Retinal neurovascular changes in chronic kidney disease
Abstract
Purpose
To examine retinal neurovascular changes in patients with chronic kidney disease (CKD).
Methods
Case–control study. A total of 171 CKD cases and 40 controls were recruited (mean age 62.9 ± 10.3 versus 60.8 ± 9.2, p = 0.257). Retinal neural parameters, including parafoveal retinal thickness (PfRT), macular ganglion cell complex thickness (GCCt), global loss volume (GLV), focal loss volume (FLV) and peripapillary retinal nerve fibre layer thickness (RNFLt), were measured using optical coherence tomography (OCT). Microvascular parameters, including foveal avascular zone size, vessel density over the parafoveal superficial vascular plexus (SVP-VD), parafoveal deep vascular plexus (DVP-VD) and radial peripapillary capillary (RPC-VD), were measured using OCT angiography.
Results
Chronic kidney disease (CKD) patients showed reduced PfRT, GCCt and RNFLt and increased GLV and FLV compared with the controls (all p < 0.005). Among patients with CKD, estimated glomerular filtration rate was an independent factor associated with PfRT (coefficient 0.19, p = 0.015), GCCt (coefficient 0.10, p = 0.006), GLV (coefficient − 0.08, p = 0.001), FLV (coefficient − 0.02, p = 0.006) and RNFLt (coefficient 0.15, p = 0.002). Parafoveal retinal thickness (PfRT), GCCt, GLV, FLV and RNFLt were correlated with SVP-VD (all p < 0.001) but not with DVP-VD (all p > 0.1).
Conclusions
Chronic kidney disease (CKD) patients demonstrated a significant reduction in macular thickness and changes in retinal neural parameters. These changes were associated with the severity of CKD and correlated with the microvascular rarefaction in the parafoveal SVP.
Introduction
Chronic kidney disease (CKD) is a common systemic comorbidity in ophthalmology patients who are elderly or have systemic diseases such as diabetes mellitus (DM) or hypertension. The prevalence of CKD is estimated to be 8–16% in the general population,(Jha et al. 2013) but may be even higher than 30% in patients aged 70 years and older, and more than 50% in patients with diabetes (Zhang et al. 2008; Collins et al. 2009; Jha et al. 2013).
It is well known that patients with CKD have an increased risk of several major neurological diseases, including cerebrovascular disease, cerebral cortical thinning, cognitive impairment, dementia and peripheral neuropathy (Arnold et al. 2016; Chen et al. 2017; Lau et al. 2017; Ito et al. 2018; Hamed 2019). In the eye, CKD has been associated with an increased risk of glaucoma (Wong et al. 2014; Shim et al. 2016; Wong et al. 2016), a common form of optic neuropathy. Chronic kidney disease (CKD) has also been linked with specific optic neuropathies such as uraemic optic neuropathy and ischaemic optic neuropathy (Winkelmayer et al. 2001). However, it is unclear why patients with CKD have an increased risk of neurological complications or how neural structure in the retina may be affected in different stages of CKD.
Patients with CKD often show reduced macular thickness on optical coherence tomography (OCT) (Balmforth et al. 2016). However, their clinical implications are unknown. We hypothesize that macular thinning in patients with CKD may represent an early retinal neural impairment, which can increase with the severity of CKD. There is increasing recognition that the neural and microvascular structures are intimately linked as ‘neurovascular units’ within the retina. Thus, we further hypothesize that CKD patients may also have early microvascular alternation which parallels neural impairment. We designed this study to examine these two hypotheses in patients with different stages of CKD.
Materials and Methods
Study design
We conducted a case–control study in a tertiary hospital in Taiwan (Keelung Chang Gung Memorial Hospital) between August 2017 and July 2018. The study was approved by the Chang Gung Memorial Hospital Institutional Review Board (IRB No.: 201602022B0) and followed the tenets of the Declaration of Helsinki. The overall goal of the study was to evaluate both retinal neural and microvascular changes in different stages of CKD. The present report focuses on neural impairment within the retina as well as its relationship to microvascular alterations. Other details of the microvascular changes and their associated risk factors will be discussed in a separate paper.
Cases and controls
Chronic kidney disease (CKD) cases were defined as patients with estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 for >3 months (Stevens & Levin 2013). Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine concentration (Cr) using the CKD Epidemiology Collaboration equation (Earley et al. 2012). Severity of CKD was categorized based on the eGFR values into stage 3 (30–59 ml/min/1.73 m2), stage 4 (15–29 ml/min/1.73 m2) and stage 5 (<15 ml/min/1.73 m2). The CKD group included patients with age ≥21 years, CKD stages 3–5 (including end-stage renal disease) and no visual symptoms.
Controls were age-matched healthy patients with age ≥21 years, no major systemic disease, no visual symptoms and absence of retinal disease in a 4:1 ratio. We excluded patients with (1) significant ocular media opacity; (2) inadequate quality of spectral-domain OCT (SD-OCT) or OCT angiography (OCTA) image (signal strength index <50 or quality score <6 or presence of significant artefact); or (3) pregnancy. Written informed consent was obtained from each patient. In patients in whom both eyes were eligible, the eye with better OCTA image quality was considered the study eye. Patients using a different OCTA imaging protocol, with high refractive error (myopia ≤−6 D or hyperopia ≥5 D), with diabetic retinopathy (DR), or with an epiretinal membrane in both eyes, were excluded from this part of the analysis.
For cases and controls, we measured the following neural parameters from SD-OCT: parafoveal retinal thickness (PfRT), macular ganglion cell complex thickness (GCCt), global loss volume (GLV), focal loss volume (FLV) and peripapillary retinal nerve fibre layer thickness (RNFLt). We measured microvascular parameters from OCTA: parafoveal superficial vascular plexus vessel density (SVP-VD), parafoveal deep vascular plexus vessel density (DVP-VD), radial peripapillary capillary vessel density (RPC-VD) and foveal avascular zone (FAZ) size.
Besides, medical histories and the most recent 3 months of laboratory data were collected. On the date of enrolment, each patient received a blood pressure measurement, comprehensive ophthalmic examinations and colour fundus photography. Mean arterial pressure (MAP) was calculated from systolic blood pressure (SBP) and diastolic blood pressure (DBP) by the equation ‘DP + 1/3 (SBP − DBP).’ Best-corrected visual acuity (BCVA) was measured on a Snellen chart and converted to the logarithm of the minimum angle of resolution for calculation. Intraocular pressure (IOP) was measured using a non-contact tonometer (NT-3000; NIDEK, Gamagori, Japan). Axial length was measured with an IOLMaster (Carl Zeiss Meditec, Jena, Germany).
Neural parameters from SD-OCT
A single SD-OCT (AngioVue, Optovue RTVue XR Avanti; Optovue Inc., Fremont, CA, USA) was used to acquire SD-OCT B-scan and OCTA images after pupil dilation. The machine uses an 840-nm diode laser source and has an A-scan rate of 70 kHz. Macular thickness was measured using the retina map mode and was provided in an Early Treatment DR Study chart format. Parafoveal retinal thickness (PfRT) was defined as the average thickness of a 1-mm round annulus area extended from the foveal 1-mm circle. An optic nerve head scan was obtained for the measurement of RNFLt. The average RNFLt was measured over a 3.45-mm-diameter circle centred on the ONH. A ganglion cell complex (GCC) scan was used to measure the GCCt over the macula. The scan consisted of 15 vertical line scans covering a 7-mm2 region centred on a point 1 mm temporal to the fovea. The GCC encompasses the nerve fibre layer (NFL), ganglion cell layer (GCL) and inner plexiform layer (IPL). The average GCCt within a 6-mm circle, GLV (%) and FLV (%) were automatically calculated using the AngioVue (Fig. S1).
Microvascular parameters from OCTA
Vessel densities over the macular and peripapillary regions and FAZ size were determined through OCTA images. In the macular region, a 304 × 304 A-scan covering an area of 3 × 3 mm2 centred on the fovea was performed. The angiovue software (version: A2017,1,0,151, Optovue Inc.) segmented the vascular area into the SVP and DVP. The default segmentation for the SVP includes the vasculature between the internal limiting membrane (ILM) and 10 μm above the IPL; for the DVP, the default segmentation includes the vasculature between 10 μm above the IPL and 10 μm below the outer plexiform layer (OPL). Vessel density was defined as the percentage area occupied by the large and small vessels in the macular region. The angiovue software calculated the SVP-VD and the DVP-VD over the parafoveal 1-mm-wide circular annulus area (Fig. S1). The FAZ size was also automatically provided by this software.
In the optic disc and peripapillary region, a 304 × 304 A-scan covering an area of 4.5 × 4.5 mm2 was performed. An OCTA image of the RPC segment, which extends from the ILM to the posterior boundary of the NFL, was used for analysis. The peripapillary region was defined as a 1-mm-wide round annulus extending from a 2-mm-diameter circle centred on the optic disc. The RPC-VD was measured using imagej software (National Institutes of Health, USA; http://rsb.info.nih.gov/ij) as previously reported (Rodrigues et al. 2019). Briefly, Gaussian blur operation (sigma = 2), global thresholding and binarization were used to create a mask for the larger vessels. A median filter (radius of 1 pixel) was used to remove high-intensity small vessels, and dilation was employed to remove the edges of the large capillaries. Subsequently, this mask was subtracted from the original OCTA image. Finally, a threshold for capillary detection was calculated, and the image was binarized to create a small capillary mask. The capillary density was calculated in the region of interest, which excluded the large vessels and contained only small capillaries (Fig. S1).
Statistical analysis
Demographic data and clinical characteristics between CKD cases and controls were compared using Pearson's chi-squared test for categorical variables and the independent sample t-test for continuous variables. Multiple linear regression models were used to determine the factors associated with PfRT, GCCt, GLV, FLV and RNFLt. Age, gender, smoking status, DM, hypertension, MAP, IOP, axial length and eGFR were the independent variables included. Linear regression was also used for determining (1) the p for trends among the controls and the different stages of CKD; and (2) the p-values between each stage of CKD in all neural and microvascular parameters after controlling for age, gender, DM and axial length. Partial correlation was used to determine the relationship between neural parameters (PfRT, RNFLt, GCCt, GLV and FLV) and microvascular parameters (SVP-VD, DVE-VD and RPC-VD) after controlling for age, gender, DM, hypertension and axial length. A two-tailed p value < 0.05 was considered statistically significant. All the data were analysed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
We initially recruited 200 CKD patients and 50 healthy controls in this study. Of these, 11 patients in the CKD group and eight subjects in the control group were excluded from the analysis due to the use of different scan protocols for the peripapillary OCTA images. Additionally, 18 patients in the CKD group and two subjects in the control group were excluded due to high refractive error, DR or epiretinal membrane. This leaves 171 CKD cases and 40 control subjects for analyses.
For CKD cases, the mean Cr, blood urea nitrogen (BUN), BUN/Cr ratio and eGFR were 4.8 ± 4.4 mg/dl, 44.6 ± 27.6 mg/dl, 13.3 ± 6.2 and 26.6 ± 19.8 ml/min/1.73 m2, respectively. There were 68 (40%) patients with stage 3 CKD, 38 (22%) with stage 4 and 65 (38%) with stage 5. Among the patients with stage 5 CKD, 21 (32%) and 29 (45%) patients were receiving haemodialysis and peritoneal dialysis, respectively. The aetiology of CKD was as follows: DM in 62 (36%) patients, hypertension in 43 (25%), gout in 10 (6%), other systemic diseases in 5 (3%), chronic glomerulonephritis in 20 (12%), polycystic kidney disease in 9 (5%), other renal or urinary tract diseases in 12 (7%) and unknown in 10 (6%). The mean SBP, DBP and MAP during enrolment were 138.8 ± 20.0, 77.4 ± 13.5 and 97.8 ± 14.4 mmHg, respectively. Among the 154 patients with hypertension, the anti-hypertensive drugs taken include angiotensin-converting enzyme inhibitors/angiotensin receptor blockers in 110 (71%) patients, calcium channel blockers in 81 (53%) patients, beta-blockers in 61 (40%) patients, diuretics in 41 (27%) patients, alpha-1 blockers in 15 (10%), vasodilators in 8 (5%) patients and alpha-2 agonists in 2 (1%) patients.
Table 1 summarizes the demographic data and ocular characteristics in the CKD and control groups. The mean age was 62.9 years in CKD cases and 60.8 years in controls (p = 0.257). No significant differences were noted in other characteristics, including gender, smoking status, axial length, IOP, RPC-VD and FAZ size between cases and controls. Compared with controls, the CKD group had worse BCVA; decreased PfRT, GCCt, RNFLt, SVP-VD and DVP-VD; and increased GLV and FLV.
| CKD group (n = 171) | Control group (n = 40) | p value | |
|---|---|---|---|
| Age (mean ± SD, range) | 62.9 ± 10.3, 31–79 | 60.8 ± 9.2, 40–85 | 0.257 |
| Gender, female: male, n (%) | 67:104 (39:61) | 21:19 (53:48) | 0.124 |
| Ever-smoker, n (%) | 23 (14) | 4 (10) | 0.556 |
| Diabetes mellitus, n (%) | 73 (42) | – | |
| Hypertension, n (%) | 154 (90) | – | |
| Cardiovascular disease, n (%) | 38 (22) | – | |
| Cerebrovascular disease, n (%) | 2 (1) | – | |
| LogMAR BCVA (mean ± SD, range) | 0.121 ± 0.143, 0.000–0.699 | 0.069 ± 0.105, 0.000–0.398 | 0.009 |
| Axial length (mean ± SD, range) | 23.71 ± 1.14, 20.91–26.24 | 24.04 ± 0.95, 21.82–25.74 | 0.100 |
| IOP, mmHg (mean ± SD, range) | 14.95 ± 2.67, 7.6–21.6 | 14.90 ± 2.38, 10.0–19.5 | 0.906 |
| PfRT, μm (mean ± SD, range) | 299 ± 19, 237–348 | 314 ± 16, 271–345 | <0.001 |
| GCCt, μm (mean ± SD, range) | 91.6 ± 8.1, 67–113 | 96.0 ± 7.6, 82–111 | <0.001 |
| GLV, %, (mean ± SD, range) | 6.11 ± 5.74, 0.03–28.37 | 3.20 ± 3.72, 0.03–13.95 | 0.001 |
| FLV, %, (mean ± SD, range) | 1.94 ± 2.10, 0.00–10.95 | 0.74 ± 0.98, 0.00–5.13 | <0.001 |
| RNFLt, μm (mean ± SD, range) | 94.1 ± 11.7, 57–125 | 99.9 ± 7.6, 78–118 | <0.001 |
| SVP-VD, %, (mean ± SD, range) | 46.87 ± 4.38, 33.2–54.4 | 49.53 ± 3.39, 39.7–55.8 | <0.001 |
| DVP-VD, %, (mean ± SD, range) | 50.44 ± 3.92, 37.9–58.9 | 52.97 ± 3.17, 46.2–58.2 | <0.001 |
| RPC-VD, %, (mean ± SD, range) | 35.10 ± 4.83, 18.7–45.6 | 34.30 ± 4.92, 22.6–41.0 | 0.351 |
| FAZ size, mm2, (mean ± SD, range) | 0.336 ± 0.128, 0.038–0.736 | 0.301 ± 0.094, 0.072–0522 | 0.110 |
- BCVA = best-corrected visual acuity, CKD = chronic kidney disease, DVP-VD = parafoveal deep vascular plexus vessel density, FAZ = foveal avascular zone, FLV = focal loss volume, GCCt = ganglion cell complex thickness, GLV = global loss volume, IOP = intraocular pressure, LogMAR = logarithm of the minimum angle of resolution, PfRT = parafoveal retinal thickness, RNFLt = retinal nerve fibre layer thickness, RPC-VD = radial peripapillary capillary vessel density, SD = standard deviation, SVP-VD = parafoveal superficial vascular plexus vessel density.
- The bold letters indicate a p value < 0.05.
Table 2 shows the results of the multiple linear regression models. Estimated glomerular filtration rate (eGFR) was a factor consistently associated with all neural parameters (i.e. PfRT, CGGt, GLV, FLV and RNFLt; all p < 0.05). Figure 1 displays the distributions of all neurovascular parameters and IOP among the controls and different stages of CKD groups. We observed that the progression of CKD stage is associated with all neural parameters (i.e. PfRT, CGGt, GLV, FLV and RNFLt; all p for trend < 0.005) and SVP-VD (p for trend = 0.011) after controlling for age, gender, DM and axial length. It is not associated with DVP-VD (p for trend = 0.118), RPC-VD (p for trend = 0.501) and FAZ size (p for trend = 0.131).
| PfRT | GCCt | GLV | FLV | RNFLt | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | |
| Age | −0.07 (−0.4 to 0.26) | 0.671 | −0.12 (−0.27 to 0.02) | 0.087 | 0.07 (−0.03 to 0.17) | 0.168 | 0.02 (−0.02 to 0.06) | 0.257 | −0.16 (−0.36 to 0.04) | 0.115 |
| Gender | 7.76 (1.39 to 14.14) | 0.017 | −0.51 (−3.29 to 2.27) | 0.717 | 1.35 (−0.6 to 3.3) | 0.173 | 0.62 (−0.07 to 1.31) | 0.077 | −2.87 (−6.77 to 1.03) | 0.148 |
| Smoking | −2.84 (−7.06 to 1.38) | 0.186 | −1.57 (−3.41 to 0.27) | 0.094 | 1.08 (−0.21 to 2.36) | 0.101 | 0.34 (−0.11 to 0.8) | 0.138 | −2.42 (−5 to 0.17) | 0.066 |
| DM | −7.65 (−13.51 to − 1.79) | 0.011 | −0.96 (−3.52 to 1.59) | 0.458 | 0.64 (−1.15 to 2.43) | 0.480 | 0.84 (0.2 to 1.47) | 0.010 | −0.83 (−4.42 to 2.75) | 0.647 |
| HTN | 3.45 (−6.36 to 13.26) | 0.489 | −0.71 (−4.99 to 3.57) | 0.744 | 0.72 (−2.27 to 3.72) | 0.635 | 0.13 (−0.93 to 1.19) | 0.807 | −2.04 (−8.05 to 3.96) | 0.503 |
| MAP | −0.05 (−0.29 to 0.19) | 0.675 | −0.03 (−0.13 to 0.08) | 0.607 | 0 (−0.07 to 0.07) | 0.960 | 0 (−0.03 to 0.02) | 0.824 | −0.05 (−0.19 to 0.1) | 0.526 |
| eGFR | 0.2 (0.04 to 0.35) | 0.013 | 0.1 (0.03 to 0.16) | 0.005 | −0.08 (−0.13 to − 0.03) | 0.001 | −0.02 (−0.04 to − 0.01) | 0.005 | 0.15 (0.05 to 0.24) | 0.002 |
| IOP | 0.01 (−1.12 to 1.14) | 0.986 | −0.12 (−0.61 to 0.38) | 0.637 | 0.07 (−0.28 to 0.41) | 0.710 | −0.05 (−0.17 to 0.07) | 0.402 | −0.18 (−0.87 to 0.52) | 0.617 |
| AL | −0.26 (−2.87 to 2.34) | 0.842 | −1.03 (−2.17 to 0.1) | 0.075 | 0.47 (−0.33 to 1.26) | 0.249 | −0.33 (−0.62 to − 0.05) | 0.020 | −2.04 (−3.64 to − 0.45) | 0.012 |
- AL = axial length, CI = confidence interval, DM = diabetes mellitus, eGFR = estimated glomerular filtration rate, FLV = focal loss volume, GCCt = ganglion cell complex thickness, GLV = global loss volume, HTN = hypertension, IOP = intraocular pressure, MAP = mean arterial pressure, PfRT = parafoveal retinal thickness, RNFLt = retinal nerve fibre layer thickness.
- The bold letters indicate a p value < 0.05.
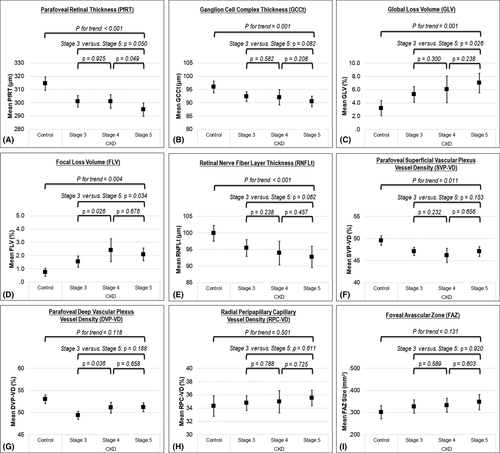
Table 3 shows the correlations between the neural and microvascular parameters. Superficial vascular plexus (SVP)-VD and RPC-VD were correlated with all the neural parameters (all p < 0.01 after controlling for age, gender, DM, hypertension and axial length). Deep vascular plexus (DVP)-VD was not correlated with any of the neural parameters (all p > 0.1). Parafoveal retinal thickness (PfRT) was correlated with RNFLt, GCCt, GLV and FLV (all p < 0.001 after controlling for age, gender, DM, hypertension and axial length). Their relationships are also illustrated in Fig. S2.
| PfRT* | SVP-VD* | DVP-VD* | RPC-VD* | |||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | p value | Coefficient | p value | Coefficient | p value | Coefficient | p value | |
| PfRT | – | – | 0.33 | <0.001 | −0.06 | 0.483 | 0.22 | 0.005 |
| RNFLt | 0.52 | <0.001 | 0.46 | <0.001 | −0.02 | 0.803 | 0.41 | <0.001 |
| GCCt | 0.68 | <0.001 | 0.34 | <0.001 | −0.12 | 0.111 | 0.42 | <0.001 |
| GLV | −0.67 | <0.001 | −0.36 | <0.001 | 0.09 | 0.245 | −0.42 | <0.001 |
| FLV | −0.60 | <0.001 | −0.44 | <0.001 | 0.05 | 0.503 | −0.31 | <0.001 |
- DVP-VD = parafoveal deep vascular plexus vessel density, FLV = focal loss volume, GCCt = ganglion cell complex thickness, GLV = global loss volume, PfRT = parafoveal retinal thickness, RNFLt = retinal nerve fibre layer thickness, RPC-VD = radial peripapillary capillary vessel density, SVP-VD = parafoveal superficial vascular plexus vessel density.
- * Partial correlation controlled for age, gender, diabetes mellitus, hypertension and axial length.
- The bold letters indicate a p value < 0.05.
Figure 2 and Figs S3 and S4 show representative cases with different severities of neural impairment and accompanying microvascular damages among patients with different stages of CKD.
Discussion
In this study, we showed significant retinal neural and microvascular changes in CKD patients, demonstrated on SD-OCT and OCTA. Macular thinning (i.e. decreased PfRT) was found to be strongly correlated with retinal neural impairment (i.e. decreased GCCt and RNFLt and increased GLV and FLV). Retinal neural impairment was associated with decreased eGFR and increased CKD stage. Concomitant microvascular alterations were present in the parafoveal superficial retinal layers (i.e. SVP-VD). This supports the concept that the parafoveal inner retinal ‘neurovascular unit’ may be affected in CKD.
Few prior studies showed the decreased macular thickness and RNFLt in patients with CKD (Demir et al. 2009; Balmforth et al. 2016). Recently, Prakasam et al. (2019) evaluated 15 paediatric patients with mild-to-moderate CKD and found that the parafoveal thickness, GCL and IPL were reduced compared with healthy controls. Our study found that macular thinning was strongly associated with decreased GCCt and RNFLt as well as with increased GVL and FLV in adult patients with CKD. In addition, we determined that the risk of this neural impairment is associated with CKD severity. To the best of our knowledge, this is the first observation of a correlation of retinal neural impairment with severity of CKD.
Ganglion cell complex thickness (GCCt) and RNFLt are sensitive parameters for the detection of neural damage in early glaucoma, even in the preperimetric status (Mwanza et al. 2012; Lisboa et al. 2013). Focal loss volume (FLV) and GLV represent the local significant GCC loss and the average amount of GCC loss over the GCC map, respectively. A previous study indicated that FLV may be a more accurate parameter than GCCt and RNFLt to distinguish glaucomatous eyes from normal eyes (Rao et al. 2012). Defects in the parameters as mentioned above represent neural damage in the inner retina. Our results suggest that decreased macular thickness could be characteristic of neural damage within the retina in patients with CKD. Macular thickness might thus be a potentially useful marker for screening for retinal neural defects in patients with CKD (Fig. S2).
Multiple linear regression models in our study showed eGFR to be a significant independent risk factor consistently for the changes in all neural parameters (Table 2). The risk of neural impairment is also associated with increased CKD stage in the overall study population, even after adjusting for potential confounding factors. When comparing each stage of CKD, however, the difference may not be statistically significant (Fig. 1). This could be due to the small patient number in each subgroup and/or the disproportional distribution of eGFR (30–59 versus 15–29 versus <15) among different stages of CKD.
There are several possible mechanisms for the neural damage in CKD patients (Wong et al. 2014). It could be related to comorbid systemic diseases (such as DM and hypertension) or other pathological conditions related to CKD.
Diabetes mellitus (DM) was present in 42% of our patients. Studies have shown that neurodegeneration may occur early in DM, even before clinically notable retinopathy or microangiopathy (Wong et al. 2014; Gundogan et al. 2016; Jonsson et al. 2016; Sohn et al. 2016), which may lead to findings of decreased PfRT and increased FLV in this study (Table 2). However, unlike eGFR which was associated with all neural parameters, DM was not significantly associated with GCCt, GLV and RNFLt. Because all patients with DR had been excluded from our analysis, the appearance of DM's influence might have been artificially reduced in our study.
Hypertension is also very common among patients with CKD, being present in approximately 85% of such patients. Hypertension might also be correlated with neural impairment in patients with CKD, although the relationship is still controversial (Balmforth et al. 2016; Kalaitzidis & Elisaf 2018). The inconsistent conclusions may have resulted from different study designs that enrolled patients with different durations and severities of hypertension. In our study, hypertension and blood pressure during enrolment were not significantly associated with neural impairment. This may be because 90% of our patients had hypertension and most of these hypertensive patients already had their blood pressure under control with treatment. As a result, it is difficult to evaluate the effect of hypertension and blood pressure with the current study design. Future longitudinal studies could further elucidate the influence of blood pressure on the neural damage among CKD patients.
Other possible mechanisms include increased inflammation and oxidative stress, dysregulation of the renin–angiotensin system and presence of uraemic toxins. These pathways might also cause neural damage by increasing neuroinflammation, oxidative stress, vascular dysfunction or neuron and astrocyte death (Wong et al. 2014; Arnold et al. 2016; Balmforth et al. 2016; Chen et al. 2017; Lau et al. 2017; Ito et al. 2018; Hamed 2019).
The relationship between CKD and IOP remains controversial. In the Singapore Malay Eye Study, lower eGFR was associated with higher IOP (Nongpiur et al. 2010), but no such association was found in the Korean National Health and Nutrition Examination Survey (Shim et al. 2016). In our study, IOP showed no association with the neural impairment in CKD patients (Table 2). No difference of IOP was noted between CKD and control group (Table 1). Thus, IOP changes may be not able to explain the neural impairment in CKD patients. However, given that patients with CKD have a higher risk of glaucoma (Wong et al. 2014; Shim et al. 2016; Wong et al. 2016), regular follow-up of IOP and changes in neural defects could be crucial in patients with CKD with decreased macular thickness.
In this study, the neural parameters were associated with SVP-VD and RPC-VD, but not with DVP-VD. This may be because the neural parameters (CGGt, GLV, FLV and RNFLt) used in this study mainly reflect the neural changes in the superficial retinal layer (i.e. GCL and NFL axons). Superficial vascular plexus (SVP) consists of vessels primarily in the GCL and NFL (Campbell et al. 2017). The RPC runs in parallel with the NFL axons and supplies the densely packed nerve fibre bundles in this region (Campbell et al. 2017). It is, therefore, reasonable that the neural parameters in this study are associated with SVP-VD and RPC-VD. On the other hand, the DVP contains the ‘intermediate’ and ‘deep’ capillary plexuses, which are located within the IPL–inner nuclear layer (INL) and INL–OPL, respectively (Campbell et al. 2017). Thus, although DVP-VD decreased in the CKD group, it was not associated with the ‘superficial’ neural parameters in this study.
The strong correlation between neural impairment and vascular alteration in the parafoveal superficial retina layers (i.e. SVP-VD) suggests a concomitant impairment in the neural and microvascular elements within the inner retina over the parafoveal area in patients with CKD. The term ‘neurovascular unit’ refers to the intimate physical and biochemical relationship among neurons, glia and vasculature within the retina (Metea & Newman 2007; Antonetti et al. 2012). Close coupling of the neurovascular unit is critical for autoregulation of retinal blood flow and metabolic activities (Metea & Newman 2007; Antonetti et al. 2012; Cubuk et al. 2018). Damage in either the neural element or vascular element may disintegrate this unit and lead to loss of autoregulation in the retina (Antonetti et al. 2012; Gardner & Davila 2017; Cubuk et al. 2018). Disruption of the neurovascular unit occurs in the pathogenesis of DR (Antonetti et al. 2012; Gardner & Davila 2017). Our study showed that progressive damage of the neurovascular unit may also occur in patients with CKD.
It is interesting that despite decreased RNFLt in CKD patients and that RNFLt is correlated with RPC-VD, the level of RPC-VD is not significantly different between CKD patients and controls. One possible reason could be that RNFLt may be more sensitive than RPC-VD in detecting early neurovascular changes with the current OCT and OCTA technology. Some patients with obvious focal RNFL defects could have subtle changes in the PRC (Fig. S3). The changes could be difficult to quantify in an OCTA. In a recent study, Rodrigues et al. demonstrated a significantly decreased RPC-VD in diabetic patients (Rodrigues et al. 2019). However, the RPC changes were not proportioned to the severity of DR in their data. Annular RPC density was 32.37%, 28.87%, 31.12% and 29.32% in control, no DR, mild non-proliferative DR (NPDR) and moderate NPDR groups, respectively. Further improvement in OCTA quality and segmentation algorism may improve the accuracy of detecting subtle peripapillary microvascular changes and help confirm this hypothesis.
Enlarged FAZ or loss of perifoveal capillaries had been shown to be early signs of several retinal vascular diseases (Balaratnasingam et al. 2016; Lynch et al. 2018). In our study, although FAZ size seems to enlarge with increased CKD severity, the statistical analyses were not significant. This may be attributed to our patients' relative early stages of retinal disease. Patients with DR and visual symptoms had been excluded from our study. This may also support that the retinal neural impairment could occur early in CKD patients.
In the multiple linear regression model, gender was associated with PfRT (Table 2), which agrees with prior studies that macular thickness is lower in women than in men (Hashemi et al. 2017; Cubuk et al. 2018). Another noteworthy finding was that DM was associated with higher FLV, whereas longer axial length was associated with lower FLV (Table 2). Longer axial length may have a protective effect against DR (Fu et al. 2016). We speculate that this protective effect may have led to lower FLV in the patients with longer axial length in our study.
Our study had several limitations. First, information about inflammation markers, oxidative stress and uraemic toxins was incomplete, and the exact pathophysiological mechanism underlying this neural defect remains unclear. Second, we could not determine the role of hypertension in neural impairment. However, our results could still apply to daily clinical practice because hypertension commonly coexists in patients with CKD. Finally, the rate of progression in these neural defects is unknown. Further animal model or longitudinal cohort studies are necessary to elucidate the pathway of injury and evaluate the rate and risks of progression.
In summary, our study demonstrated that macular thinning is a characteristic of retinal neural impairment in patients with CKD. This retinal neural impairment was strongly associated with decreased eGFR. We also demonstrated accompanying microvascular alterations in the parafoveal superficial retinal layers, which implied damage of the ‘neurovascular unit’ in this region. Our study suggests CKD patients may benefit from an ophthalmological assessment including SD-OCT and OCTA examinations.