Interactions between ocular and systemic disease using national register-based data in the Danish Excellence Centre in Ophthalmic Epidemiology (DECODE-EYE): study perspective
Abstract
Purpose
The Danish Excellence Centre in Ophthalmic Epidemiology (DECODE-EYE) is a national research collaboration formed in order to study the real-life interaction between ocular and systemic disease based on the entire Danish population. Here, we aim to describe the study design and the methodology, which will be used.
Methods
We will extract data from various healthcare registers and databases including the Danish Civil Registration System (unique personal identifier), the Danish National Patient Register (inpatient and outpatient visits), the Danish National Prescription Registry (redeemed prescription drugs), the National Health Service Register (data on health services in primary health care), the Danish Register of Cause of Death (data on cause of death), Statistics Denmark (demographic and socioeconomic data), the Danish Registry of Diabetic Retinopathy (level of diabetic retinopathy (DR) in diabetes patients) and the database of the Danish Association of the Blind (date and cause of blindness). Relevant registers will be linked by the unique personal identifier, and associations will be examined cross-sectional and/or longitudinally, in principle in 1:5 age- and gender-matched case–control cohort studies.
Conclusion
Denmark has a strong tradition in register-based healthcare research, given a high number of validated national registers and databases. DECODE-EYE will provide Danish, large-scale data on associations between ocular and systemic disease.
With a target population of 5.8 million individuals, twelve separate studies (Protocols A–L) have initially been designed to be studied in the upcoming years. These will provide novel data on interactions between systemic disease and relevant ophthalmological end-points like blindness, DR, glaucoma, corneal disease, retinal vascular disease, cataract and intravitreal angiostatic treatment.
Introduction
Ocular manifestations are well-known in systemic disease. Prominent examples of this include diabetes, hypertension, hemoglobinopathies, and a variety of infectious, inflammatory, inherited and malignant diseases. In some cases, the systemic disease is even diagnosed based on ophthalmologic findings. For instance, optic neuritis can be the initial symptom of multiple sclerosis (Kurtzke 1985), and diabetic retinopathy (DR) will often be present at the time of onset of type 2 diabetes (Kostev & Rathmann 2013).
As an extended part of the brain, the eye has the potential to provide valuable information about vascular and neurogen dysfunction. The retina is the only part of the body available for in vivo inspection of the vasculature and the neural tissue down to single-cell imaging. This has in particular been important in recent decades, which have introduced a variety of non-invasive methods like retinal wide-field imaging, optical coherence tomography (OCT), (Fujimoto & Swanson 2016) OCT angiography, (Koustenis et al. 2017) and retinal oximetry (Stefansson et al., 2019).
In general, the association and interaction between ocular and systemic disease are not fully understood given a limited number of studies, which are often based upon small-size, cross-sectional cohorts with a selected group of patients. In these terms, register-based studies of an entire population would be able to contribute longitudinal, real-life interactions between ocular and systemic disease.
Denmark has a leading position within the field of register-based research in healthcare science. This is founded upon a long tradition of utilizing national healthcare registers with disease-related data at an individual level. With a well-characterized national population of 5.8 million inhabitants, it is possible to perform large-scale, real-life studies at a national level. The Danish Excellence Centre in Ophthalmic Epidemiology (DECODE-EYE) has been founded by a group of Danish ophthalmologists, epidemiologists and national database experts. The mission of the consortium is to obtain a better understanding of the interactions between ophthalmological conditions and systemic diseases. The premises of this are (1) that the eye enables direct in vivo inspection and measurements of neural (optic nerve, retinal nerve layer thickness) and vascular (retinal vessels) tissue, and (2) systemic conditions and treatment are likely to have a more profound effect of the eye than earlier expected.
The present protocol outlines the upcoming studies of DECODE-EYE and provides a detailed description of the proposed methodology, including descriptions of the studies and the underlying national registers to be used. By accessing the unique Danish national registers and health databases, we aim to conduct series of large population-based studies to examine important relationships between ocular and systemic disease to obtain a better understanding of the subtle interaction between the eye and the rest of the body.
Materials and Methods
The DECODE-EYE study is principally designed as matched case–control cohort studies based on data in the Danish National registers. The DECODE-EYE study examines several ocular diseases and their association with systemic disease in individually restricted study populations. We will include relevant ocular diseases, conditions and treatments like blindness, DR, glaucoma, corneal disease, retinal vascular disease, cataract and intravitreal angiostatic treatment. Specific diagnostic codes and medication specifically for the treatment of an eye disease will be used to identify relevant cases.
Study protocols
Twelve study protocols have been designed. Lists of protocols, with principal and secondary end-points as well as relevant registers, are presented in Table 1. Protocols have been designed to address ocular conditions, diseases and treatments like blindness (Protocol A), diabetes (Protocols A, B, C, D, J, K and L), glaucoma (Protocols A, G and H), retinal vascular disease (Protocol E), cataract (Protocol F), cornea and angiostatic treatment (Protocol I).
| Protocol | Ocular field | Principal end-point | Secondary end-point | National registers |
|---|---|---|---|---|
| A |
Blindness Diabetes Glaucoma |
The overall and disease-specific yearly incidence of blindness 1999–2019 | Disease-specific 10-year incidence rate for the development of blindness in diabetes, age-related macular degeneration, glaucoma and other ocular diseases |
Danish Civil Registration System Danish Association of the Blind Danish National Patient Registry Danish National Prescription Registry |
| B |
Diabetes CVD |
DR as a marker of 5-year risk of micro and macrovascular disease and mortality | Association between DR and micro and macrovascular disease |
Danish Civil Registration System Danish Register of Cause of Death Danish National Patient Registry Danish Registry of Diabetic Retinopathy |
| C | Diabetes |
The yearly incidence of diabetes-induced vitrectomy in relation to socioeconomic markers 1999–2019 |
The 5-year incidence rate for vitrectomy in patients with DR |
Danish Civil Registration System Danish National Patient Registry Danish Registry of Diabetic Retinopathy Statistics Denmark |
| D | Diabetes | DR as marker of 5-year risk of neurodegenerative disease | Association between DR and neurodegenerative disease |
Danish Civil Registration System Danish National Patient Registry Danish Registry of Diabetic Retinopathy |
| E | Retinal vascular occlusion | Retinal vascular occlusion as a marker of 20-year risk of CVD and mortality | The yearly incidence of retinal vascular occlusion 1999-2019 |
Danish Civil Registration System Danish Register of Cause of Death Danish National Patient Registry |
| F | Cataract | Cataract as a marker of 18-year risk of socioeconomic level and all-cause mortality | Early cataract surgery as a marker of systemic ageing |
Danish Civil Registration System Danish Register of Cause of Death Danish National Patient Registry The Danish National Health Service Registry Statistics Denmark |
| G | Glaucoma | Glaucoma as a marker of 5-year risk of systemic neurodegenerative disease | Association between glaucoma and neurodegenerative disease |
Danish Civil Registration System Danish National Patient Registry Danish National Prescription Registry |
| H | Cornea | Keratoconus and corneal dystrophy as markers of 10-year risk of systemic connective tissue disease | Association between keratoconus and corneal dystrophy and systemic connective tissue disease |
Danish Civil Registration System Danish National Patient Registry |
| I | Angiostatic treatment | Intravitreal angiostatic treatment as a marker of 10-year CVD and mortality | Correlation between a load of intravitreal angiostatic treatment and CVD and mortality |
Danish Civil Registration System Danish Register of Cause of Death Danish National Patient Registry |
| J | Diabetes | DR as a marker of 5-year risk of cerebral disease | Association between DR and cerebral disease |
Danish Civil Registration System Danish National Patient Registry Danish Registry of Diabetic Retinopathy |
| K | Diabetes | Systemic diabetic treatment as a marker of 5-year development in the level of DR | Differences between the systemic treatment of the same drug class and 5-year development in the level of DR |
Danish Civil Registration System Danish National Patient Registry Danish National Prescription Registry Danish Registry of Diabetic Retinopathy |
| L | Diabetes | Systemic intervention (bariatric surgery, insulin pump and coronary bypass surgery) as markers of 3-year development in the level of DR | Systemic intervention (bariatric surgery, insulin pump and coronary bypass surgery) as markers of 1-year development in the level of DR |
Danish Civil Registration System Danish National Patient Registry Danish Registry of Diabetic Retinopathy |
- CVD = cardiovascular disease, DR = diabetic retinopathy.
The majority of protocols aim to test ocular disease in association with and in the prediction of systemic disease. Examples of this are retinal vascular occlusion in relation to cardiovascular morbidity and mortality (Protocol E), cataract as a marker of all-cause mortality and morbidity (Protocol F), glaucoma as a marker of neurodegenerative disease (Protocol G) and corneal disease as a predictor of systemic connective tissue disease (Protocol H). This also includes DR in relation to micro and macrovascular morbidity and mortality (Protocol B), neurodegenerative disease (Protocol D) and various cerebral diseases (Protocol J). In DR, reverse interactions will also be studied (i.e. systemic disease/intervention as a marker of the incident/altered DR). This includes Protocol K and L with DR in response to pharmacological treatment and systemic intervention, respectively.
Protocol I will study the use of intravitreal angiostatic treatment (given for neovascular age-related macular degeneration, retinal vein occlusion and diabetic macular oedema) as a potential marker of systemic morbidity and mortality. Finally, some protocols aim to determine trends of time with yearly incidences of ocular disease (blindness in Protocol A and diabetes-induced vitrectomy in Protocol C).
While some associations will be cross-sectional, most studies will include longitudinal analysis with a follow-up time between three (Protocol L), five (Protocols B, D, G, J and K), 10 (Protocols H and I), 18 (Protocol F) and 20 years (Protocol E).
Data
Data from eight target registers and two databases will be included in DECODE-EYE. A brief introduction of the data sources is provided below and an overview of data sources available for the relevant study protocol is provided in Table 2 and Fig. 1.
| Register and database | Period available | Ocular disease, condition or treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Blindness | Diabetic retinopathy | Glaucoma | Retinal vascular occlusion | Cataract | Corneal disease | Angiostatic treatment | ||
| Protocol | A | A, B, C, D, J, K, L | A, G | E | F | H | I | |
| The Danish Civil Registration System | 1968–2018 | X | X | X | X | X | X | X |
| The Danish National Patient Register | 1977–2018 | X | X | X | X | X | X | X |
| MiniPas | 2004–2018 | X | X | X | X | X | X | X |
| DUSAS | 2002–2018 | X | ||||||
| The Danish National Health Service Register | 1990–2018 | X | ||||||
| The Danish National Prescription Registry | 1995–2018 | X | X | |||||
| The Danish Register of Cause of Death | 1970–2018 | X | X | X | X | |||
| Statistics Denmark | 1976–2018 | X | X | |||||
| The Danish Registry of Diabetic Retinopathy | 2003–2018 | X | X | |||||
| The Danish Association of the Blind | <1973–2018 | X | X | |||||
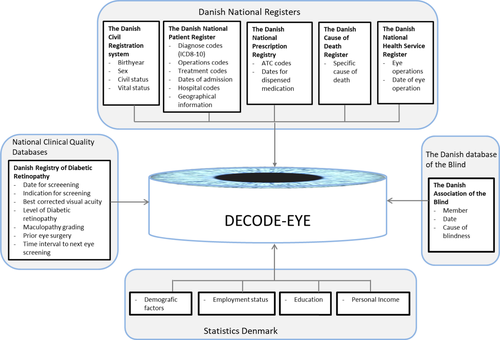
We use recognized International Classification Systems (ICD and ATC) from the World Health Organisation (WHO) to identify and classify the relevant disease of interest, which enhances the generalizability to other populations. The specific diagnostic codes and classification will be presented in relevant papers.
Target registers
The Danish Civil Registration System
Since 1968, the Danish Civil Registration System has allocated a unique personal identifier, the Central Personal Registration (CPR) number to each citizen in Denmark (Schmidt et al. 2014). The CPR follows the individuals throughout life and is used for registration in all health registers. CPR serves as the key to accurately link data from multiple data sources on an individual level. From the Danish Civil Registration System, we will extract individual information on sex, birth year, civil and vital status. The civil status indicates whether the individual is married, divorced, living alone (single status) or a widow. The vital status indicates whether the individual is alive, emigrated, disappeared (residence unknown to Danish authorities) or dead, along with the date of these events.
The Danish National Patient Register
The Danish National Patient Register was established in 1977 as electronic data sources with information on all patient contacts at somatic departments within the Danish hospital facility as part of, for example examination or treatment (Schmidt et al. 2015). Since 1995, the register was expanded to include all hospital contact – inpatient and outpatient – at somatic and psychiatric departments. Furthermore, the register includes information on hospital admission and discharge for inpatient and outpatient and emergency room contacts (Schmidt et al. 2015). Dates for admission and diagnostic codes in accordance with the International Classification of Diseases (ICD), version 8 until 1994 and version 10 onwards (WHO 2018), are also registered in Danish National Patient Register. The diagnostic codes are registered with a primary diagnostic code for the main reason for hospital contact, secondary codes for underlying diseases and supplementary codes used to supplement the primary diagnostic codes (e.g. right and/or left eye for ocular diseases). Furthermore, the reason for hospital referral is registered by referral codes. Surgical procedures are classified based on the Nordic Medico-Statistical Committee (NOMESCO) codes. (2011) The Danish National Patient Register is also used as a source register for the specialized MiniPas and DUSAS register. Admissions to private hospitals and clinics, including for patients where the contact to the healthcare system are covered by private insurance, are registered in the MiniPas registry, which was established in 2004. Surgeries and treatment by medical specialist that are financed by private insurances or outside Denmark are registered in the DUSAS registry, which was established in 2002. MiniPas and DUSAS emanate from the Danish National Patient Register, and the data and structure match with the Danish National Patient Register, with a few exceptions.
Originally, the Danish National Patient Register was an instrument to monitor hospital activities, but since the beginning of 2000, it has also served as an instrument for the payment of activities in public as well as private hospitals. In Denmark, the healthcare system is tax-funded and free of charge for citizens in Denmark. This provides an unselected study population for research. (Schmidt et al. 2019) In general, the validity of data is considered high, since data are collected prospectively and used for quality control of the hospital activities. However, patients treated in the primary care for, for example uncomplicated diseases and less severe complications are not registered in the Danish National Patient Register. We, therefore, link the study population to other registers and databases to identify these sub-cohorts.
A limitation of the Danish National Patient Registers is the lack of information on lifestyle risk factors, such as dietary intake, smoking and alcohol, given that these data could provide important information related to ocular and systemic diseases.
From the Danish National Patient Register, MiniPas and DUSAS, we plan to collect information related to the individual’s hospital contacts. These include the relevant codes for diagnosis, examination, treatment, surgery, admission dates and geographical information.
The Danish National Health Service Register
The Danish National Health Service Register (Andersen et al. 2011) contains information regarding contact with local healthcare providers, such as private hospitals and clinics. These are registered by specialty and service. In Denmark, some eye surgeries (e.g. cataract surgery) can be performed by a private practicing ophthalmologist. We will collect data from the Danish National Health Service Register to capture patients who had their eye operation performed in private hospitals or clinics.
The Danish National Prescription Registry
The Danish National Prescription Registry (Kildemoes et al. 2011) was established by the Danish Medicine Agency in 1994 and enclosed information on reimbursed medication classified in accordance to the Anatomical Therapeutic Chemical Classification system (ATC) (Lagerlund et al. 2020) and the date for dispensed medication. The register only includes information on filed prescription dispensed at Danish community pharmacies. Medication administered in-hospital, and over-the-counter medication is not recorded in the registry. In this study, we will abstract ATC codes and dates relevant to the disease of interest.
The Danish Register of Cause of Death
The Danish Register of Causes of Death (Helweg-Larsen 2011) was established by the National Board of Health in 1970 as an electronic data source. The register includes data collected from the death certificates on Danish residents dying in Denmark and comprises data on the date of death and the condition (underlying and contributory cause) that directly causes the death. For DECODE-EYE, we will collect information on the underlying cause of death, that is the disease or condition that led to death.
Statistics Denmark
Statistics Denmark is a national organization in Denmark responsible for creating a long list of statistical information about the Danish society. This includes demographical and socioeconomic data at individual levels. In the present studies, we will collect data on demographic factors, employment status (available since 1976), education (available since 1981) and personal income (available since 1980).
Target databases
The Danish Registry of Diabetic Retinopathy
The Danish Registry of Diabetic Retinopathy (Andersen et al. 2016) is a National Clinical Quality Database that comprises all patients above the age of 18 years who are diagnosed with diabetes in Denmark and has attended the screening programme for diabetic eye disease.(Grauslund et al., 2018) It was established between 2003 and 2006 as a regional database. Since then, the registry has been developed and extended to a nationwide database and, furthermore, to include data on patients screened by private practicing ophthalmologists. The registry aims to monitor the development of DR in Denmark and to evaluate the diabetic eye screening programme. Data include the level of DR, visual acuity, presence/absence of diabetic macular oedema, prior eye surgery (cataract extraction and vitrectomy) and planned time interval to next eye screening.
The Danish Association of the Blind
The Danish Association of the Blind is a database that comprises all patients alive that are members of the Danish Association of the Blind. This is a private organization, and membership is offered to all patients who meet the Danish definition of legal blindness [best-corrected visual acuity in the best eye equal to or below 6/60 or equally debilitating eye complications (eg visual fields lower than 10 degrees)]. Data include date and cause of blindness (reported by referring ophthalmologist). Even though this is a voluntary organization, various benefits to members ensure that for instance, 88% of all blind patients in Denmark with type 1 diabetes are members of the association (Sjolie & Green 1987).
Sampling plan
In general, the target populations of cases in the specific sub-studies in each protocol are Danish residents registered with one of more diagnostic codes for specific eye diseases at a hospital or by a private practicing ophthalmologist. In each sub-study, we aim to examine the association between the specific eye disease and systemic disorder(s). Therefore, the specific diagnostic codes (ICD 8 and 10) and/or the ATC codes will be used to identify individuals with the condition of interest, and the CPR number will be used to link with relevant registers and databases.
Cases
For each of the sub-studies included, cases in all relevant age groups will be included. The Danish Registry of Diabetic Retinopathy will contribute with cases for the studies of DR. These cases will be diabetes patients who have attended eye screening in relation to diabetes and have been registered in the Danish Registry of Diabetic Retinopathy database between 2013 and 2018. Otherwise, cases will be identified by diagnostic codes and the use of specific medication registered in the national health registers. An impartial institution (the Board of Health Data, managing the national registers) will carry out the identification of cases and controls based on predefined codes (diagnostic, operational and ATC) as well as inclusion and exclusion criteria. Primary, secondary (and supplementary) diagnostic codes from the Danish National Patient Register will be used to identify cases and outcome for the specific sub-studies. We will use the ATC codes registered in the Danish National Prescription Registry to identify individuals medically treated with medication (e.g. glaucoma).
An index date will be identified for each case and will be defined as the date for first screening for DR, or the first date of a diagnose codes for the specific eye disease in question or the first date of redeemed medication at the pharmacy.
Control persons
Eligible control persons are residents in Denmark who are alive on the index date of their respective case and who are not diagnosed with the specific exposure and outcome in question before index date. Controls will be assigned with the index date of their matched cases. Information on the vital status from the Danish Civil Registration System will be used to determine eligible control persons. Cases and controls will be matched by sex and birth year. In general, controls will be selected with the ratio 5:1. For rare eye diseases (e.g. hereditary corneal dystrophies), the ratio of control will be 10:1.
Analysis plan
The specific sub-studies will be analysed and published separately. We will use descriptive analyses and a multivariable conditional logistics regression approach to calculate the odds ratio in order to control for potential confounding by the matching factors (sex and birth year).
Comorbidity
Based on the diagnostic codes and the Charlson comorbidity index by Quan and colleagues(Quan et al. 2011), we will examine the burden of diseases among cases and control persons (Brusselaers & Lagergren 2017).
Geographical variation
Information on the geographical location of the hospital and the specific hospital department will be extracted from the registers and used to examine for geographical variation in diagnoses and outcome.
Ethics and dissemination
In the present paper, we present the study design and methodology of DECODE-EYE. To illustrate our upcoming work on the interactions between ocular and systemic disease by real-life, register-based, national healthcare data, we have included descriptions of the first 12 study protocols. In specific, we have described the eight target registers and the two target databases of the studies and presented the methodology of the upcoming analysis. Some interactions are biologically plausible but have not been tested before. Others have been studied in other studies but have not been investigated in large-scale, real-life populations like in DECODE-EYE.
We expect results from these, and potentially other, protocols for the upcoming years. In addition, we imagine DECODE-EYE will lay the foundation for upcoming prospective studies within the area. Finally, we encourage similar studies in other national cohorts, and we would be eager to perform worldwide collaborations to unlock intriguing, new discoveries in the interphase of ocular and systemic disease.




