Ophthalmological findings in neuroborreliosis – a prospective study performed in western Sweden
Abstract
Purpose
To evaluate and follow-up ophthalmological findings in individuals diagnosed with neuroborreliosis, confirmed by cerebrospinal fluid (CSF) analysis.
Methods
Twenty-four individuals (13 males), mean age 43.5 ± 18.2 years, with strong clinical suspicion of neuroborreliosis, were referred to the Department of Ophthalmology by the Department of Infectious Diseases at Sahlgrenska University Hospital, Gothenburg, Sweden. All subjects underwent serological and CSF analysis. A structured history taking and a detailed ophthalmological examination were performed prospectively.
Results
Diagnosis for neuroborreliosis was confirmed as definite in 16, possible in two and negative in four individuals, while two had unknown diagnosis. The majority (n = 14/18) with definite and possible diagnoses had ophthalmological symptoms and/or findings either in history or at examination. The most common findings were visual disturbance, diplopia, red eyes, photophobia, facial palsy with palpebral diastasis, strabismus and sixth nerve palsy. The number of symptoms and findings was correlated with immunoglobulin G (IgG)/IgM in CSF (r = 0.6, p = 0.009/0.016; Spearman's correlation). All subjects improved, except one with initially fulminant papilloedema, who still suffered from optic disc atrophy and affected visual fields at the last follow-up.
Conclusion
The majority of patients diagnosed with neuroborreliosis had ophthalmological symptoms and/or findings. Facial palsy with palpebral diastasis was a common finding. Onset of diplopia and/or sixth nerve affection may be a first sign of neuroborreliosis. Number of ophthalmological findings was correlated with the CSF antibody titre. Ticks are becoming more widespread and abundant, resulting in a higher incidence of neuroborreliosis. Hence, the knowledge of ophthalmological symptoms and findings is of great importance.
Introduction
In 1909, the Swedish dermatologist Arvid Afzelius was the first to describe the skin manifestation erythema migrans (EM) (Afzelius 1910). However, it was only in 1981 that Willy Burgdorfer discovered a spirochaete in ticks, thereby establishing the aetiological agent (Burgdorfer et al. 1982). Borreliosis, also referred to as Lyme disease, is a zoonosis caused by the spirochaete Borrelia burgdorferi complex which is transmitted via a tick vector (Stanek et al. 2012). The most common tick (Ixodes ricinus) occurring in Europe has increased its geographical spread in northern Europe. Speculations on possible explanations for this include warmer winters due to climate change and greater abundance of host animals such as roe deers (Jaenson et al. 2012). An epidemiological study in southern Sweden concluded that borreliosis incidence varies according to endemic area and age, with people aged 5–9 and 60–74 having the highest incidence rates. Sixteen per cent of all cases of borreliosis in that study developed neuroborreliosis. The study also showed a link between bite site in the head and neck region among children, and neuroborreliosis (Berglund et al. 1995).
The first stage of clinical infection usually involves the skin and EM is the most common finding, radiating out from the bite site. However, the absence of EM does not rule out the infection. Malaise, fever, myalgia, arthralgia and headache may accompany the early stages of infection, but are less common. Spreading of the infection may affect other organ systems such as the nervous (i.e. in neuroborreliosis) and cardiovascular system (Stage 2). Although rare, borrelial lymphocytoma, likewise classified as Stage 2, is a blue-reddish nodule mostly occurring in the ear lobe or nipple. Stage 3, or late borreliosis, includes acrodermatitis chronica atrophicans and arthritis (Marques 2015). In 1998, a study conducted by Oschmann et al. identified the general order in which the disease starts and progresses. Starting with a tick bite, EM develops and as the infection continues it disseminates, causing radicular pain and eventually focal neurological symptoms as the nervous system is affected. However, the clinical findings can vary considerably. The most common presentation of neuroborreliosis in Europe is Bannwarth's syndrome (i.e. lymphocytic meningoradiculitis). Some of the other symptoms or findings reported by Oschmann et al. were cranial nerve palsies, headache, muscle ache and fever. Further, seventh nerve palsy was common both unilaterally and bilaterally. Another interesting feature was the sensation of an unusual pain (often described as a burning sensation with dysesthesia), migrating to the back, typically with nocturnal exacerbations (Oschmann et al. 1998). Even though EM is a distinct characteristic of the infection, the majority of patients do not remember either being bitten by a tick or having had the skin efflorescence, therefore often making early neuroborreliosis, which develops within 2–18 weeks of the infection, a first finding (Koedel et al. 2015).
Ophthalmological involvement may occur during any stage of the infection. However, it is most often reported during stages 2 (i.e. neuroborreliosis) and 3 (Mora & Carta 2009). Most of our knowledge about ophthalmological involvement is based on case reports or smaller case series, and a variety of different symptoms and findings have been reported. During Stage 1, local symptoms such as periorbital swelling, conjunctivitis and photophobia have been noted. Symptoms from the ocular system in general may be pain, reduced visual acuity (VA) or blurred vision and diplopia (Klig 2008; Mora & Carta 2009). During stages 2 and 3, the following are examples of ophthalmological manifestations which have been reported: seventh nerve palsy with palpebral diastasis, keratitis, episcleritis, uveitis, vitreitis, choroiditis, optic disc oedema, macular oedema, affection of the retinal vessels, optic neuritis, decreased colour vision and reduced visual fields. Other ocular findings from case reports are paralytic mydriasis, Horner's syndrome, Argyll Robertson pupil and orbital myositis (Klig 2008). Overall, if a patient is infected and the eye or visual system is affected, it is likely to cause symptoms. It is highly likely that the patient will seek medical care. Children may, for reasons related to the development of the brain and visual system, not be aware of (or tell about) or not even experience symptoms (Zaidman 1997; Mora & Carta 2009).
In Europe, modern consensus guidelines for neuroborreliosis state that clinical symptoms suggesting neuroborreliosis and cerebrospinal fluid (CSF) analysis showing lymphocytic pleocytosis and specific intrathecal antibodies against B. burgdorferi are needed to confirm a diagnosis as definite (Mygland et al. 2010). It should be noted that diagnostic criteria have become stricter over time. During the 1990s, diagnostic criteria were more liberal. For example, intrathecal antibody production was not mandatory for the definite diagnosis (Oschmann et al. 1998). Even today, there are differences between European and US criteria, one example of which is that testing for antibodies in the CSF is not mandatory in the United States (Wormser et al. 2006; Marques 2015).
As ticks are increasing in number and becoming more widespread in northern Europe, this may lead to a larger number of infected individuals and hence result in a higher incidence of neuroborreliosis (Lindgren et al. 2000). The ophthalmological symptoms and findings in patients with neuroborreliosis may vary considerably and, except for case studies (Amer et al. 2009; Sauer et al. 2011), there are not many studies that profoundly investigate ocular involvement in these patients (Zaidman 1997; Mora & Carta 2009). Therefore, the purpose of this study was to further evaluate and follow-up ophthalmological symptoms and findings in individuals diagnosed with neuroborreliosis confirmed by CSF analysis.
Patients and Methods
Over 6 years, 24 individuals with strong clinical suspicion of neuroborreliosis or newly diagnosed neuroborreliosis were referred to the Department of Ophthalmology by the Department of Infectious Diseases at Sahlgrenska University Hospital, Gothenburg, Sweden. All subjects were serologically tested for B. burgdorferi antibodies no later than 2 days after admittance (three subjects were tested prior to referral). All patients underwent lumbar puncture no later than 3 days after admittance. At the time of prospective ophthalmological assessment, all patients were either untreated or had just started antibiotic therapy.
Diagnostic criteria
The European consensus guidelines for neuroborreliosis state that all of the following criteria need to be fulfilled for a definite diagnosis: clinical symptoms suggesting neuroborreliosis and CSF analysis showing lymphocytic pleocytosis (≥5 × 106/L, mononuclear count >90%) and specific intrathecal antibodies, either immunoglobulin G (IgG) or IgM or both, against B. burgdorferi (Mygland et al. 2010). These IgG and IgM antibodies were analysed using an enzyme-linked immunosorbent assay (ELISA) kit (Lyme Borreliosis Kit; DakoCytomation, Glostrup, Denmark). To assess intrathecally produced antibodies, blood serum and CSF samples should be collected at the same time. Where intrathecal antibodies are absent, the diagnosis is possible if specific antibodies in serum are detected after 6 weeks (Mygland et al. 2010). Furthermore, Swedish national guidelines published by the Medical Products Agency state that new neurological symptoms after EM may be classified as possible neuroborreliosis.
Ophthalmological examination
The ophthalmological investigation followed a specific protocol including anamnesis, clinical findings, treatment and follow-up. Data regarding age, sex, current medication and previous medical and ophthalmological history were obtained. Recent history, including exposure to endemic areas, tick bite, general symptoms (e.g. fatigue, headache, neck pain, fever, gastrointestinal symptoms, migrating muscular and/or joint ache), EM, borrelial lymphocytoma, acrodermatitis chronica atrophicans, facial palsy and other neurologic symptoms and findings, was recorded as well as specific ophthalmological symptoms such as subjectively reduced VA, diplopia, red eyes, photophobia, blepharospasm and strabismus. The ophthalmological examination included best corrected VA, pupillary light reaction, ocular motility and nystagmus, strabismus, slit-lamp inspection and ophthalmoscopy (conjunctiva, cornea, anterior chamber flare, lens, vitreous body, fundus inspection under cycloplegia), test of visual fields with high-pass resolution perimetry (Frisén & Nikolajeff 1993) and, on clinical suspicion of involvement, colour vision and fluorescein angiography. Patients with ophthalmological symptoms and/or findings were followed up at the Department of Ophthalmology at Sahlgrenska University Hospital, Gothenburg, Sweden.
Statistical analyses
Mean ± standard deviation (SD), median and range of the continuous variable were calculated for descriptive purposes and number and percentages for categorical variables. Antibody (IgG or IgM) results (graded 0–4) and mononuclear cells were compared with the number of ophthalmological findings and symptoms using Spearman's correlation analysis. The groups with a definite (n = 16) and possible (n = 2) diagnosis were merged to one group (n = 18) when performing correlation analysis. A value of p < 0.05 was considered statistically significant.
Ethical approval
The study was approved by The Regional Ethical Review Board in Gothenburg, and written informed consent was obtained from all subjects or the parent(s)/guardian(s) of the participating children. All procedures were performed in accordance with the tenets of the Declaration of Helsinki.
Results
Twenty-four individuals, 13 males and eleven females, were included in the study, mean age 43.5 ± 18.2 years (range 8.2–69.7 years). All participants were treated with oral doxycycline 200–400 mg daily (children received adequate doses) for 10–14 days, according to standard treatment in Sweden (Dotevall & Hagberg 1999). Two subjects in the possible group and seven of 16 in the definite group did not use any other medication. No known ophthalmological side effects were registered for the medications used by the nine subjects in the definite group.
Diagnostic criteria
Table 1 shows the four diagnostic groups using revised consensus criteria: definite diagnosis (n = 16), possible diagnosis (n = 2), negative diagnosis (i.e. not neuroborreliosis) (n = 4) and unknown diagnosis (n = 2). Patients with a definite diagnosis had specific antibodies in their CSF, while those with a possible diagnosis had new neurological symptoms after EM. Negative and unknown diagnoses meant no antibodies in CSF. The majority of patients had had exposure to an endemic area, but even among the definite and possible diagnoses, fewer than half of the patients (seven out of 18) reported noticing a tick bite and EM (Table 2). No subject was found to have either borrelial lymphocytoma or acrodermatitis chronica atrophicans.
| Subject (No./sex/age) | Diagnosis | Serum and CSF analysis (yes/no) | Specific serum ABs (±) | 1st CSF mono (x 106/L) | 1st CSF-IgG (0–4)b | 1st CSF-IgM (0–4)b | 1st CSF-specific ABs i.t. (±) | Follow-up, CSF analysis (yes/no) | Last follow-up, LP (months) | Last follow-up, CSF mono (x106/L) | Last follow-up, CSF-IgG (0–4)b | Last follow-up, CSF-IgM (0–4)b | Follow-up, CSF-specific ABs i.t. (±) | Follow-up, serum IgG (±) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/F/8.2 | Definite | Yes | + | 28 | 2 | 0 | + | No | ||||||
| 2/M/8.8 | Definite | Yes | + | 208 | 0 | 0 | − | Yes | 6.8 | n/a | 3 | 0 | + | |
| 3/M/68.5 | Definite | Yes | + | 363 | 3 | 3 | + | No | ||||||
| 4/F/50.6 | Definite | Yes | + | 284 | 4 | 1 | + | Yes | 0.2a | 121 | n/a | n/a | ||
| 5/F/45.5 | Definite | Yes | + | 98 | 4 | n/a | + | Yes | 1.4 | 15 | n/a | n/a | ||
| 6/M/51.2 | DEFINITE | Yes | + | 488 | 4 | 2 | + | Yes | 1.0 | 112 | n/a | n/a | ||
| 7/M/38.6 | Definite | Yes | + | 291 | 4 | 4 | + | Yes | 1.7 | 20 | n/a | n/a | ||
| 8/F/67.6 | Definite | Yes | + | 28 | 2 | 0 | + | Yes | 1.4 | 5 | n/a | n/a | ||
| 9/F/55.3 | Definite | Yes | + | 156 | 4 | 4 | + | Yes | 1.4 | 21 | n/a | n/a | ||
| 10/F/27.6 | Definite | Yes | + | 379 | 3 | 4 | + | Yes | 1.5 | 9 | n/a | n/a | ||
| 11/F/66.8 | Definite | Yes | + | 56 | 3 | 0 | + | No | ||||||
| 12/F/59.7 | Definite | Yes | + | 69 | 2 | 2 | + | Yes | 1.6 | 6 | 0 | 0 | − | |
| 13/F/58.2 | Definite | Yes | + | 117 | 3 | 4 | + | n/a | ||||||
| 14/F/69.7 | Definite | Yes | + | 214 | 4 | 2 | + | n/a | ||||||
| 15/M/47.4 | Definite | Yes | + | 156 | 4 | 0 | + | Yes | 1.3 | 18 | n/a | n/a | ||
| 16/M/50.8 | Definite | Yes | − | 116 | 3 | 0 | + | n/a | ||||||
| 17/M/45.6 | Possible | Yes | + | 94 | 0 | 0 | − | No | n/a | |||||
| 18/M/22.4 | Possible | Yes | + | 340 | 0 | 0 | − | n/a | n/a | |||||
| 19/M/46.0 | Negative | Yes | − | 19 | 0 | 0 | − | n/a | n/a | |||||
| 20/M/35.3 | Negative | Yes | − | 10 | 0 | 0 | − | Yes | 1.6 | n/a | 0 | 0 | − | − |
| 21/M/35.3 | Negative | Yes | − | 108 | 0 | 0 | − | Yes | 1.7 | − | 0 | 0 | − | − |
| 22/M/29.4 | Negative | Yes | − | 5 | 0 | 0 | − | Yes | 6.1 | n/a | 0 | 0 | − | n/a |
| 23/F/14.6 | Unknown | Yes | − | 114 | 0 | 0 | − | No | − | |||||
| 24/M/40.5 | Unknown | Yes | + | 17 | 0 | 0 | − | Yes | 1.3 | 8 | 0 | 0 | − | − |
- All subjects underwent lumbar puncture (LP) no later than 3 days postadmittance. Definite diagnosis means that the patient had specific antibodies in cerebrospinal fluid (CSF), possible means new neurological symptoms appeared after erythema migrans (EM), while patients with negative and unknown diagnoses had no antibodies in CSF.
- a Short follow-up due to hospitalization (evaluate treatment effect).
- b antibody results were graded 0-4 (0 = negative; 1 = limit value; 2 = lightly elevated; 3 = elevated; 4 = strongly elevated).
- AB = antibody, CSF = cerebrospinal fluid, F = female, IgG = immunoglobulin G, IgM = immunoglobulin M, i.t. = intrathecal, M = male, mono = mononuclear cells, n/a = not available, LP = lumbar puncture, + = positive, − = negative.
| Subject (No./sex/age/diagnosis) | Previous general history | Exposure to tick-endemic area | Observed tick bite/EM | Time connectiona | Fatigue | Fever | GI symptoms | Headache | Neck pain | Migrating muscular/joint or other ache | Other neurological symptoms |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/F/8.2/D | None | ? | +/? | + | + | + | ? | + | + | ? | None |
| 2/M/8.8/D | EM 2 years prior to admittance | + | +/− | + | + | + | − | + | − | + | General weakness |
| 3/M/68.5/D | Myocardial infarction, cardiac arrest, peptic ulcer ×4. Kidney surgery | − | ?/? | ? | ? | − | − | + | + | + | Allodynia |
| 4/F/50.6/D | Sciatica at some point. Hypertension | + | −/? | + | + | − | ? | + | ? | + | None |
| 5/F/45.5/D | Pyelonephritis. Vestibular neuritis. Hereditary rheumatism | + | ?/ − | ? | + | − | + | + | − | + | None |
| 6/M/51.2/D | None | + | +/− | + | + | − | − | + | + | − | Paraesthesia |
| 7/M/38.6/D | None | + | +/+ | + | + | + | + | + | + | + | None |
| 8/F/67.6/D | Thyroidectomy, migraine | + | ?/ − | ? | + | + | + | − | + | + | Allodynia |
| 9/F/55.3/D | Musculoskeletal ache, shoulders and back | + | +/+ | + | + | + | − | + | + | + | Balance difficulties. Hearing impairment. Asthenic arms. Sensory alteration. Dysphagia. Weight loss |
| 10/F/27.6/D | Sporadic migraine | + | ?/ − | ? | + | + | − | + | + | + | Vertigo. Nausea, balance impairment |
| 11/F/66.8/D | Tachycardia | + | +/+ | ? | + | − | − | + | + | + | Vertigo. |
| 12/F/59.7/D | Rheumatoid arthritis. Malaria | + | +/+ | + | + | + | + | + | + | − | Dysphagia. Asthenic arms. Numbness |
| 13/F/58.2/D | Pain difficulties, depression | + | −/+ | ? | + | + | + | − | + | + | Sensory loss, arm |
| 14/F/69.7/D | Hypertension. Hypercholesterolaemia | + | +/+ | + | + | − | − | + | + | + | None |
| 15/M/47.4/D | Seborrheic eczema | + | −/+ | + | + | − | − | + | + | + | Hearing impairment. Head (scalp) pain |
| 16/M/50.8/D | Hiatus hernia | + | +/+ | + | + | ? | − | + | + | + | Sound susceptibility. Hearing impairment |
| 17/M/45.6/P | Lumbago, sciatica previously | + | +/+ | − | + | − | − | − | − | + | Allodynia. Sensory alteration |
| 18/M/22.4/P | None | + | ?/+ | ? | − | − | − | − | − | + | Allodynia. |
| 19/M/46.0/N | None | + | +/+ | + | + | + | − | + | − | + | None |
| 20/M/35.3/N | None | + | −/− | ? | + | − | − | + | − | + | Numbness: left leg, sole of the foot bilaterally, right thigh and scrotum. Slight numbness, right facial half |
| 21/M/35.3/N | Lumbar spondylolisthesis | − | −/− | − | − | + | + | + | + | − | Vertigo. Paraesthesia, superficially, head |
| 22/M/29.4/N | Whiplash | + | ?/ − | + | + | − | − | + | + | + | Sound susceptibility. Balance impairment. Sensory loss in the perioral region |
| 23/F/14.6/U | Headache now and again for 1 year. More, constant headache at admittance | ? | −/− | ? | + | + | ? | + | + | − | Vertigo, balance impairment, painful sensation, right facial half |
| 24/M/40.5/U | Headache sometimes. No migraine. | ? | −/− | − | + | − | − | + | + | − | Sensory loss upper lip. Head (scalp) pain. |
- Patients with a negative or unknown diagnosis did not have antibodies in cerebrospinal fluid (CSF).
- CSF = cerebrospinal fluid, D = definite, EM = erythema migrans, F = female, GI = gastrointestinal, M = male, N = negative, P = possible, U = unknown, + = yes, − = no, ? = subject does not know.
- a Time connection indicates whether there was reasonable time elapsed from exposure to an endemic area (or tick bite or EM) until the perceived illness debuted.
Ophthalmological symptoms and findings
The most common ophthalmological symptoms (Fig. 1) and findings (Fig. 2) in this study were facial palsy with palpebral diastasis, blurred vision, strabismus, diplopia and abducens nerve affection. In the definite group, twelve of 16 individuals had both symptoms and objective findings. In the possible diagnosis group (n = 2), one subject had only findings and the other subject had neither ophthalmological symptoms nor ophthalmological findings. In the negative diagnosis group (n = 4), all but one had both symptoms and findings. Finally, in the unknown group (n = 2), one subject had both symptoms and findings and the other one had only symptoms (Tables 3 and 4). No subjects had anterior chamber flare, vitreous opacities or abnormal pupillary light reaction. None needed further investigation with fluorescent angiography. Colour vision was only investigated in five of 24 subjects; all were considered normal.
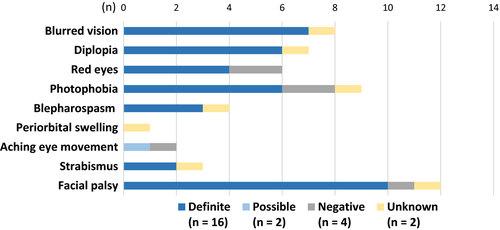
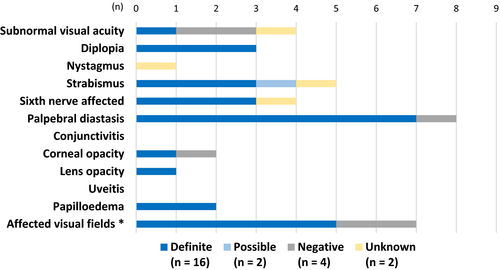
| Subject (No./sex/age/diagnosis) | Previous ocular history | Subjective visual disturbance | Diplopia | Red eyes | Photophobia | Facial palsy | Blepharospasm | Strabismus |
|---|---|---|---|---|---|---|---|---|
| 1/F/8.2/D | None | n/a | n/a | n/a | n/a | − | n/a | n/a |
| 2/M/8.8/D | None | + | + | − | − | + | − | − |
| 3/M/68.5/D | None | − | − | − | − | − | − | − |
| 4/F/50.6/D | None | + | + | n/a | + | n/a | n/a | + |
| 5/F/45.5/D | None | − | − | − | + | − | − | − |
| 6/M/51.2/D | None | + | + | − | − | − | − | + |
| 7/M/38.6/D | None | − | − | + | − | + | + | − |
| 8/F/67.6/D | Difficulties closing left eye lids during endocrine ophthalmopathy | − | − | − | − | − | − | − |
| 9/F/55.3/D | None | − | + | + | + | + | + | − |
| 10/F/27.6/D | None | − | + | − | − | + | − | + |
| 11/F/66.8/D | None | + | − | + | + | +a | − | − |
| 12/F/59.7/D | Macula affected by chloroquine treatment (OS) and vitreous detachment (OD) many years previously | n/a | + | − | + | + | − | − |
| 13/F/58.2/D | Slow-healing corneal erosion and pre-septal cellulitis 4 years previously. Star-shaped opacity | − | − | − | − | + | − | − |
| 14/F/69.7/D | None | + | − | + | + | +a | + | − |
| 15/M/47.4/D | Perceived visual disturbance over the last 2 years | + | − | − | − | + | − | − |
| 16/M/50.8/D | None | + | − | − | n/a | + | − | − |
| 17/M/45.6/P | None | − | − | − | − | − | − | − |
| 18/M/22.4/P | None | − | − | − | − | − | − | − |
| 19/M/46.0/N | None | − | − | − | + | − | − | − |
| 20/M/35.3/N | None | − | − | − | − | − | − | − |
| 21/M/35.3/N | Reduced VA (OD) due to trauma when 5 years old (corneal cicatrization) | − | − | + | − | − | − | − |
| 22/M/29.4/N | None | − | − | + | + | +a | − | − |
| 23/F/14.6/U | None | + | + | − | + | − | − | + |
| 24/M/40.5/U | None | − | − | − | − | + | + | − |
- D = definite, F = female, M = male, N = negative, n/a = not available, OD = oculus dexter, OS = oculus sinister, P = possible, U = unknown, VA = visual acuity, + = yes, or presence of finding, − = no, or no finding.
- a Bilaterally.
| Subject (No./sex/age/diagnosis) | VA (LogMAR), OD/OS | Eye lid diastasis (mm), OD/OS | Conjunctiva, OD/OS | Cornea, OD/OS | Lens, OD/OS | Fundus, OD/OS | PO (0–5), OD/OS | Motility affected, OD/OS | Strabismus | Nystagmus | Affected VF | Colour vision (points), OD/OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/F/8.2/D | 0.0/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | 10/10 |
| 2/M/8.8/D | 0.0/0.05 | 0/6–7; no BP | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | 15/20 |
| 3/M/68.5/D | 0.0/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | n/a |
| 4/F/50.6/D | 0.0/0.0 | 0/0 | −/− | −/− | −/− | −/+a | 0/2 | Sixth nerve, OU | − | − | n/a | n/a |
| 5/F/45.5/D | 0.0/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | Exophoria | − | +/+ | n/a |
| 6/M/51.2/D | 0.4/0.3 | 0/0 | −/− | −/− | −/− | +a/+a | 5/5 | −/− | Esotropia OD | − | +/+ | 0/5 |
| 7/M/38.6/D | −0.1/−0.2 | 2/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | n/a |
| 8/F/67.6/D | 0.0/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | n/a |
| 9/F/55.3/D | 0.0/0.0 | 0/1 | −/− | −/− | −/− | −/− | 0/0 | −/sixth nerve | − | − | −/− | n/a |
| 10/F/27.6/D | 0.0/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/sixth nerve | Hypophoria | − | −/− | n/a |
| 11/F/66.8/D | 0.05/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | n/a |
| 12/F/59.7/D | 0.0/0.0 | 0/0 | Pinguecula OU | −/− | −/− | −/− | 0/0 | −/− | − | − | +/+ | 5/5 |
| 13/F/58.2/D | 0.05/0.0 | 0/10; no BP | −/− | −/Opacity | Mild opacity OU | −/− | 0/0 | −/− | − | − | +/+ | n/a |
| 14/F/69.7/D | 0.0/0.0 | 0/1 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | n/a | n/a |
| 15/M/47.4/D | 0.1/0.1 | 1/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | +/+ | n/a |
| 16/M/50.8/D | 0.0/0.0 | 3/0; BP | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | n/a |
| 17/M/45.6/P | 0.0/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | n/a |
| 18/M/22.4/P | −0.1/−0.1 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | Exophoria | − | −/− | n/a |
| 19/M/46.0/N | 0.0/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | +/+ | 10/10 |
| 20/M/35.3/N | 0.0/−0.1 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | −/− | n/a |
| 21/M/35.3/N | 0.3/0.0 | 0/0 | −/− | Opacityb/− | −/− | −/− | 0/0 | −/− | − | − | +/+ | n/a |
| 22/M/29.4/N | 0.1/0.0 | 8/8; BP, OU | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | n/a | n/a |
| 23/F/14.6/U | 0.3/0.2 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/sixth nerve | Esotropia OS | + | −/− | n/a |
| 24/M/40.5/U | −0.1/0.0 | 0/0 | −/− | −/− | −/− | −/− | 0/0 | −/− | − | − | n/a | n/a |
- BP = Bell's phenomenon, D = definite, F = female, LogMAR = logarithm of the minimum angle of resolution, M = male, N = negative, n/a = not available, OD = oculus dexter, OS = oculus sinister, OU = oculus uterque (i.e. both eyes), P = possible, PO = papilloedema, U = unknown, VA = visual acuity, VF = visual fields, + = yes, or presence of finding, − = no, or no finding.
- a Papilloedema.
- b Due to previous trauma.
A significant positive correlation was found between both IgG (r = 0.6, p = 0.009) and IgM (r = 0.6, p = 0.016) antibody titres (grading 0–4) and number of ophthalmological symptoms and findings in patients with confirmed neuroborreliosis, that is patients with a definite or possible diagnosis (n = 18). However, no statistically significant correlation was found with mononuclear cells in CSF (r = 0.4, p = 0.089).
Follow-up
Patients with ophthalmological symptoms and/or findings were followed up. Thus, in the definite diagnosis group, eight subjects were considered in need of follow-up, having findings such as large palpebral diastasis, papilloedema, abnormal visual fields, diplopia and reduced VA. However, three declined, leaving five subjects who participated in follow-up (mean 60 ± 73 days, range 12–180 days). Two of the five subjects had remaining findings at last examination. One who initially had fulminant bilateral papilloedema was found to have optic disc atrophy and remaining affection of the visual fields at last examination (Fig. 3); the other subject at last examination had a palpebral diastasis in regression. In addition, two patients, one from the negative and one from the unknown group, were followed up and both were restored to normal. Participants with normal or trivial findings were not followed up. Overall, follow-up for all subjects ranged from 14 to 472 days, with a median of 97 days.

Discussion
In this population of 24 individuals with clinical suspicion of neuroborreliosis, the vast majority from the definite and possible diagnosis groups had ophthalmological symptoms in their history and/or ophthalmological findings at examination. The most frequent symptoms and findings were palpebral diastasis due to facial palsy, blurred vision, red eyes, photophobia, diplopia and sixth nerve affection. Findings and symptoms were positively correlated with the CSF antibody titres of IgG and IgM.
Interestingly, neither conjunctivitis nor uveitis were found in any of the patients in the present study. A Finnish study with 20 subjects with somewhat similar diagnostic methods for neuroborreliosis found ten cases of uveitis. Other manifestations also found in the same study were conjunctivitis, episcleritis, keratitis, sixth nerve palsy, paralytic mydriasis, affected colour vision and even ocular vascular occlusion. Similar to the present study, the Finnish study found EM in ten of 20 participants with neuroborreliosis (Mikkila et al. 2000). In Germany, a prospective study of adolescents identified 84 patients with Lyme arthritis (like neuroborreliosis, a later stage of borrelial infection). Only six of 84 patients had conjunctivitis, and another three of those 84 had intermediate uveitis with retinal detachment, keratitis and anterior uveitis. However, the patient sample in the German study included individuals with Lyme arthritis and not neuroborreliosis (Huppertz et al. 1999). The reasons why no uveitis was found in the present study remain uncertain. This study is strengthened by two facts: firstly, the majority of subjects were prospectively examined by one ophthalmologists according to a specific protocol. Secondly, all patients underwent lumbar puncture and antibody analysis. For comparison, the German study on Lyme arthritis was a prospective multicentre study. Whether lumbar puncture was performed was not specified (Huppertz et al. 1999). Although the present study is a single-centre study, being more prone to selection bias and a possible lack of including relevant patients from the Department of Infectious Diseases, as well as patients with ophthalmological symptoms were probably more willing to participate, still the strengths of the study overweight its limitations.
In Denmark, Correll et al. carried out a retrospective study before and after antibiotic treatment for neuroborreliosis in six children also having ocular motor disturbance. Their findings were sixth nerve palsy (n = 2), partial third nerve palsy (n = 1) and nystagmus (n = 3) with symptoms such as diplopia (n = 2), mild photophobia (n = 1) and Adie's syndrome (n = 1). The authors, in discussing limitations of a small sample size, consider the possibility that their findings may not be related to neuroborreliosis per se; however, they conclude that this is unlikely as all six children in their study showed rapid improvement with antibiotic treatment, with five of the six children restored to normal no later than 7 months. Moreover, four of the six had noted a tick bite and two had noticed EM (Correll et al. 2015). When looking at the children in our study, two children, both aged 8, recalled having been bitten but had not noticed EM. Both had accompanying general symptoms listed in the protocol, such as fatigue, fever and headache. Only one of them reported blurred vision, diplopia, palpebral diastasis and facial nerve palsy.
In the present study, five individuals were followed up because of ophthalmological findings. The individual with fulminant papilloedema had optic disc atrophy and affected visual fields at the end of follow-up, but was otherwise restored. All subjects improved to such a point where they were either considered no longer in need of follow-up or themselves declined follow-up. Seven of the investigated individuals had visual field affection at first examination, but only one was found to have remaining visual field affection at the last follow-up (Fig. 2 and Table 4). The affected visual fields (Fig. 2) were due to lack of compliance, papilloedema with optic disc atrophy, myopia or previous ocular trauma.
In the present study, ophthalmological examinations should have been performed before the start of antibiotic treatment. However, four patients (Nos. 2, 10, 11 and 12) underwent ophthalmological examination long after the antibiotic treatment was over. This could of course have affected the outcome of the ophthalmological examination, as signs could have gone into full regression by the time of the examination. However, the assessment point ranged from 2 days before to 34 days after antibiotic treatment start-up, with a median of 4.5 days. Nevertheless, all of these patients had ophthalmological symptoms in the history and two still had findings on examination.
To our knowledge, there are few studies investigating prevalence of specific antibodies against B. burgdorferi intrathecally among healthy individuals. A Swedish epidemiological study among farmers and forest workers compared with office clerks found that in the outdoor group, 7.6% had positive IgG antibodies against B. burgdorferi compared with 5.3% of the clerks (Werner et al. 2001). The ophthalmological symptoms and findings reported in this study were correlated with the CSF antibody titre of IgG and IgM in patients from the definite and possible diagnosis group. Hence, higher titres of specific antibodies against B. burgdorferi in CSF result in more ophthalmological symptoms and findings, at least in patients with confirmed neuroborreliosis, that is subjects with a definite and possible diagnosis.
In conclusion, most of the patients with neuroborreliosis in the present study turned out to have a large number of ophthalmological findings and symptoms, some of which were considered severe. Overall, when looking at the results from the present study and previously reported results, one can conclude that neuroborreliosis is often difficult to diagnose initially, as many of the symptoms and findings are vaguely related. The take-home message for the ophthalmologist is that there might be reason to suspect neuroborreliosis, not only in subjects with facial palsy but also in patients with onset of diplopia and sixth nerve affection.




