Oral consumption of Garcinia kola(Bitter kola) lowers intraocular pressure
Abstract
Purpose
Garcinia kola (bitter kola) is locally ingested across the West African subregion. It has ocular hypotensive effects similar to some commonly used glaucoma medications when administered topically. The study assessed the effect of oral ingestion of G. kola on intraocular pressure (IOP).
Method
A randomized, single-blind, placebo-controlled, cross-over design was used in this study. Forty-six healthy subjects, aged between 19 and 27 years were recruited and randomized into two groups (A and B). Subjects in group A ingested 100 mg/kg body weight bitter kola in a 200 ml solution on their first visit and group B ingested 200 ml of water. On the second visit, the order of treatment was reversed, IOP was measured at baseline and every 45 min interval for 135 min. The mean difference between the baseline and post-treatment IOP measurements were tested for statistical significance using repeated-measures analysis of variance (95% confidence intervals [CIs]).
Results
Mean IOP measurements decreased by 7.9, 18.2 and 20.6% at 45, 90 and 135 min, respectively, after G. kola treatment. The reduction, though variable across subjects, was statistically significant (F [2.13, 95.62] = 90.35, p < 0.0001) across the respective time points. Repetition of an identical protocol without G. kola caused clinically negligible changes in IOP. There was no statistically significant influence of gender or age in G. kola effect on IOP reading.
Conclusion
Oral ingestion of G. kola lowered the intraocular pressure of healthy young adults by 21%. Such an effect may be of therapeutic benefit to patients with POAG or ocular hypertension in low-income settings.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide (Quigley & Broman 2006). Its most common form, primary open-angle glaucoma (POAG), is characterized by progressive optic nerve degeneration and affects more than 60 million people worldwide. In Africa, glaucoma accounts for 15% of blindness and it is the region with the highest prevalence of blindness relative to other regions worldwide (Resnikoff et al. 2004). Intraocular pressure (IOP) is the only modifiable factor in patients with glaucoma; therefore, treatment with IOP-lowering medication has been critical to prevent blindness. However, several authors reported that long-term use of glaucoma medication resulted in ocular surface disease in some patients, and subsequently led to poor adherence to the glaucoma treatment regimen in these patients (Leung, Medeiros & Weinreb 2008). The development of a biologically active natural product that is effective in lowering IOP and have fewer side effects might be critical to improving glaucoma treatment compliance. One of such natural products is Garcinia kola, a member of the Guttiferae plant species found throughout West and Central Africa (Heckel & Schlagdenhauffen 1884).
Garcinia kola(G. kola), colloquially referred to as ‘bitter kola’ because of its typical distinct bitter taste, has anti-inflammatory, antiparasitic, antimicrobial and antiviral properties (Heckel & Schlagdenhauffen 1884; Adegbehingbe et al. 2008). The seeds have been shown to contain a complex mixture of polyphenolic compounds, biflavonoids, prenylated benzophenones and xanthones which account for its wide pharmacological properties (Hussain et al. 1982; Iwu & Igboko 1982). Kolaviron (KV), the major component isolated from the seeds of G. kola, is regarded as the pharmacodynamics behind G. kola action. It has been previously reported that kolaviron is a likely inhibitor of acetylcholinesterase (Ijomone & Obi 2013).
In Africa, G. kola is prevalently used for traditional hospitality and serve a variety of roles in ethnomedicine in the treatment of several ailments including coughs, colds, voice hoarseness, aphrodisiac and liver diseases (Farombi 2011; Iwu 2014). Its systemic blood pressure lowering effects has also been reported in a previous study (Naiho & Ugwu 2009). It has also shown potential utility for fighting virulent diseases, including Ebola, by halting viral replication (BBC 1999). In addition, there is significant scientific evidence suggesting that G. kola is safe in humans at normal consumption level (Adefule-Ositelu, Adefule & Giwa 1996; Adefule-Ositelu et al. 2004).
The IOP-lowering effect of topical aqueous solutions of G. kola has been demonstrated in both animal (Adefule-Ositelu, Adefule & Giwa 1996) and human studies, including a recent randomized clinical trial which examined different IOP-lowering medications (Adefule-Ositelu et al. 2010). Although the previous studies have demonstrated the therapeutic effectiveness of topically administered G. kola solutions in reducing IOP, the effect of the oral ingestion has not been studied previously. In the current study, we examined the acute effect of oral intake of G. kola on the intraocular pressures of normal young adults.
Methods
Study design
The study utilized a randomized, investigator-masked, placebo-controlled, cross-over experimental design. Subjects were randomized to receive either G. kola or water-treatments in two different sessions. The researcher performing the Goldmann applanation tonometry was masked to the treatment that had been administered to the subjects in a bid to eliminate any possible researcher bias. However, it was not possible to mask the subjects to the treatments because of G. kola’s characteristic bitter taste.
Inclusion and exclusion criteria
Subjects were eligible if they were aged between 19 and 27 years, and emmetropes with normal ocular examinations using hand-held slit-lamp biomicroscope (BX 900, Haag-Streit USA) and direct and indirect ophthalmoscope (Keeler, Halma UK), as appropriate. They had a baseline IOP measures of between 11 and 24 mmHg in both eyes, and none of the subjects was a casual or habitual G. kola consumer.
Subjects were excluded if they had a family history of glaucoma, any ocular or systemic disease, or were taking any forms of medication at the time of the study. Subjects were also excluded if they reported allergic reactions to G. kola as previously documented (Adegbehingbe et al. 2008) or have potential risk factors for angle-closure glaucoma. Other exclusion criteria included ocular trauma or previous eye surgery, corneal abnormalities or dry eyes that could prevent accurate applanation tonometry.
Setting
Data collection procedures were carried out in a patient care setting at the Optometry Clinic of the University of Cape Coast, Ghana. The clinic provides quality eye care to the University and its surrounding communities. It also serves as a teaching facility for Doctor of Optometry clinical students.
Study subjects
The study subjects were recruited from the University of Cape Coast student population. Fifty-five students volunteered to take part in the study after explanation of the nature and possible consequences of the study. Each participant had a systemic health review and comprehensive ocular health assessment including dry eyes assessment. Three had corneal abnormalities (advanced pterygium) and did not qualify and two withdrew consent before randomization. Of the 50 remaining individuals, two withdrew consent before receiving the first treatment and two were withdrawn for scheduling constraints (they could not be contacted). Forty-six subjects met the study inclusion criteria and were selected for randomization. The randomization was done by a neutral second party with no involvement in the study, at the Optometry Clinic of the University of Cape Coast, Ghana.
Ethical consideration
The study was approved by the institutional review board (IRB) of the University of Cape Coast, Ghana, and the experimental procedures were conducted in accordance with good clinical practice guidelines and the principles of the Declaration of Helsinki. Informed consent was obtained from each subject before the commencement of data collection.
Data collection procedures
The experiments took place within a week after recruitment. Subjects were instructed to abstain from coffee, any form of cola, and kola nuts, for at least 1 week before receiving the first treatment and also did the same until they received the second treatment. Subjects were also asked to abstain from food, water and other beverages in the morning before, and during the experimental interval and to arrive at the clinic at 9 AM on the study day. Participants were reminded of these instructions and their scheduled time, a day before the experiment through phone calls. Body mass was measured for each subject after they had provided written informed consent. Afterwards, subjects were randomly assigned to A or B treatment groups following simple randomization procedures by a computer-generated random number list (Excel 2007, Microsoft, Redmond, WA, USA).
On their first visit, group A subjects ingested 100 mg/kg bodyweight of anhydrous G. Kola (Herb Store USA, Walnut, CA) which was mashed and dissolved in 200 ml of distilled water as the solvent and then served in a glass cup whereas group B subjects ingested 200 ml of distilled water. The order of treatment was reversed on the second visit, with group A subjects ingesting 200 ml distilled water while those in group B ingested 100 mg/kg bodyweight of anhydrous G. kola which was mashed and dissolved in 200 ml of distilled water as the solvent and then served in a glass cup over the same time period. The dosage of 100 mg/kg was used in this study which is equivalent to 5.0–9.6 g dose of G. kola depending on body weight (Davies & Mohammed 2013; Maňourová et al. 2019) and falls within the average daily consumption of two nuts of bitter kola. This dosage was considered to be safe because some authors speculate that high doses (400 mg/kg) of G. kola can be toxic to human organs and may cause liver damage (Biliaminu et al. 2016), and peptic ulceration (Nwafor & Ogheneaga 1992). Furthermore, most human (Esimone et al. 2002) and animal (Nwafor & Ogheneaga 1992) studies had given low and moderate doses of 100 mg/kg and 200 mg/kg of G. kola. A sufficient recovery period of at least 5 days was provided before subjects received the second treatment to eliminate any potential carryover effect. Five days was chosen as the washout phase because it was anticipated that the concentration of G. kola in the blood would have fallen to a pharmacologically insignificant level by the sixth day.
Over 135 min, IOP was measured by an experienced personnel every 45 min. Intraocular pressure (IOP) was measured in both eyes with a Goldmann applanation tonometer (AT 900D, Haag-Strait USA). The baseline IOPs were measured bilaterally after which the subjects were exposed to a treatment. Topical 0.4% Proparacaine Hydrochloride was applied as the local anaesthetic and sodium fluorescein strips (OPTITECH Eye Care, India) were used to outline the area of flattening. All data were collected during the day, between 9:00 AM and 12:00 PM. This period was chosen to minimize the influence of diurnal variations in IOP. Three consecutive readings obtained with variations of less than 3mmHg was considered to be an acceptable result and averaged for analysis. The information on which subject received the experimental or control treatment was not revealed to the examiner measuring IOP until the end of the investigation.
Data analysis
Data were analysed using repeated-measures ANOVA with the statistically significant level set at p < 0.05. The assumption of data normality was checked with the Shapiro-Wilk test of normality. Greenhouse-Geisser corrected degrees of freedom were computed to minimize the effects of violating assumptions about data sphericity for repeated-measures ANOVA. Pairwise t-tests with a post hoc Bonferroni correction were used when applicable. Test of correlation was performed to determine associations between the measured variables. As the Spearman correlation coefficient for IOP readings in the left and right eye was high (r = 0.87, p = 0.001), only right eye data (n = 46) were used for analyses. All data were presented as mean differences between treatments and 95% confidence intervals (95% CI).
Results
Demographic characteristics of subjects
All 46 subjects enrolled in the study completed all two sessions of the study. They had a mean age of 23.29 ± 2.10 years (range, 19–27 years). Twenty-five (54%) were male, and 21 (46%) were female. Males had a mean age of 24.36 ± 1.55 years and were slightly older than females by a mean age difference of 2.36 ± 0.51 years (p < 0.001, t = 4.64, 95% CI, 1.34–3.38). The weight of the subjects range from 50–86 kg, with a mean of 64.00 ± 9.314 kg (95%CI 61.23–66.77). Males and females weigh 63.0 ± 8.07 kg and 65.9 ± 10.70 kg, respectively (p = 0.433).
Normality of measured variables
The mean IOP measured before and after G. kola intake were all normally distributed (p > 0.05, Shapiro-Wild test of normality); however, age data of study subjects were not normally distributed (p = 0.003). Table 1 summarizes the results of Shapiro-Wilk test of normality.
| Variable | Statistics | p |
|---|---|---|
| Age | 0.914 | 0.003 |
| Body mass | 0.955 | 0.076 |
| Baseline IOP (G. kola) | 0.944 | 0.067 |
| IOP at 45 min (G. kola) | 0.94 | 50.071 |
| IOP at 90 min (G. kola) | 0.967 | 0.133 |
| IOP at 135 min (G. kola) | 0.996 | 0.230 |
| Baseline IOP (water) | 0.950 | 0.073 |
| IOP at 45 min (water) | 0.953 | 0.070 |
| IOP at 90 min (water) | 0.947 | 0.061 |
| IOP at 135 min (water) | 0.954 | 0.101 |
The effect of G. kola on IOP
The results of the experiment are summarized in Tables 2 and 3. The mean baseline IOP reading was 15.80 ± 2.47 mmHg (95% CI, 15.08–16.53) before exposure to G. kola. The IOP readings measured after G. kola’s treatments were 13.55 ± 2.42 mmHg (95% CI, 12.83–14.28), 12.93 ± 2.42 mmHg (95% CI, 12.21–13.66) and 12.54 ± 2.38 mmHg (11.83–13.26) after 45, 90 and 135 min, respectively. These represent percentage reductions in IOP of 7.9, 18.2 and 20.6% at the respective time points. Mauchly’s Test of Sphericity indicated that the assumption of sphericity had been violated, χ2 (5) = 31.82, p < 0.0001, and therefore, a Greenhouse-Geisser correction for degrees of freedom was used to assess the observed F-ratio. Oral ingestion of G. kola had a statistically significant effect on IOP, (F (2.13, 95.62) = 90.35, p < 0.0001). Post hoc tests using the Bonferroni correction revealed that G. Kola intake elicited a marked reduction in IOP from baseline to 45 min of ingestion (15.80 ± 2.47 mmHg versus 13.55 ± 2.42, respectively), which was statistically significant (p < 0.0001). Another statistically significant reduction in IOP was found between baseline and 90 min post-G. kola intake (15.80 ± 2.47 mmHg versus 12.93 ± 2.41, respectively; p < 0.0001). There was a marked decrease in IOP reading at 135 min after G. kola intake (15.80 ± 2.47 mmHg versus 12.54 ± 2.38 mmHg), which was statistically significant (p < 0.0001). Table 2 represents the results of the Bonferroni post hoc test to determine the effect of G. kola on IOP. The influence of gender and age in G. kola’s effect on IOP reading was also assessed. There was no statistically significant gender or age influence in G. kola effect on IOP reading (p > 0.05).
| Pair | Mean difference ± SEM | p | 95% CI for difference | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| 1 | 2.25 ± 0.24 | <0.001 | 1.60 | 2.90 |
| 2 | 2.87 ± 0.24 | <0.001 | 2.22 | 3.56 |
| 3 | 3.26 ± 0.29 | <0.001 | 2.49 | 4.06 |
- 1 = Baseline – 45 min, 2 = Baseline – 90 min, 3 = Baseline - 135 min, 4 = Baseline – 180 min.
| Pair | Mean difference ± SEM | p | 95% CI for difference | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| 1 | 0.28 ± 0.124 | 0.195 | −0.070 | 0.620 |
| 2 | 0.15 ± 0.076 | 0.340 | −0.062 | 0.362 |
| 3 | 0.30 ± 0.096 | 0.020 | −0.033 | 0.567 |
- 1 = 45 min - Baseline; 2 = 90 min - Baseline; 3 = 135 min - Baseline; 4 = 180 min – Baseline.
The effect of water on IOP readings
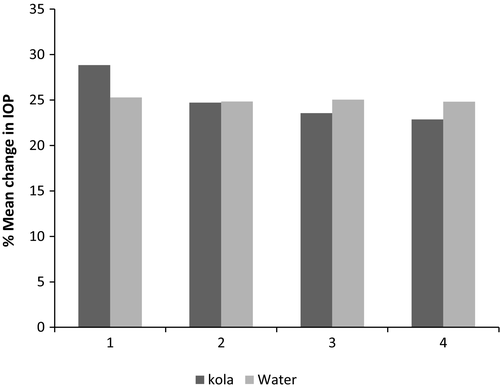
The mean baseline IOP reading was 15.83 ± 2.76 mmHg (95% CI, 14.94–16.71) before exposure to water. The readings measured after water treatments were 15.55 ± 2.82 mmHg (95% CI, 14.65–16.45), 15.68 ± 2.78 mmHg (95% CI, 14.79–16.56) and 15.53 ± 2.76 mmHg (95% CI, 14.64–16.45) after 45, 90 and 135 min, respectively. These represent percentage reductions in IOP of 1.8, 0.95 and 1.9% at the respective time points. The effect of water on IOP readings was similarly analysed with repeated-measures ANOVA. Mauchly’s Test of sphericity indicated that the assumption of sphericity had been violated, χ2 (5) = 16.72, p = 0.005, and therefore, a Greenhouse-Geisser correction for degrees of freedom was used to assess the observed F-ratio. There was a significant effect of time on IOP measurements after water intake, (F (2.27, 24.98) = 3.54, p = 0.026). Post hoc tests using the Bonferroni correction revealed that water intake elicited a slight reduction in IOP of 0.275 ± 0.124 from baseline to 45 min of intake (15.80 ± 2.76 mmHg versus 15.55 ± 2.82 mmHg, respectively) which was not statistically significant (p = 0.195). Another statistically non-significant reduction in IOP of 0.150 ± 0.076, was found between baseline and 90 min postwater intake (15.80 ± 2.76 mmHg versus 15.67 ± 2.78 mmHg, respectively; p = 0.340). There was also a slight reduction in IOP of 0.30 ± .096 mmHg, at 135 minutes after water intake (15.80 ± 2.47 mmHg vs. 15.53.54 ± 2.76 mmHg), which was statistically significant (p = 0.020). Table 3 represents the results of the Bonferroni post hoc test to determine the effect of water on IOP. Figure 1 represents the mean change in IOP after G. kola and water treatments.
Association between measured variables
The Spearman correlation test was used to analyse any association of age and body mass with the IOP readings measured at the various time points after G. kola intake. The analysis revealed no statistically significant correlation between the measured variables. Table 4 summarizes the results of the Spearman correlation test.
| IOP_0 | IOP_45 | IOP_90 | IOP_135 | ||
|---|---|---|---|---|---|
| Age | Spearman correlation | 0.005 | 0.031 | 0.005 | 0.023 |
| P (2-tailed) | 0.973 | 0.838 | 0.975 | 0.878 | |
| Body mass | Spearman correlation | −0.156 | −0.253 | −0.274 | −0.289 |
| P (2-tailed) | 0.302 | 0.090 | 0.065 | 0.051 | |
| Gender | Spearman correlation | 0.081 | 0.037 | 0.032 | 0.022 |
| P (2-tailed) | 0.591 | 0.809 | 0.835 | 0.887 | |
- IOP_0 = IOP reading at baseline, IOP_45 = IOP reading at 45 min, IOP_90 = IOP reading at 90 min, IOP_135 = IOP reading at 135 min.
Discussion
Although the IOP-lowering effect of topically administered G. kola (bitter kola) has been sufficiently substantiated with scientific evidence, the effect of its oral ingestion on IOP has not been studied. Because oral ingestion is the widely used route for the consumption of G. kola, any IOP-lowering effect could be important for patients with POAG especially in low-income settings.
Therefore, we have used a randomized controlled cross-over experimental model to determine whether G. kola administered orally in a dose comparable to normal daily consumption levels will reduce IOP significantly in healthy young adults. We found that IOP decreased significantly at 45 min of oral ingestion of G. kola and remained reduced for over 2 hr. In comparison with changes observed after water intake, there was an approximately 21% significant reduction in IOP from baseline to at least 135 min of oral administration of G. kola, and there appears to be no effect of gender or age.
These results support the conclusions of two previous studies. Specifically, Adebukunola and colleagues (Adefule-Ositelu et al. 2010) reported mean IOP reductions of 24% in G. kola -topically treated eyes of newly diagnosed patients with POAG or ocular hypertension after 6th week of follow-up. The slight difference between our absolute percentage IOP reduction effect of G. kola and theirs maybe because they investigated cases with primary open-angle glaucoma or ocular hypertension. Second, the animal studies by Adefule-Ositelu, Adefule & Giwa (1996) demonstrated a decrease in IOP of 62.9%–76.2% in normal eyes of guinea pigs, rabbits, sheep and dogs over a two-week period of treatment with G. kola 0.5% aqueous eye drops. It would seem likely that the relatively short period of post-treatment observation in our study limited the magnitude of therapeutic effect that should have been achieved with the oral ingestion of G. kola. However, the American Academy of Ophthalmology's Practice Guidelines for open-angle glaucoma (Prum et al. 2016) recommend an IOP reduction of at least 20% below baseline intraocular pressure as clinically relevant IOP reduction in a POAG patient, and this was achieved in G. kola-treated group.
The significant mean decrease in IOP of 0.30 ± 0.096 mmHg (1.9% decrease) observed in the water-treated group at 135 min was clinically negligible, and so we could not conclude that water produced the observed effect in the G. kola-treated group. Repeated tonometry may induce a decline in estimated IOP as a result of eye rubbing (Liu & Lin 2009) or body tilting (Nagaraju & Porciatti 2008; Malihi & Sit 2012), although other mechanisms could have contributed to this response. To distinguish between the IOP changes attributable to the therapeutic effect of G. kola and that attributable to repeated IOP fluctuations, we compared the percentage of subjects achieving reductions in the water and G. kola-treated groups. The percentage of subjects who achieved clinically relevant changes in IOP of at least 20% decrease from baseline at 135 min was approximately 70% in the G. kola-treated group and none in the water-treated group; among those in the G. kola-treated group, 35% achieved a decrease in IOP of ≥25%. Clinically relevant repeated IOP variations occur only over time frames of 12–24 hr, as well as over visits separated by days, weeks or months (Realini, Barber & Burton 2002). Moreover, no significant decrease in IOP after 200 ml of oral water intake have been reported in cited research studies (Kronfeld 1975; Brucculeri et al. 1999).
The exact mechanism by which G. kola lowers IOP, presumably by the action of kolaviron, is unknown. A recent study demonstrated that kolaviron, isolated from G. kola, is a likely inhibitor of acetylcholinesterase (AChE) activities in the hippocampus and striatum of wistar rats8. Again, AChE inhibitors are known hypotensive agents which have been shown to have an IOP-lowering effect in animal studies (Goldblum, Garweg & Bohnke 2000). As has been postulated for other AChE inhibitors, the IOP-lowering effect of G. kola probably results from the activity of kolaviron which inactivates AChE and potentiate the action of endogenous acetylcholine, leading to contraction of the ciliary body, which, by pulling on the scleral spur, opens up the trabecular meshwork and thereby enhances aqueous humour outflow (Kaufman & Barany 1976). Conversely, G. kola might act by inhibiting the active process of aqueous secretion through the action of carbonic anhydrase and/or sodium potassium activated adenosine triphosphate (Na+ K+ ATPase). Chromatography and pharmacodynamics analyses of G. kola nut extract is compatible with this mechanism (Adefule-Ositelu et al. 2010). Also, roles for common physiologic factors, such as vasodilation which reduces aqueous production through the lowering of perfusion pressure, and a miotic effect which increases outflow facility have also been proposed (Adefule-Ositelu et al. 2005). Our study design did not allow for an exploration of the mechanisms underlying the observed IOP-lowering effect of G. kola. Further studies are required to investigate these proposed actions.
Our study has all the characteristics of a good experiment. Subjects were randomly assigned to receive two different treatments of either 100 mg/kg bodyweight of bitter kola mashed in 200 ml of water or 200 ml water for the first visit and the alternate treatment for the second visit, in a randomized controlled examiner-masked manner. However, the fact that subjects knew which study treatment (Garcinia kola or water) they received can affect physiological and psychological responses, and thus influence the IOP values. Also, randomization was done without stratification for gender but any influence of gender was reduced to a minimum because of the cross-over design. Furthermore, we recruited normal patients, taking into account that patients with glaucoma are relatively more likely to show a spontaneous decrease in IOP on repeated applanation tonometry measurements. Nevertheless, results in normal patients may not represent the effects that would be present in glaucoma patients due to differences in susceptibility and sensitivity to changes of net movement of fluid into the eye. It is also likely that the magnitude of reduction in IOP after oral consumption of G. kola will be higher in glaucoma patients than in normal patients.
This study has several limitations. First, the participants may not be representative of the wider population, the cohort size was small and their selection may be biased because they were recruited for study inclusion based on convenience sampling. The participants were consecutively selected in order of appearance until the estimated number of participants was reached. The sample size was further limited by the restrictive exclusion criteria applied. The internal validity of the study, however, will not be affected, because there was randomization of subjects. Moreover, our results were consistent between the water- and G. kola- treated eyes over the 3-time points in the experiment despite the masking. Also, statistical analyses showed significant reductions in IOP from the interventions despite the small sample.
Second, Goldmann applanation tonometry, although regarded as the gold standard for IOP measurement in clinical practice, is an invasive method which employs the use of fluorescein to outline the area of flattening, and errors in measurement may occur if the fluorescent ring is too wide or narrow. Also lacrimation during IOP measurement after dye application, reflexive attempts by patients to wipe away the flowing dye, and patients squinting during measurement may lead to measurement errors. The main outcome measure in this experiment was IOP; therefore, a number of measures were taken to improve measurement reliability and accuracy. Intraocular pressure (IOP) was measured by the same examiner with the use of a fluorescein strip to standardize the amount of fluorescein applied and this led to a significant reduction in sensation caused by the blink reflex in the patient. According to Goldmann, deviations in IOP measurement occur if the mires are not maintained ‘meticulously’ at a width of 0.2 mm (Goldmann 1961). Fluorescein strips yield an average mire width of 0.30 mm whereas the drops yield an average of 0.44 mm (Goldmann 1961).
Lastly, our study may have been compromised by the relatively short period of post-treatment observation. This limits the extrapolation of the results to patients with glaucoma who require the lifelong application of anti-glaucoma medications. Nonetheless, it is possible that several weeks of G. kola treatment may result in better therapeutic effects on IOP, as was demonstrated in a previous study (Adefule-Ositelu et al. 2010). Further investigations and clinical studies are required to evaluate the clinical utility of these findings and possible long-term effects of oral consumption of G. kola on intraocular pressure.
In conclusion, our study results suggest that oral consumption of 100 mg/kg body weight of bitter kola lowered intraocular pressure by 21% over a 2 hr period. Such an effect may be of therapeutic benefit to patients with glaucoma or ocular hypertension in resource deficient settings.




