Drusenoid pigment epithelial detachment volume is associated with a decrease in best-corrected visual acuity and central retinal thickness: the Norwegian Pigment Epithelial Detachment Study (NORPED) report no. 1
Abstract
Purpose
To investigate the association of drusenoid pigment epithelial detachment (DPED) volume and change in best-corrected visual acuity (BCVA) and central retinal thickness (CRT) during the growth phase of large DPEDs.
Methods
Patients from an ongoing prospective observational study, the Norwegian Pigment Epithelial Detachment Study (NORPED), with 1 year of follow-up and DPEDs ≥1000 µm in diameter, examined with the Heidelberg Spectralis HRA-OCT were included. Patients with DPEDs in the regression phase were excluded. Multicolour, near-infrared reflectance, optical coherence tomography (OCT) and OCT angiography images were obtained every 6 months. Fluorescein angiography and indocyanine green angiography were performed at baseline and yearly to exclude choroidal neovascularization (CNV).
Results
Forty-four patients and 66 eyes were included. In the statistical model for BCVA, every 1.0 mm3 increase in DPED volume led to a decrease in BCVA of 4.0 ETDRS letters (95% CI, −7.0 to −1.0, p = 0.008). A decrease in BCVA was significantly associated with older patient age, the presence of acquired vitelliform lesions and subfoveal location of the DPEDs. In the model for CRT, every 1.0 mm3 increase in DPED volume led to a decrease in CRT of 26.7 µm (95% CI, −44.4 to −9.0, p = 0.003). Two eyes had progression of geographic atrophy and none developed CNV.
Conclusion
The increasing volume of DPEDs during the growth phase is associated with a decrease in BCVA and CRT. The subfoveal location of DPEDs and the presence of acquired vitelliform lesions appear to be associated with a further reduction in BCVA.
Introduction
Drusen and retinal pigment epithelium (RPE) abnormalities are the defining features of age-related macular degeneration (AMD) (Bird et al. 1995). The extent of drusen and RPE abnormalities identified on colour fundus photography is a known risk factor for progression to late AMD with choroidal neovascularization (CNV) and geographic atrophy (Gass 1972; Ferris et al. 2013; Chew et al. 2014). Drusenoid pigment epithelial detachment (DPED) is thought to result from the confluence of large drusen and in part by the formation of a hydrophobic barrier in Bruch’s membrane resulting in the accumulation of fluid (Casswell et al. 1985; Roquet et al. 2004). Previous retrospective studies have observed that DPEDs could persist, regress with or without the development of geographic atrophy, or develop CNV (Hartnett et al. 1992; Roquet et al. 2004; Alexandre de Amorim Garcia Filho et al. 2013). Other studies of the natural history of eyes with DPED have shown high rates of progression to geographic atrophy, CNV and vision loss (Cukras et al. 2010; Yu et al. 2019). DPEDs are usually located in the foveal region and associated with a loss of best-corrected visual acuity (BCVA), with or without progression to central geographic atrophy (Cukras et al. 2010; Balaratnasingam et al. 2016b; Yu et al. 2019). DPEDs typically have an initial growth phase followed by a regression phase with progressive outer retinal atrophy on optical coherence tomography (OCT), ultimately leading to complete RPE and outer retinal atrophy (cRORA) (Balaratnasingam et al. 2016b; Sadda et al. 2018). During the last years, several risk factors for the progression of geographic atrophy have been identified. Drusen disrupt the integrity of the photoreceptor layer on OCT (Hartmann et al. 2012), which has been shown to be thinner on top of the drusen (Schuman et al. 2009; Sadigh et al. 2013). Intraretinal hyperreflective foci, loss of RPE and outer photoreceptor segments, increasing DPED volume and abnormal DPED thinning on OCT are associated with progression of geographic atrophy on colour fundus photography (Sleiman et al. 2017). Subretinal drusenoid deposits on OCT are independent risk factors for loss of visual function both with and without associated geographic atrophy and CNV (Garg et al. 2013).
To our knowledge, the present study is the first prospective observational study of DPEDs. In this report, we have investigated which OCT features that could predict a change in BCVA and central retinal thickness (CRT) during the growth phase of DPEDs. We hypothesized, as previously suggested by Mrejen et al. (2013), that the larger the DPED volume the greater the distance from the RPE to the choriocapillaris, resulting in reduced microvascular support and subsequent progressive outer retinal atrophy with a decrease in BCVA and CRT.
Material and Methods
This is the first study report on the 1-year results from an ongoing prospective, observational, multicentre study, the Norwegian Pigment Epithelial Detachment Study (NORPED). The patients were included from March 2016 to December 2017, and the planned follow-up period is 5 years. Ophthalmologists in central and western Norway referred patients with DPEDs to the University Hospitals of Trondheim, Bergen and Stavanger. The research study was approved by the Regional Committee for Medical and Health Research Ethics Central Norway (2012/1743) and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from the study participants after explanation of the nature and possible consequences of the study.
Only patients examined with the Spectralis HRA-OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) were included in this report. Other inclusion criteria were age of ≥50 years, DPED with a diameter ≥1000 µm and BCVA ≥20/400. Exclusion criteria were previous vitrectomy, corticosteroid therapy, intravitreal anti-vascular endothelial growth factor injections and photodynamic therapy. Eyes with subfoveal fibrosis, central geographic atrophy, CNV on fluorescein angiography (FA) or indocyanine green angiography (ICGA), glaucoma with central visual field defects, diabetic macular oedema, proliferative diabetic retinopathy and uveitis were also excluded.
Patients with a pigment epithelial detachment that appeared yellow on clinical fundus examination, with predominantly hyperreflective contents on OCT, staining on FA and hypofluorescence on ICGA were diagnosed with DPED (Casswell et al. 1985; Arnold et al. 1997). Patients with subretinal fluid and intraretinal cysts were not excluded if FA and ICGA did not exhibit features of CNV. Baseline FA and ICGA images were pseudonymized and sent to an external reading centre to ensure a correct diagnosis of avascular DPED. Eyes with leakage on FA and ‘hotspots’ or ‘plaques’ on ICGA were diagnosed with CNV. Central geographic atrophy was defined as a well-demarcated circular lesion with visible choroidal vasculature, early hyperfluorescence on FA and ICGA, and ≥250 µm in size measured on OCT with foveal involvement.
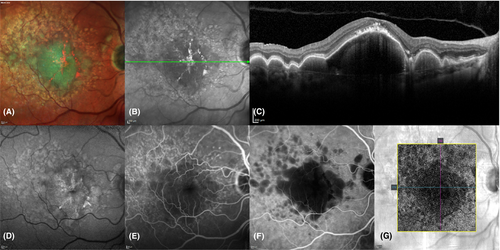
Patients were examined by slit-lamp biomicroscopy and multimodal imaging at baseline, 6 and 12 months. The multimodal imaging was performed with the Spectralis HRA-OCT and consisted of FA, ICGA, near-infrared reflectance imaging, multicolour imaging, fundus autofluorescence imaging and OCT. Macular cube scans were acquired with the follow-up mode, and a dense scan pattern of 49 scans with eye-tracking enabled. Each line of the macular cube scans consisted of 18–30 averaged images recorded with the automatic real-time function. High definition line scans centred on the fovea with 100 averaged images were also obtained. OCT angiography (OCTA) was performed at every study visit with the Cirrus HD-OCT (Carl Zeiss Meditec AG, Jena, Germany) (Fig. 1). FA and ICGA were done at baseline and after 12 months and if CNV was suspected.
BCVA was measured with an Early Treatment Diabetic Retinopathy Study (ETDRS) chart in an illuminated cabinet (ESV3000; Good-Lite, Elgin, IL, USA) at 4 m. The cabinet automatically adjusts lighting to 85 cd/m2. Room lighting was dimmed so that 100−110 Lux could be measured directly in front of the cabinet. The refraction was measured at baseline and yearly by the investigating physician.
Volumetric measurements of the DPEDs and intraretinal hyperreflective foci were done with a semi-automatic segmentation program (ReV Analyzer version 3.0.5; ADCIS SA, Saint-Contest, France). The DPEDs and intraretinal hyperreflective foci were in each OCT scan manually delineated, which enabled automatic calculation of their volumes. Intraretinal hyperreflective foci were defined as hyperreflective areas in the retina above the ellipsoid zone on OCT (Fig. 2A). The calliper available in the viewing software of the Spectralis HRA-OCT (Spectralis Viewing Module version 6.0.9.0; Heidelberg Engineering GmbH, Heidelberg, Germany) was used to measure subfoveal DPED height, diameter and CRT. The three centremost scans, centred in the middle of fovea guided by OCT, were used for measurements, and the mean was used for statistical analysis. The fovea was defined as the innermost guiding circle (712 µm in diameter) of the ETDRS grid, available in the Spectralis Viewing Module, centred in the middle of the fovea guided by OCT. The DPEDs were classified as subfoveal if their location were within this circle. CRT was measured from the internal limiting membrane to, but not including, the RPE. Subfoveal thickening of the RPE, subretinal fluid and acquired vitelliform lesions were not included in the measurements. DPED height was measured from the RPE to Bruch’s membrane. During measurement, the OCT images were magnified 400−800% in the 1:1 µm format.
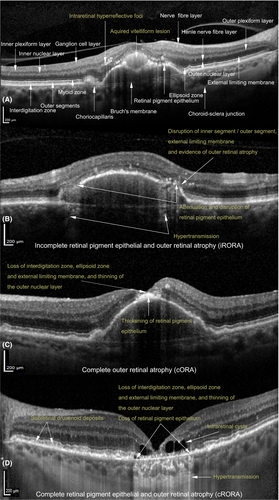
Subretinal drusenoid deposits (Fig. 2D) were present if there were ≥5 subretinal deposits in >1 OCT scan identified on OCT as previously described by Zweifel et al. (2010). Acquired vitelliform lesions were defined as present if there was foveal subretinal hyperreflective material on OCT (Fig. 2A). Foveal thickening of the RPE (Fig. 2B) was defined on OCT as thickened RPE, with shadowing of the sub-RPE structures, also visible as hyperreflective areas on near-infrared reflectance images.
Grading of atrophy on OCT was done as described by the Classification of Atrophy Meeting consensus group (Sadda et al. 2018; Guymer et al. 2020). cRORA (Fig. 2D); (1) region of hypertransmission of at least 250 µm in diameter in any lateral dimension, (2) zone of attenuation or disruption of the RPE of at least 250 µm in diameter and (3) evidence of overlying photoreceptor degeneration. Complete outer retinal atrophy (cORA) (Fig. 2C); evidence of photoreceptor degeneration, but with intact or discontinuous RPE. Features of photoreceptor degeneration included all of the following: loss of interdigitation zone, ellipsoid zone, and external limiting membrane, and thinning of the outer nuclear layer, which also could be identified by a descending outer plexiform layer. Incomplete RPE and outer retinal atrophy (iRORA) (Fig. 2B); the criteria for cRORA were not met, but there was hypertransmission <250 µm in diameter and the RPE-line was discontinuous with evidence of photoreceptor degeneration.
Statistical analysis
Descriptive statistics and linear mixed models were used for statistical analysis. In the linear mixed model, eyes of patients were nested within patients with repeated measurements to account for the correlated eye data (Ying et al. 2017). Restricted maximum likelihood estimation was used for the calculation of fixed effects. R2 statistics were calculated by running the full models and models including only DPED volume, diameter and height with restricted maximum likelihood estimation. The variance of the fixed effects was calculated after predicting fitted values based on the fixed effects alone. As described by Nakagawa and Schielzeth, R2 for the fixed effect(s) and for the full model including random effects were calculated and named R2 marginal and R2 conditional, respectively (Nakagawa & Schielzeth 2013). The residuals were visually inspected regarding normal distribution with histograms and Q–Q plots. In the building of the statistical models, only observations in the growth phase of the DPED life cycle were included. The DPEDs were determined to be in the regression phase if the DPED volume decreased and atrophy progressed after 6−12 months of follow-up. Three of 188 observations were excluded due to the regression of DPED volume and foveal cRORA or cORA. Predictors with a p-value ≥0.05 were excluded from the statistical models unless the exclusion worsened model fit. The model fit was evaluated with Akaike’s information criterion (Akaike 1974). The primary statistical models had DPED volume as the measurement unit of DPED size. However, secondary analyses of foveal DPED diameter and height were also performed as these parameters were readily available and considered clinically relevant. stata 15.1 (StataCorp LLC, College Station, TX, USA) was used for statistical analysis.
Results
A total of 66 eyes from 44 patients were included in the study. Four eyes (6%) in three patients (7%) were lost to follow-up. One patient died, another was diagnosed with dementia, and the last patient did not want further follow-up. One observation at 6 months was missing because the OCT image could not be found in the database. In two eyes (3%), we observed regression of the DPED and development of geographic atrophy, where one progressed to foveal cORA and the other to cRORA. There was no foveal iRORA, cORA or cRORA at baseline. Three eyes (5%) had extrafoveal iRORA at baseline, which increased to six (10%) at 12 months. None of the eyes developed CNV. Baseline patient and OCT characteristics are summarized in Tables 1 and 2, respectively.
| Range | ||
|---|---|---|
| Age, years, mean (SD) | 72.6 (7.5) | 54.9–87.5 |
| BCVA, ETDRS letters, mean (SD) | 76.8 (7.5) | 58.0–93.0 |
| Metamorphopsia, n (%) | 9 (21) | |
| Pseudophakia, n (%) | 9 (21) | |
| Female sex, n (%) | 33 (75) | |
| Diabetes mellitus, n (%) | 2 (5) | |
| Hypertension, n (%) | 18 (42) | |
| Hypercholesterolaemia, n (%) | 11 (26) | |
| History of smoking, n (%) | 24 (56) | |
| Cardiovascular disease, n (%) | 7 (16) |
- BCVA = best-corrected visual acuity, ETDRS = Early Treatment Diabetic Retinopathy Study, n = number; SD = standard deviation.
| Range | ||
|---|---|---|
| DPED volume, mm3, median (IQR) | 0.24 (0.40) | 0.01–2.40 |
| HRF volume, mm3, median (IQR) | 0.001 (0.04) | 0–0.051 |
| Subfoveal DPED height, µm, median (IQR) | 134 (102) | 13–531 |
| Subfoveal DPED diameter, µm, mean (SD) | 1929 (853) | 129–3932 |
| Central retinal thickness, µm, median (IQR) | 158 (60) | 49–280 |
| Subfoveal DPED, n (%) | 62 (94) | |
| HRF, n (%) | 56 (85) | |
| Interruption of ellipsoid zone, n (%) | 52 (79) | |
| Subretinal drusenoid deposits, n (%) | 42 (64) | |
| Subfoveal thickening of the RPE, n (%) | 27 (41) | |
| Aquired vitelliform lesion, n (%) | 18 (27) | |
| Subretinal fluid, n (%) | 7 (11) | |
| Intraretinal cysts, n (%) | 6 (9) |
- DPED = drusenoid pigment epithelial detachment, HRF = intraretinal hyperreflective foci, IQR = interquartile range, n = number of eyes, RPE = retinal pigment epithelium, SD = standard deviation.
Best-corrected visual acuity and drusenoid pigment epithelial detachment volume
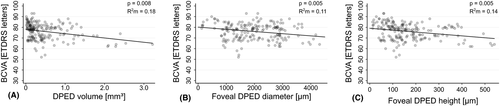
In the linear mixed model, each 1.0 mm3 increase in DPED volume led to a decrease in BCVA of 4.0 ETDRS letters (95% CI, −7.0 to −1.0, p = 0.008) (Fig. 3A), and every patient-year led to a decrease in BCVA of 0.4 ETDRS letters (95% CI, −0.6 to −0.2, p < 0.001). The presence of acquired vitelliform lesions reduced BCVA by 5.6 ETDRS letters (95% CI, −8.1 to −3.1, p < 0.001), and subfoveal location of the DPEDs reduced BCVA by 5.5 ETDRS letters (95% CI, −10.6 to −0.3, p = 0.036). Follow-up duration, the presence of subretinal drusenoid deposits, foveal subretinal fluid and intraretinal cysts, and the volume of hyperreflective foci were not significant predictors of BCVA. The proportion of the variance in BCVA predicted by the fixed effects in the model was 0.40 (R2 marginal) and by the full model 0.83 (R2 conditional).
Best-corrected visual acuity and foveal drusenoid pigment epithelial detachment diameter
In the linear mixed model, each 1000 µm increase in foveal DPED diameter led to a decrease in BCVA of 2.1 ETDRS letters (95% CI, −3.5 to −0.6, p = 0.005) (Fig. 3B), and every patient-year led to a decrease in BCVA of 0.4 ETDRS letters (95% CI, −0.6 to −0.1, p = 0.003). The presence of acquired vitelliform lesions reduced BCVA by 5.6 ETDRS letters (95% CI, −8.2 to −3.1, p < 0.001), whereas the presence of subretinal drusenoid deposits increased BCVA by 3.5 ETDRS letters (95% CI, 0.4 to 6.6, p = 0.027). Follow-up duration, the presence of foveal subretinal fluid and intraretinal cysts, and the volume of hyperreflective foci were not significant predictors of BCVA. R2 marginal and R2 conditional were 0.35 and 0.84, respectively.
Best-corrected visual acuity and foveal drusenoid pigment epithelial detachment height
In the linear mixed model, each 100 µm increase in foveal DPED height led to a decrease in BCVA of 1.8 ETDRS letters (95% CI, −3.0 to −0.5, p = 0.005) (Fig. 3C), and every patient-year led to a decrease in BCVA of 0.3 ETDRS letters (95% CI, −0.6 to −0.1, p = 0.005). The presence of acquired vitelliform lesions reduced BCVA by 6.1 ETDRS letters (95% CI, −8.6 to −3.6, p < 0.001). Follow-up duration, the presence of subretinal drusenoid deposits, foveal subretinal fluid and intraretinal cysts, and the volume of hyperreflective foci were not significant predictors of BCVA. R2 marginal and R2 conditional were 0.36 and 0.82, respectively.
Central retinal thickness and drusenoid pigment epithelial detachment volume
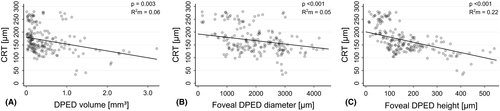
In the linear mixed model, each 1.0 mm3 increase in DPED volume led to a decrease in CRT of 26.7 µm (95% CI, −44.4 to −9.0, p = 0.003) (Fig. 4A). Patient age, subfoveal location of the DPEDs, follow-up duration, the presence of acquired vitelliform lesions, subretinal drusenoid deposits, foveal subretinal fluid and intraretinal cysts, and the volume of hyperreflective foci were not significant predictors of CRT. R2 marginal and R2 conditional were 0.10 and 0.95, respectively.
Central retinal thickness and foveal drusenoid pigment epithelial detachment diameter
In the linear mixed model, each 1000 µm increase in foveal DPED diameter led to a decrease in CRT of 13.1 µm (95% CI, −19.5 to −6.6, p < 0.001) (Fig. 4B), and the presence of subretinal fluid in the fovea led to an increase in CRT of 8.8 µm (95% CI, 0.4 to 17.3, p = 0.042). Patient age, subfoveal location of the DPEDs, follow-up duration, the presence of acquired vitelliform lesions, subretinal drusenoid deposits and intraretinal cysts, and the volume of hyperreflective foci were not significant predictors of CRT. R2 marginal and R2 conditional were 0.08 and 0.95, respectively.
Central retinal thickness and foveal drusenoid pigment epithelial detachment height
In the linear mixed model, each 100 µm increase in foveal DPED height led to a decrease in CRT of 20.2 µm (95% CI, −26.6 to −13.7, p < 0.001) (Fig. 4C). Patient age, subfoveal location of the DPEDs, follow-up duration, the presence of acquired vitelliform lesions, subretinal drusenoid deposits, foveal subretinal fluid and intraretinal cysts, and the volume of hyperreflective foci were not significant predictors of CRT. R2 marginal and R2 conditional were 0.23 and 0.95, respectively.
Discussion
This is the first prospective observational study of DPEDs examined with multimodal imaging including OCT. The main findings of this study are that the increasing volume of DPEDs during their growth phase is associated with a reduction in both BCVA and CRT. Further analysis showed that the increasing foveal DPED diameter and height had a similar effect on BCVA and CRT.
The natural history of large DPEDs is typically characterized by a growth phase, followed by a regression phase leading to geographic atrophy (Balaratnasingam et al. 2016b). The observations from our study suggest that within 1 year, there is progressive atrophy and a reduction in visual function in the majority of eyes with DPEDs during the growth phase. These findings support the hypothesis that the RPE function decreases over time as a consequence of the long-term separation from the underlying choriocapillaris, leading to RPE atrophy and photoreceptor loss (Mrejen et al. 2013). In a previous study, it has also been observed that retinal atrophy is associated with DPEDs, as patients with DPED and poor visual acuity at baseline had focal foveal atrophy within the DPED (Roquet et al. 2004). The association between decreasing BCVA and increasing DPED volume is in part supported by the retrospective DPED cohort studies of the Age-Related Eye Disease Study (AREDS) Research Group, where a decline in visual function was observed in eyes not progressing to late AMD (Cukras et al. 2010; Yu et al. 2019). In the study by Yu et al., there was a 26% estimated proportion of patients without progression to late AMD who would lose ≥15 ETDRS letters, and in the study by Cukras et al., patients not progressing to late AMD lost eight ETDRS letters over 5 years (Cukras et al. 2010; Yu et al. 2019). These studies used stereoscopic colour fundus photography to assess late AMD, and some of the DPEDs may thus have been in the regression phase, explaining the vision loss. A study that used OCT to retrospectively measure DPED height, diameter and volume in 21 eyes, found that maximum DPED size was inversely correlated with final visual acuity. However, the final visual acuity measurements in this study were performed during the regression phase of the DPEDs (Balaratnasingam et al. 2016b). A cross-sectional study evaluating macular function in patients with DPED found a reduction in visual acuity, microperimetry sensitivity and multifocal electroretinography responses in patients with DPEDs compared to healthy controls (Ogino et al. 2014), and our results support these findings.
There was an association between decreasing CRT and increasing DPED volume, which is plausible as there is a gradual progression of geographic atrophy related to the DPED life cycle. Previous natural history studies of DPEDs have not reported CRT changes when evaluating the progression of geographic atrophy. However, a few studies based on stereoscopic fundus photography have shown that the presence of DPEDs and increasing DPED area are significant risk factors for the development of geographic atrophy (Cukras et al. 2010; Yu et al. 2019).
The occurrence of acquired vitelliform lesions on top of the DPEDs was in the present study associated with an additional reduction in BCVA. This finding is in part supported by a retrospective study where patients with both AMD and acquired vitelliform lesions had a higher risk of foveal geographic atrophy and a decline in BCVA compared to those with only adult-onset foveomacular dystrophy (Balaratnasingam et al. 2016a). The vitelliform lesions are thought to arise from defective phagocytosis of the RPE, resulting in the accumulation of outer segment debris and RPE organelles, and can be considered as a marker of maximal RPE disturbance with a focal loss of photoreceptors (Arnold et al. 2003; Chen et al. 2016; Balaratnasingam et al. 2017).
The results of our study suggest that the subfoveal location of the DPEDs is unfavourable for visual function. Even though the majority of DPEDs were located in the fovea (94%), the few perifoveal located DPEDs had markedly better BCVA, which demonstrates the negative impact of DPED volume on the foveal outer retina.
The presence of intraretinal hyperreflective foci is a known risk factor for the progression to late AMD (Nassisi et al. 2018). Interestingly, we did not find that increasing volume of intraretinal hyperreflective foci predicted a change in BCVA or CRT. On the other hand, intraretinal hyperreflective foci are probably a late manifestation of the disease. The DPEDs in this report have only 1 year of follow-up, and the DPEDs in the regression phase were excluded from the statistical models, which may explain why we were unable to document such an association.
DPED is a well-known risk factor for the development of CNV (Cukras et al. 2010; Yu et al. 2019). However, none of our patients developed CNV during the first year. This may be due to the relatively short follow-up and the exclusion of patients with CNV based on several types of angiography at baseline.
The strength of this study is its prospective design, the use of multimodal imaging and a low loss to follow-up. The number of eyes included was fairly large, considering the asymptomatic nature of intermediate AMD and the rarity of large DPEDs. Only 3% of the patients in the AREDS study had DPED at baseline (Cukras et al. 2010). The patients were also thoroughly investigated with FA, ICGA and OCTA to exclude patients with CNV. Subtle type 1 CNV could still have been missed since 11% and 9% of the eyes at baseline had sub- and intraretinal fluid, respectively. Limitations of the study include the absence of a control group of AMD patients without DPED and the lack of blinding of the study investigator when measuring the primary outcomes. Another limitation and future direction is the possibility to measure the rod-mediated dark adaptation function. Rod-mediated vision is affected earlier and more severely with progression of AMD, especially in the presence of subretinal drusenoid deposits (Chen et al. 2019).
In conclusion, this study has shown that increasing DPED volume and foveal DPED diameter and height are associated with a reduction of BCVA and CRT during the growth phase of DPEDs. The subfoveal location of DPEDs and the presence of acquired vitelliform lesions appear to be associated with a further reduction in BCVA.




