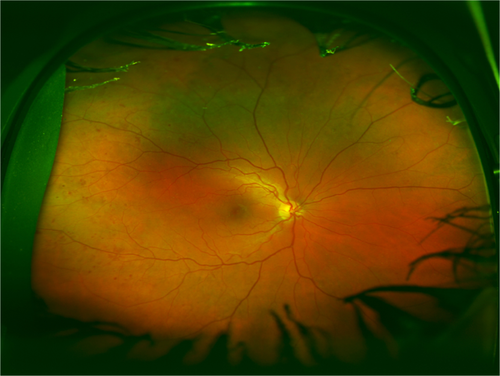
Fundus fluorescein angiography (FFA) evaluates the retinal circulation by capturing images at different time points after the administration of a fluorescent dye, allowing the visualization of the circulation, including ischaemia and leakage of fluorescent dye. Identifying the sources of leakage in DME can guide treatment by focal laser photocoagulation (ETDRS 1991b). Macular ischaemia associates with visual loss (Sim et al. 2013). Peripheral ischaemia is a risk marker for future PDR and subsequent need for panretinal laser photocoagulation (Fig. 3).
The ETDRS research group developed a DR classification system based on FFA grading for the presence and severity of capillary loss and dilation, arteriolar abnormalities, leakage of fluorescein dye, along with several other features (ETDRS 1991b). The comprehensive nature of the classification makes it a suitable research tool. However, it is time-consuming, requires excellent quality images, training in image analysis and so it is not widely used in daily clinical practice. For routine clinical assessment of FFA, including the severity of peripheral ischaemia, there is currently no gold standard.
Newer UWFP and UWFP FFA have the advantage of examining the periphery for features, such as neovascularization not visualized on standard 30 or 45 degrees FFA (Fig. 4). With the periphery visible it is possible to analyse peripheral non-perfusion that is known to highly correlate with presence of PPL and DR severity (Silva et al. 2015b; Antaki et al. 2020). It has been shown that a non-perfusion area of 107.3 disc area increases the risk for proliferative DR. The same study also reported that the non-perfused area was the largest in patients with NVD (Nicholson et al. 2019).

Fundus fluorescein angiography is a dynamic investigation that has the unique ability to show leakage independently of blood flow, a characteristic that aided the planning of treatment both for DME and PDR for several decades. Automated FFA segmentation techniques are being developed to reduce image grading time and therefore labour costs and they may provide reproducible measurements independent of clinician judgement (Rabbani et al. 2015). Due to FFA's invasive and time-consuming nature with potential for uncommon but possibly severe side effects, examination techniques without the use of injectable dyes are perhaps more preferable and are becoming more widely available in the form of OCT and OCTA.
Optical Coherence Tomography (OCT)
Optical coherence tomography provides non-invasive capture of high resolution, cross-sectional and three-dimensional retinal images and has been widely used in diagnosing and monitoring certain aspects of DR, mainly, but not exclusively DME (Fig. 5). Currently, spectral domain and swept source OCT are the most commonly employed OCT systems, enabling higher resolution imaging than the initially developed time-domain OCT systems.
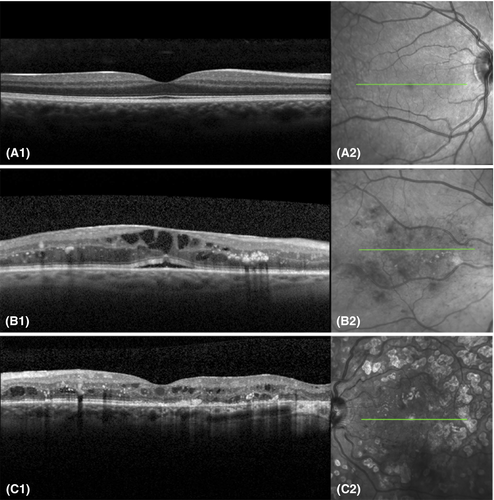
Optical coherence tomography allows both for quantitative measurements and qualitative assessment of several DME-related changes. Using OCT, DME can be quantified and evaluated for centre-involvement, a factor crucial for determining the need for injectable or laser treatments. The most frequently used quantitative measurement in DME evaluation is the central subfield thickness (CST), defined as the mean thickness of the combined retinal layers within the central 1000 μm diameter area around the fovea (Virgili et al. 2015). Determining CST on OCT is fast, simple and objective. These factors have made it a convenient measure of DME in regular clinical practice (Virgili et al. 2015). However, correlation between CST and visual acuity has shown to be modest at best and CST therefore cannot replace visual acuity as a surrogate biomarker (Ou et al. 2017).
Optical coherence tomography enables evaluation of additional morphological DR changes, mostly qualitative traits, such as different types of oedema (diffuse retinal swelling, cystoid oedema or subretinal fluid), hyperreflective material that is associated with hard exudates on CFP, or small hyperreflective foci that are thought to be precursors of hard exudates or inflammatory products and subfoveal neuroretinal detachment in inflammatory condition (Bolz et al. 2009; Vujosevic et al. 2020). Optical coherence tomography provides an opportunity to understand retinal layer integrity, such as that of the external limiting membrane, photoreceptor region and the retinal pigment epithelium in more detail. It is now understood that disorganization of the inner retinal layers is associated with macular capillary non-perfusion, and this is a potentially useful biomarker of visual acuity in DME (Sun et al. 2015) but further research is required to better understand the contribution of other retinal layers to DME pathophysiology. Additionally, inner retinal layer thinning has been described in PwDM without apparent DR and has been suggested to be a sign of neurodegeneration (De Clerck et al. 2015; Santos et al. 2017). As well as patients with early DR shows thicker macular GCL and thinner RNFL around the optic disc (Frydkjaer-Olsen et al. 2018). Furthermore, reduction in choroidal thickness has been reported, using enhanced depth imaging OCT systems, although this finding was not consistent in all studies (Melancia et al. 2016).
In DME clinical trials, use of OCT data is mainly focused on assessing treatment eligibility by determining macular thickness measurements, but other OCT features are rarely taken into account. Several research groups proposed more inclusive OCT-based DME classification scales, including macular volume, hyperreflective foci, and integrity of external limiting membrane and photoreceptor layers (Panozzo et al. 2004; Helmy & Atta Allah 2013). These classification systems are yet to be applied in clinical practice or clinical trials, as the additive value of detailed morphological characteristics in defining specific DME phenotypes is still awaiting.
Future directions for OCT include automated image analysis, using machine learning techniques (Schlegl et al. 2017). This could facilitate clinical decision making, as the images can be analysed more objectively both for retinal and choroidal features. The development of a portable, handheld OCT is potentially making OCT a valuable instrument in settings where financial considerations play a role, for example in a primary healthcare setting or in developing countries (Shelton et al. 2014). However, clinical application of such a device is yet to be validated in DR and purchase prices must be further reduced before it becomes a clinically meaningful tool in all settings.
The OCT is helpful in therapeutic decision making and follow-up of DME treatment response and must be part of a multimodal imaging approach in DR, and it may become even more valuable when other abnormalities besides CST are routinely taken into account.
Optical Coherence Tomography Angiography
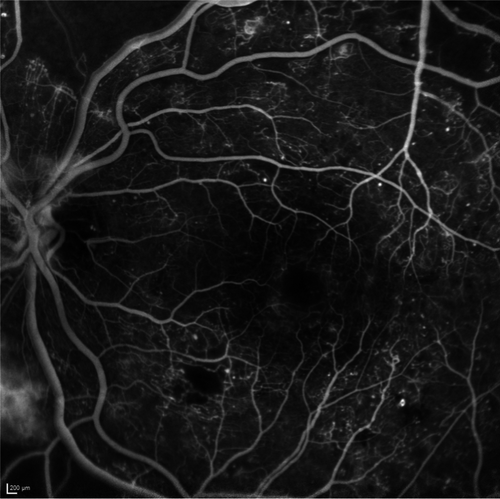
Optical coherence tomography angiography (OCTA), like OCT, is a non-invasive imaging modality that generates high-resolution three-dimensional images of the retinal and choroidal vasculature, providing insight into blood flow patterns (Fig. 6). With OCTA, the OCT signal is used to image the exact same cross-section multiple times. While the OCT signal backscattered from static tissue will remain identical, the signal from moving erythrocytes changes over time. This difference in OCT signal between the images is used to construct an image delineating blood flow.
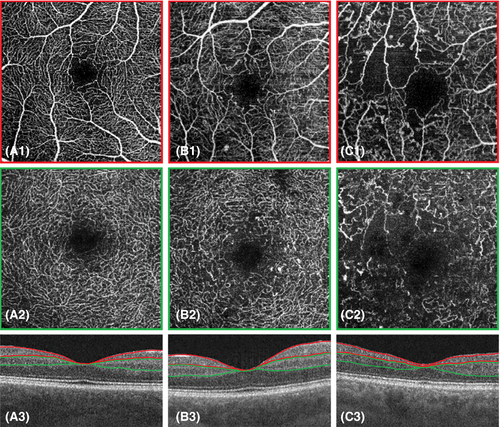
Fundus fluorescein angiography is currently considered the gold standard for imaging the retinal vasculature. However, comparative histological and angiographic studies reported that FFA lacks in the visualization of the deeper capillary network (Snodderly et al. 1992). OCTA images provide more details than FFA and enable depth-resolved analysis of the capillary plexi. This is of particular importance as microvascular DR abnormalities are commonly more pronounced in the deeper than in the superficial capillary plexi (Hasegawa et al. 2016; Vujosevic et al. 2021). For OCTA, administration of contrast agents is not needed, making it a non-invasive, low risk imaging modality. Optical coherence tomography angiography's major limitation at present is its inability to show vascular leakage (Fig. 7A). In addition, structures with slow blood flow that do not meet the speed threshold may not be delineated. Furthermore, this technique is prone to a range of artefacts, including image acquisition, intrinsic characteristics of the eye (e.g. media opacities), motion, image processing and display strategies, none of which should be beyond the capabilities of software engineers and so OCTA's image quality is likely to increase (Spaide et al. 2015).
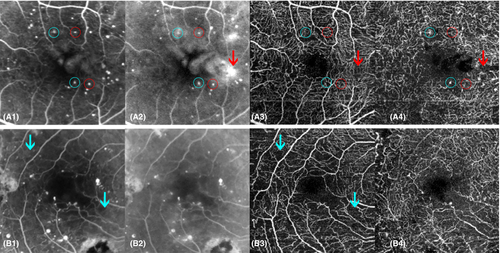
Although OCTA is a relatively new technique (since 2012), it has already proven its additional value in DR evaluation. Areas of impaired capillary perfusion can readily be identified and accurately quantified (Fig. 7B) (Ishibazawa et al. 2015). A frequently used measure of capillary dropout is vessel density, which is decreased in patients with DR and correlates with DR severity and visual acuity (Durbin et al. 2017; Samara et al. 2017). Reduced vessel density was even shown to precede clinically detectable DR and may be used as a prognostic biomarker for prediction of early onset DR (Czako et al. 2018). Optical coherence tomography angiography can identify and quantify the foveal avascular zone (FAZ), an enlargement of which has shown to be associated with worse visual acuity (Samara et al. 2017). There have been shown good agreement in measuring FAZ between FFA and OCTA (Enders et al. 2020). Fractal dimension, a measure of vessel branching complexity, is also shown to be reduced in DR (Zahid et al. 2016). Qualitative traits, such as detection of microaneurysms is also possible, although FFA was found to detect more microaneurysms than OCTA, possibly due to slow or turbulent blood flow (Fig. 7A) (Ishibazawa et al. 2015). In addition, OCTA visualizes other vascular abnormalities, such as vessel beading, tortuosity and neovascularization (Ishibazawa et al. 2015). Optical coherence tomography angiography gives the advantages of 3-dimensional images making it possible to distinguishing between IRMA and NV, based on whether it stays within the retinal layers (as IRMA) or breaches the inner limiting membrane (ILM) forming NV (Arya et al. 2020).
A promising OCTA feature is the ability to quantify flow speed. Generating perfusion indices based on flow speed and vessel density provides the ability to detect flow impairment and might allow for new ways of monitoring disease progression and therapeutic effects in the future (Choi et al. 2015). Other future directions for OCTA include the acquisition of large data sets with DR subjects, in order to gain additional insight into disease aetiology, progression and treatment response. Subsequently, additional diagnostic markers for development and progression of DR could be set and a new classification system established. Introduction of faster and more accurate widefield OCTA could also make them more useful in clinic as the alternative to FFA in terms of distinguishing between IRMA and NV in the periphery.
Functional Testing
Although electrophysiology and microperimetry are not imaging techniques as such, they play a role in the multimodal assessment of a patient, and as they generate what could be classified as images, they must be mentioned here. Currently, their use is mostly restricted to research settings, as they are time-consuming and accessibility is limited.
Electrophysiology: retinal/optic nerve function can be determined by electrophysiology of the visual system, a non-invasive technique that measures the electrical response of the retina/optic nerve to photic stimulations. Studies on electrophysiology of the visual system provided compelling evidence that neurodegenerative alterations are present in pre-clinical stages of DR (EUROCONDOR Project; Santos et al. 2017).
Microperimetry: with microperimetry, the visual field of the macula can be determined, through the emission of visual stimuli on very specific locations on the retina. In DR, and in DME in particular, macular sensitivity can be reduced, due to specific lesions, such as cotton wool spots, areas of macular ischaemia, macular hard exudates, cystoid oedema or due to degeneration of retinal tissue (De Benedetto et al. 2014).
Adaptive Optics
Adaptive optics (AO) is a non-invasive scanning laser ophthalmoscope to visualize microcirculations and retinal structures. It allows visualization of photoreceptors and have shown lower cone density in patients with DM even without signs of DR (Lombardo et al. 2016; Soliman et al. 2016). Cone density decreases with DR severity (Zaleska-Zmijewska et al. 2019). Adaptive optics is still not an integrated part of DR screening and testing, this likely because it is costly and time-consuming (Akyol et al. 2021), its functionality is more often used in inherited retinal diseases (Shah et al. 2019).
Summarization of Imaging Modalities
In the sections above, we have viewed the different imaging modalities used in DR/DME. An overview of the recent advances in current imaging techniques for DR discussed in this review is provided in Table 2.
| Imaging modality | Recent advances |
|---|---|
| CFP |
|
| (U)WFP |
|
| FFA |
|
| OCT |
|
| OCTA |
|
In Table 3, we summarize the imaging modalities and how they can be used in primary and/or secondary care, in treatment setting and to monitor patients post-treatment.
| SLB | CFP | UWFP | FFA | OCT | OCTA | ERG | MP | Comments | Usability in research | |
|---|---|---|---|---|---|---|---|---|---|---|
| Description | Manual 3-dimensional examination of anterior and posterior segment of the eye | Colour photograph of a selected part of the fundus | Colour photography comprising a larger area than CFP, peripheral retina also visualized | Dynamic test with contrast agent to visualize retinal circulation | Non-invasive, cross-sectional image of the central macular area | Extension of OCT; non-invasive image of (macular) micro-circulation | Functional test: measurement of electrical response of retinal cells | Functional test: perimetry of the macular area | ||
| Screening purposes in primary care settings | Only if well trained staff is available and there is no possibility of imaging | Minimum of 2-field CFP | Yes | No | In certain settings beneficial for reducing false positive maculopathy rate | No | Currently not applicable, but new devices need to be evaluated | No | Functional testing is a reasonable requirement from the patient's point of view | 2-field CFP does not provide enough information for careful phenotyping |
| Secondary care review clinics | X | X | x | Only in certain circumstances | X | Currently not feasible | Same as Primary care | No | ||
| Treatment clinics | X | 7-field CFP | X | (X) | X | (X) | In some instances | In some instances | FFA and OCTA if indicated | Either 7-field CFP or UWFP provide enough information; OCT for evaluation of retinal layer integrity |
| Post-treatment, monitoring | X | X | X | (X) | X | (X) | FFA and OCTA if indicated |
- Abbreviations: CFP = colour fundus photography; ERG = electroretinography; FFA = fundus fluorescein angiography; MP = microperimetry; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; SLB = split lamp biomicroscopy; UWFP = ultra-wide-field photography; (X) = can be used in clinical guidance for retinal laser photocoagulation/anti-VEGF treatment and/or differential diagnosing for PDR /CNV.
Discussion
In the last decades, the various imaging techniques became integral part of DR/DME evaluation. Only two decades ago, diagnosis of diabetic eye disease relied on clinical examination and some additional imaging using CFP and FFA. Some of these imaging modalities have been partially replaced by newer techniques, such as FFA's central role in the evaluation of DME that is now taken over by OCT and OCTA. Notwithstanding replacement, clinical practice has shifted towards the use of multimodal imaging, evaluating features visualized on multiple imaging modalities at once to establish individual risk assessment. This has led to an increasing dependence on multimodal imaging characteristics for management decisions in DR/DME. However, not all settings require the same set of imaging modalities to establish individual risk assessment. In a screening setting, health care practitioners rely on different imaging characteristics (e.g. the presence of microaneurysms or haemorrhages) than ophthalmologists in a treatment setting, for whom features such as macular oedema or ischaemia play a more prominent role.
Individual risk assessment can be achieved by using the appropriate set of imaging modalities. Sensitivity and specificity of the imaging modality to detect DR features, burden of the examination on the patient and the financial burden must be balanced. For DR screening purposes, 2-field 45-degree CFP may be sufficient even for prognostication as long as there are at least two sets of images available, a year apart (Scanlon et al. 2013). For making treatment decisions, additional imaging modalities may be required. For focal or panretinal laser photocoagulation, FFA may be indicated to guide treatment, especially for targeting microaneurysms in the macula or non-perfused peripheral retina. If centre-involving DME is suspected, OCT can assist with decision making on intravitreal injections and subsequent monitoring of disease progression and treatment response.
The gold standard ETDRS classification methods we use today are based on CFPs, based on the Airlie House Classification, Virginia, USA in 1968 (ETDRS 1991b). With respect to screening for DR, this classification is still useful as it is universally accepted and understood and can feed the CFP characteristics into automated grading applications. Such technological and AI developments may facilitate a shift towards telemedicine DR screening that takes place in the living room of the patient or within a rural area using a smartphone camera.
Once a patient has been referred to an ophthalmologist; however, the CFP based modified Airlie House DR classification no longer provides enough detail for management decisions. Valuable features on other imaging modalities, such as disorganization and the thickness of retinal layers on OCT and capillary vessel density or the extent of ischaemia on FFA or OCTA, are not taken into account. For provision of appropriate diabetic eye care, a more comprehensive imaging and image analysis protocol and a comprehensive new classification system might yield improved stratification for prognostication and treatment options.
In conclusion, multimodal imaging has become an integral part of the evaluation of diabetic retinal disease. We are likely missing opportunities for lowering the impact of diabetic eye disease if we do not use all the imaging information available. Understanding how new cameras and other imaging machines such as portable and handheld devices can reduce inequalities of service provision, together with advances in AI are likely to lead to new ways of identifying and managing diabetic retinal disease.




