The CC chemokines CCL8, CCL13 and CCL20 are local inflammatory biomarkers of HLA-B27-associated uveitis
Abstract
Purpose
To determine the concentrations of the CC chemokines CCL2, CCL7, CCL8, CCL11, CCL13, CCL20, CCL24 and CCL26 in aqueous humour (AH) samples from patients with specific uveitic entities.
Methods
Aqueous humour samples from patients with active uveitis associated with Behçet's disease (BD) (n = 13), sarcoidosis (n = 8), HLA-B27-related inflammation (n = 12), Vogt–Koyanagi–Harada (VKH) disease (n = 12) and control patients (n = 9) were assayed with the use of a multiplex assay.
Results
When considering all uveitis patients as one group, all chemokine levels except CCL2 were significantly increased compared to controls. CCL8, CCL13 and CCL20 were the most strongly upregulated, 48-fold, 118-fold and 173-fold, respectively, above control AH levels. CCL8 and CCL13 levels were significantly higher in HLA-B27-associated uveitis than in sarcoidosis and VKH disease. CCL20 levels were significantly higher in HLA-B27-associated uveitis than in BD, sarcoidosis and VKH disease. In addition, CCL20 levels were significantly higher in BD than in VKH disease. In HLA-B27-associated uveitis, CCL8, CCL13 and CCL20 were upregulated 111-fold, 255-fold and 465-fold, respectively, compared with controls. CCL8, CCL13 and CCL20 levels were significantly higher in nongranulomatous uveitis (BD and HLA-B27-associated uveitis) than in granulomatous uveitis (sarcoidosis and VKH disease).
Conclusion
Immune responses mediated by CCL8, CCL13 and CCL20 appear to be more potent in nongranulomatous uveitis, particularly in HLA-B27-associated uveitis.
Introduction
Endogenous uveitis includes a wide range of diverse inflammatory eye conditions affecting not only the uvea but also the retina, optic nerve and vitreous and is a major cause of severe visual impairment. Inflammatory eye diseases often occur in conjunction with systemic inflammatory disorders, such as Behçet's disease (BD), sarcoidosis, human leukocyte antigen (HLA)-B27-associated uveitis and Vogt–Koyanagi–Harada (VKH) disease with differing clinical phenotypes. It is possible that different immunologic mechanisms are involved in each clinical subtype (El-Asrar et al. 2011a; El-Asrar et al. 2011b; Abu El-Asrar et al. 2016). The wide range in clinical manifestations and the varied response to therapy leave multiple challenges in uveitis management.
Although the aetiology of endogenous uveitis remains unresolved, the recruitment and activation of mononuclear cells is thought to be essential in the perpetuation of the inflammatory response (Butler & McMenamin 1996; Jiang et al. 1999; Abu El-Asrar et al. 2007; Kerr et al. 2008). Understanding the mechanisms of selective immune cell subtype migration to the eye in patients with uveitis offers a significant tailored therapeutic potential. Chemokines are proinflammatory cytokines which orchestrate migration and activation of different leukocyte populations during the immune response. On the basis of the number and arrangement of the conserved cysteins, chemokines can be divided into four groups: CXC, CC, C and CX3C. The CC family is generally chemotactic for monocytes, T lymphocytes, natural killer (NK) cells, dendritic cells, basophils and eosinophils (Proost et al. 1996; Bachelerie et al. 2013; White et al. 2013). These chemokines attract leukocytes by binding to the seven transmembrane-spanning G protein-coupled receptors CCR1 through CCR10 (Bachelerie et al. 2013; White et al. 2013). CC chemokines and their receptors have been shown to be involved in a variety of inflammatory diseases by recruiting leukocytes to the inflammatory site (Bachelerie et al. 2013; White et al. 2013). Therapies designed to block the activity or inhibit the production of these chemokines and their corresponding receptors and, hence, leukocyte subtypes are currently being developed (Bachelerie et al. 2013; White et al. 2013).
Analysis of CC chemokines in the aqueous humour (AH) from patients with specific clinical entities of endogenous uveitis may help in identifying their interaction in the ocular inflammatory microenvironment and is a first and essential step in dissecting the key chemokine cascades involved in the pathogenesis of each clinical subtype. To determine the signals responsible for mononuclear cell infiltration into the eye, we have screened the AH from patients with active uveitis associated with BD, sarcoidosis, HLA-B27-related intraocular inflammation and VKH disease for the CC chemokines monocyte chemotactic protein (MCP)-1/CCL2, MCP-3/CCL7, MCP-2/CCL8, eotaxin/CCL11, MCP-4/CCL13, macrophage inflammatory protein (MIP)-3α/CCL20, eotaxin-2/CCL24 and eotaxin-3/CCL26. In addition, we correlated the levels of CC chemokines with the levels of the proinflammatory cytokines interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ).
Patients and Methods
Patients with active uveitis, seen at the outpatient clinic of King Abdulaziz University Hospital, were included in the study. Patients who had undergone cataract extraction at King Abdulaziz University Hospital with no prior history of uveitis, served as control group. Patients were examined using slit-lamp biomicroscopy, indirect ophthalmoscopy, fluorescein angiography and indocyanine green angiography. Diagnoses were made using established clinical criteria, with supporting laboratory evidence as needed (Abu El-Asrar et al. 2013; Al Dhahri et al. 2015).
Aqueous humour (100–200 μl) was aspirated from each patient by means of limbic paracentesis with the use of a 27-gauge needle attached to a tuberculin syringe after the application of topical local anaesthetic oxybuprocaine hydrochloride 0.4% (Benoxinate; Chauvin Pharmaceuticals Ltd., Kingston, UK). The procedure was performed under a surgical microscope. The samples were snap frozen and maintained at −70°C until use. Aqueous humour samples from patients with uveitis were obtained before therapy. All procedures followed the tenets of the Declaration of Helsinki, and informed consent was obtained from all patients and the control subjects. The study was approved by the Research Center, College of Medicine, King Saud University.
Aqueous humour samples were obtained from 13 patients with BD, 8 with sarcoidosis, 12 with HLA-B27-associated uveitis and 12 with VKH disease. Nine patients, who had undergone cataract extraction, served as control group.
CC chemokine and cytokine assays
The chemokine expression profile in the AH samples was determined using a cocktail of antibody-coated non-magnetic beads concomitantly measuring the levels of CCL2, CCL7, CCL8, CCL11, CCL13, CCL20, CCL24, CCL26, IL-1β, TNF-α and IFN-γ (BIORAD, Hercules, CA, USA). The analysis was performed following the manufacturer's instructions, and results were generated using the Bio-Plex 200 system and software (BIORAD). The experimental detection range of the different analytes is indicated in Table 1.
| Analyte | Detection range (pg/ml) |
|---|---|
| MCP-1/CCL2 | 0.9–916 |
| MCP-3/CCL7 | 1.75–5548 |
| MCP-2/CCL8 | 0.27–1096 |
| Eotaxin/CCL11 | 0.92–1044 |
| MCP-4/CCL13 | 0.17–738 |
| MIP-3/CCL20 | 0.57–581 |
| Eotaxin-2/CCL24 | 13.63–15 675 |
| Eotaxin-3/CCL26 | 0.59–2145 |
| IL-1β | 0.73–748 |
| TNF-α | 0.87–2910 |
| IFN-γ | 2.36–25 946 |
Statistical analysis
Data management was preliminarily done using Excel 2013 (Microsoft, Redmond, WA, USA), and then all statistical analyses were performed using spss version 20.0 software (IBM, Armonk, NY, USA). Descriptive statistics of the continuous variables were presented as means ± standard deviation and fold increase, where fold increase was calculated by dividing chemokine levels of the uveitis patients by the cataract patient control levels. The Kruskal–Wallis test was used to compare different disease categories. Mann–Whitney test was then used to compare two independent groups. Pearson correlation coefficients were computed to investigate correlation between variables. A p value less than 0.05 indicated statistical significance.
Results
Levels of CC chemokines and cytokines in aqueous humour samples from all patients and controls
When considering the whole patient group, all chemokine and cytokine levels except MCP-1/CCL2 were significantly higher in the AH of uveitis patients than in controls (Table 2). Among the eight studied CC chemokines, MCP-2/CCL8, MCP-4/CCL13 and MIP-3α/CCL20 were the most strongly upregulated chemokines, being elevated 48-fold, 118-fold and 173-fold, respectively, compared with controls (Table 2).
| All patients (n = 45) (pg/ml) [Range] (Fold increase)a | Controls (n = 9) (pg/ml) [Range] | p value (Mann–Whitney test) | |
|---|---|---|---|
| MCP-1/CCL2 | 1977.9 ± 2733.2b [60.8–7325.0] (1.8) | 1104.8 ± 1365.6b [278.0–3997.5] | 0.880 |
| MCP-3/CCL7 | 163.6 ± 183.9 [14.0–913.2] (11.1) | 14.8 ± 2.3 [14.0–21.0] | <0.001c |
| MCP-2/CCL8 | 872.2 ± 2064.6 [0.0–8770.0] (47.6) | 18.3 ± 47.0 [0.0–143.6] | 0.001c |
| Eotaxin/CCL11 | 113.4 ± 68.4 [0.0–255.3] (7.6) | 14.9 ± 10.1 [7.4–31.3] | <0.001c |
| MCP-4/CCL13 | 189.0 ± 295.7 [1.6–1117.6] (117.6) | 1.6 ± 0.5 [1.4–2.8] | <0.001c |
| MIP-3α/CCL20 | 1025.6 ± 1666.9 [0.0–4650.0] (172.5) | 6.0 ± 3.2 [4.5–13.7] | <0.001c |
| Eotaxin-2/CCL24 | 665.5 ± 626.2 [0.0–3618.4] (4.1) | 161.0 ± 89.0 [109.0–331.9] | <0.001c |
| Eotaxin-3/CCL26 | 33.6 ± 42.5 [4.7–226.2] (6.8) | 4.9 ± 0.7 [4.7–6.7] | <0.001c |
| IL-1β | 35.5 ± 42.9 [5.8–186.0] (6.1) | 5.8 ± 0.0 [5.8–5.8] | 0.001c |
| TNF-α | 177.9 ± 167.1 [16.9–767.6] (16.8) | 10.6 ± 7.9 [6.9–29.3] | <0.001c |
| IFN-γ | 160.9 ± 166.1 [18.8–812.0] (8.6) | 18.8 ± 0.0 [18.8–18.8] | <0.001c |
- a Fold increase compared with controls.
- b Mean ± SD.
- c Statistically significant at 5% level of significance.
In the four disease groups, the levels of chemokines and cytokines were significantly higher than in controls except for MCP-1/CCL2 and for MCP-2/CCL8 and eotaxin-2/CCL24 in sarcoidosis. MCP-1/CCL2 levels were significantly higher in controls than in patients with VKH disease (Table 3).
| Controls (n = 9) (pg/ml) | Behçet's disease (n = 13) (pg/ml) (p value) | Sarcoidosis (n = 8) (pg/ml) (p value) | HLA-B27-associated uveitis (n = 12) (pg/ml) (p value) | VKH disease (n = 12) (pg/ml) (p value) | |
|---|---|---|---|---|---|
| MCP-1/CCL2 | 1104.8 ± 1365.6a | 2205.3 ± 2777.6a (0.442) | 415.4 ± 275.3a (0.178) | 3885.3 ± 3164.2a (0.05) | 865.8 ± 2061.8a (0.023)b |
| MCP-3/CCL7 | 14.8 ± 2.3 | 160.3 ± 128.0 (<0.001)b | 100.9 ± 83.0 (0.002)b | 318.0 ± 264.2 (<0.001)b | 54.7 ± 37.5 (0.001)b |
| MCP-2/CCL8 | 18.3 ± 47.0 | 896.4 ± 1957.1 (0.003)b | 151.3 ± 359.8 (0.922) | 2028.8 ± 3217.7 (<0.001)b | 169.9 ± 245.2 (0.001)b |
| Eotaxin/CCL11 | 14.9 ± 10.1 | 109.4 ± 57.1 (<0.001)b | 102.4 ± 73.6 (0.001)b | 149.9 ± 80.0 (0.007)b | 88.5 ± 55.2 (<0.001)b |
| MCP-4/CCL13 | 1.6 ± 0.5 | 216.8 ± 299.7 (<0.001)b | 32.3 ± 33.8 (<0.001)b | 409.7 ± 383.6 (<0.001)b | 42.6 ± 92.3 (<0.001)b |
| MIP-3α/CCL20 | 6.0 ± 3.2 | 881.5 ± 1352.1 (<0.001)b | 107.4 ± 134.3 (0.01)b | 2766.5 ± 2032.0 (0.001)b | 53.0 ± 57.4 (0.001)b |
| Eotaxin-2/CCL24 | 161.0 ± 89.0 | 709.2 ± 910.0 (0.002)b | 462.7 ± 368.2 (0.079) | 610.5 ± 321.7 (<0.001)b | 808.2 ± 646.5 (0.003)b |
| Eotaxin-3/CCL26 | 4.9 ± 0.7 | 29.5 ± 22.3 (<0.001)b | 24.3 ± 18.8 (0.002)b | 61.7 ± 70.3 (<0.001)b | 16.1 ± 15.3 (0.003)b |
| IL-1β | 5.8 ± 0.0 | 35.9 ± 37.6 (0.001)b | 13.2 ± 9.2 (0.007)b | 78.0 ± 50.9 (<0.001)b | 7.6 ± 2.7 (0.032)b |
| TNF-α | 10.6 ± 7.9 | 195.5 ± 153.2 (<0.001)b | 155.6 ± 169.5 (<0.001)b | 281.7 ± 216.6 (<0.001)b | 87.1 ± 70.0 (<0.001)b |
| IFN-γ | 18.8 ± 0.0 | 203.9 ± 209.4 (<0.001)b | 104.3 ± 84.5 (0.002)b | 258.6 ± 172.4 (<0.001)b | 54.2 ± 30.5 (<0.001)b |
- VKH, Vogt–Koyanagi–Harada.
- a Mean ± SD.
- b Statistically significant at 5% level of significance.
Levels of CC chemokines and cytokines in aqueous humour samples from patients with uveitis associated with BD, sarcoidosis, HLA-B27-related intraocular inflammation and VKH disease
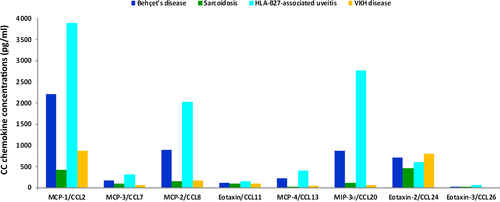
Subsequently, the distribution of chemokine and cytokine levels among the four disease groups was evaluated (Kruskal–Wallis test) and the results are shown in Table 4 and Fig. 1. Among the chemokines and cytokines analysed, MCP-1/CCL2, MCP-3/CCL7, MCP-2/CCL8, MCP-4/CCL13, MIP-3α/CCL20, eotaxin-3/CCL26, IL-1β, TNF-α and IFN-γ levels differed significantly between patients with BD, sarcoidosis, HLA-B27-associated uveitis and VKH disease (p = 0.001; p = 0.001; p = 0.005; p = 0.002; p < 0.001; p = 0.004; p < 0.001; p = 0.029; p = 0.001, respectively). Pairwise comparisons (Mann–Whitney test) (Table 5) indicated that MCP-1/CCL2, MCP-3/CCL7, MIP-3α/CCL20, eotaxin-3/CCL26, IL-1β and IFN-γ levels were significantly higher in patients with BD than in patients with VKH disease (p = 0.009; p = 0.008; p = 0.014; p = 0.019; p = 0.008; p = 0.04, respectively). MCP-1/CCL2, MCP-3/CCL7, MCP-2/CCL8, MCP-4/CCL13, MIP-3α/CCL20, eotaxin-3/CCL26, IL-1β and IFN-γ levels were significantly higher in patients with HLA-B27-associated uveitis than in patients with sarcoidosis (p = 0.004; p = 0.008; p = 0.004; p = 0.002; p = 0.003; p = 0.045; p < 0.001; p = 0.014, respectively). MCP-1/CCL2, MCP-3/CCL7, MCP-2/CCL8, MCP-4/CCL13, MIP-3α/CCL20, eotaxin-3/CCL26, IL-1β, TNF-α and INF-γ levels were significantly higher in patients with HLA-B27-associated uveitis than in patients with VKH disease (p = 0.002; p < 0.001; p = 0.007; p < 0.001; p < 0.001; p = 0.001; p < 0.001; p = 0.005; p < 0.001, respectively). In addition, MIP-3α/CCL20 and IL-1β levels in patients with HLA-B27-associated uveitis were significantly higher than in patients with BD (p = 0.016; p = 0.014, respectively). There were no statistically significant differences between patients with BD and sarcoidosis and between patients with sarcoidosis and VKH disease.
| Behçet's disease (n = 13) (pg/ml) [Fold increase]a | Sarcoidosis (n = 8) (pg/ml) [Fold increase]a | HLA-B27-associated uveitis (n = 12) (pg/ml) [Fold increase]a | VKH disease (n = 12) (pg/ml) [Fold increase]a | p value (Kruskal–Wallis test) | |
|---|---|---|---|---|---|
| MCP-1/CCL2 | 2205.3 ± 2777.6b [2.0] | 415.4 ± 275.3b [0.4] | 3885.3 ± 3164.2b [3.5] | 865.8 ± 2061.8b [0.8] | 0.001c |
| MCP-3/CCL7 | 160.3 ± 128.0 [10.9] | 100.9 ± 83.0 [6.8] | 318.0 ± 264.2 [21.5] | 54.7 ± 37.5 [3.7] | 0.001c |
| MCP-2/CCL8 | 896.4 ± 1957.1 [48.9] | 151.3 ± 359.8 [8.3] | 2028.8 ± 3217.7 [110.7] | 169.9 ± 245.2 [9.3] | 0.005c |
| Eotaxin/CCL11 | 109.4 ± 57.1 [7.3] | 102.4 ± 73.6 [6.9] | 149.9 ± 80.0 [10.1] | 88.5 ± 55.2 [5.9] | 0.150 |
| MCP-4/CCL13 | 216.8 ± 299.7 [134.9] | 32.3 ± 33.8 [20.1] | 409.7 ± 383.6 [255.0] | 42.6 ± 92.3 [26.5] | 0.002c |
| MIP-3α/CCL20 | 881.5 ± 1352.1 [148.2] | 107.4 ± 134.3 [18.1] | 2766.5 ± 2032.0 [465.2] | 53.0 ± 57.4 [8.9] | <0.001c |
| Eotaxin-2/CCL24 | 709.2 ± 910.0 [4.4] | 462.7 ± 368.2 [2.9] | 610.5 ± 321.7 [3.8] | 808.2 ± 646.5 [5.0] | 0.659 |
| Eotaxin-3/CCL26 | 29.5 ± 22.3 [6.0] | 24.3 ± 18.8 [4.9] | 61.7 ± 70.3 [12.5] | 16.1 ± 15.3 [3.3] | 0.004c |
| IL-1β | 35.9 ± 37.6 [6.2] | 13.2 ± 9.2 [2.3] | 78.0 ± 50.9 [13.5] | 7.6 ± 2.7 [1.3] | <0.001c |
| TNF-α | 195.5 ± 153.2 [18.5] | 155.6 ± 169.5 [14.7] | 281.7 ± 216.6 [26.6] | 87.1 ± 70.0 [8.2] | 0.029c |
| IFN-γ | 203.9 ± 209.4 [10.8] | 104.3 ± 84.5 [5.5] | 258.6 ± 172.4 [13.8] | 54.2 ± 30.5 [2.9] | 0.001c |
- VKH, Vogt–Koyanagi–Harada.
- a Fold increase compared with controls.
- b Mean ± SD.
- c Statistically significant at 5% level of significance.
| MCP-1/CCL2 |
BD > VKH HLA-B27 > Sarcoidosis HLA-B27 > VKH |
| MCP-3/CCL7 |
BD > VKH HLA-B27 > Sarcoidosis HLA-B27 > VKH |
| MCP-2/CCL8 |
BD > Sarcoidosis HLA-B27 > Sarcoidosis HLA-B27 > VKH |
| MCP-4/CCL13 |
HLA-B27 > Sarcoidosis HLA-B27 > VKH |
| MIP-3α/CCL20 |
BD > VKH HLA-B27 > BD HLA-B27 > Sarcoidosis HLA-B27 > VKH |
| Eotaxin-3/CCL26 |
BD > VKH HLA-B27 > Sarcoidosis HLA-B27 > VKH |
| IL-1β |
BD > VKH HLA-B27 > BD HLA-B27 > Sarcoidosis HLA-B27 > VKH |
| TNF-α | HLA-B27 > VKH |
| IFN-γ |
BD > VKH HLA-B27 > Sarcoidosis HLA-B27 > VKH |
- BD, Behçet's disease; HLA-B27, HLA-B27-associated uveitis; VKH = Vogt–Koyanagi–Harada.
Among the four disease groups, MCP-2/CCL8, MCP-4/CCL13 and MIP-3α/CCL20 were most strongly upregulated in patients with HLA-B27-associated uveitis and BD compared with controls. The levels of MCP-2/CCL8, MCP-4/CCL13 and MIP-3α/CCL20 were elevated 111-fold, 255-fold and 465-fold, respectively, in patients with HLA-B27-associated uveitis and 49-fold, 135-fold and 148-fold, respectively, in patients with BD (Table 4).
Comparisons of CC chemokine and cytokine levels in nongranulomatous uveitis versus granulomatous uveitis
When patients were divided into those with nongranulomatous uveitis (BD and HLA-B27-associated uveitis) (n = 25) and those with granulomatous uveitis (sarcoidosis and VKH disease) (n = 20), MCP-1/CCL2, MCP-3/CCL7, MCP-2/CCL8, MCP-4/CCL13, MIP-3α/CCL20, eotaxin-3/CCL26, IL-1β, TNF-α and IFN-γ levels in nongranulomatous uveitis were significantly higher than the levels in granulomatous uveitis. No statistically significant difference could be obtained for eotaxin/CCL11 and eotaxin-2/CCL24 (Table 6).
| Nongranulomatous uveitis (n = 25) (pg/ml) [Fold increase]a | Granulomatous uveitis (n = 20) (pg/ml) [Fold increase]a | p value (Mann–Whitney test) | |
|---|---|---|---|
| MCP-1/CCL2 | 3011.7 ± 3029.9b [2.7] | 685.7 ± 1593.8b [0.6] | 0.001c |
| MCP-3/CCL7 | 236.0 ± 216.0 [16.0] | 73.2 ± 62.3 [5.0] | 0.001c |
| MCP-2/CCL8 | 1440.0 ± 2644.6 [78.6] | 162.4 ± 287.4 [8.9] | 0.003c |
| Eotaxin/CCL11 | 128.8 ± 70.6 [8.6] | 94.0 ± 61.7 [6.3] | 0.077 |
| MCP-4/CCL13 | 309.4 ± 349.3 [192.6] | 38.5 ± 73.4 [23.9] | 0.002c |
| MIP-3α/CCL20 | 1786.3 ± 1931.4 [300.4] | 74.8 ± 96.5 [12.6] | 0.001c |
| Eotaxin-2/CCL24 | 661.8 ± 681.2 [4.1] | 670.0 ± 567.5 [4.2] | 0.698 |
| Eotaxin-3/CCL26 | 45.0 ± 52.8 [9.1] | 19.4 ± 16.8 [3.9] | 0.002c |
| IL-1β | 56.1 ± 48.5 [9.7] | 9.8 ± 6.6 [1.7] | <0.001c |
| TNF-α | 233.0 ± 184.1 [22.0] | 114.5 ± 120.8 [10.8] | 0.009c |
| IFN-γ | 230.2 ± 190.6 [12.2] | 74.3 ± 61.7 [3.9] | <0.001c |
- a Fold increase compared with controls.
- b Mean ± SD.
- c Statistically significant at 5% level of significance.
Among the two disease groups, MCP-2/CCL8, MCP-4/CCL13 and MIP-3α/CCL20 were most strongly upregulated in patients with nongranulomatous uveitis (79-fold, 193-fold and 300-fold, respectively) (Table 6).
Correlations
Significant correlations were found between AH levels of IL-1β and levels of MCP-1/CCL2 (r = 0.48; p < 0.001), MCP-3/CCL7 (r = 0.8; p < 0.001), MCP-2/CCL8 (r = 0.6; p < 0.001), eotaxin/CCL11 (r = 0.66; p < 0.001), MCP-4/CCL13 (r = 0.7; p < 0.001), MIP-3α/CCL20 (r = 0.92; p < 0.001) and eotaxin-3/CCL26 (r = 0.6; p < 0.001). The levels of TNF-α correlated significantly with the levels of MCP-1/CCL2 (r = 0.4; p = 0.004), MCP-3/CCL7 (r = 0.66; p < 0.001), MCP-2/CCL8 (r = 0.33; p = 0.016), eotaxin/CCL11 (r = 0.74; p < 0.001), MCP-4/CCL13 (r = 0.63; p < 0.001), MIP-3α/CCL20 (r = 0.7; p < 0.001) and eotaxin-3/CCL26 (r = 0.8; p < 0.001). In addition, there were significant correlations between levels of IFN-γ and levels of MCP-1/CCL2 (r = 0.4; p = 0.003), MCP-3/CCL7 (r = 0.77; p < 0.001), MCP-2/CCL8 (r = 0.82; p < 0.001), eotaxin/CCL11 (r = 0.7; p < 0.001), MCP-4/CCL13 (r = 0.66; p < 0.001), MIP-3α/CCL20 (r = 0.75; p < 0.001) and eotaxin-3/CCL26 (r = 0.63; p < 0.001). In contrast, the correlations between levels of the proinflammatory cytokines and levels of eotaxin-2/CCL24 were not significant.
Discussion
Among the CC chemokines analysed, CCL8, CCL13 and CCL20 were the most strongly upregulated chemokines. The MCPs (CCL2, CCL7, CCL8 and CCL13) represent a major subgroup of the CC chemokines that share biochemical and biological characteristics distinct from other CC chemokines. Based on the structural similarity of the mature proteins, showing more than 60% identical amino acids in the human species, MCPs represent a separate branch of the phylogenetic tree of CC chemokines whose genes are predominantly localized on human chromosome 17q11 (Nomiyama et al. 2013). In contrast, the weakly related CCL24 protein shows only 36% identity with CCL2 and its gene is localized on chromosome 7q11 (Nomiyama et al. 2013). Nevertheless, within the MCP subgroup, the chemokines exert different biological properties based upon a distinct receptor recognition pattern. Indeed, CCL2 which is the first identified monocyte attracting chemokine (Decock et al. 1990) binds to its unique receptor CCR2, which is functionally expressed on monocytes, but also on other mononuclear leukocyte types (Charo et al. 1994). In contrast, CCL7 and CCL8 discovered later on based on monocyte chemotactic activity (Van Damme et al. 1992), bind to several receptors, including CCR1, CCR2, CCR3 and CCR5 (Combadière et al. 1995; Gong et al. 1998), which explains the pluripotent chemotactic activities of these MCPs on most leukocyte types including lymphocytes, eosinophils and basophils (Alam et al. 1994; Baggiolini & Dahinden 1994; Noso et al. 1994; Taub et al. 1995). CCL11, which has been identified as a selective eosinophil chemoattractant in vivo (Jose et al. 1994), is only functioning via CCR3. CCL13 shows a broader spectrum of responsive leukocyte types by signalling through CCR1, CCR2, CCR3 and CCR5 (Garcia-Zepeda et al. 1996; Uguccioni et al. 1996).
Our subgroup analysis showed that CCL8 and CCL13 levels were found to be significantly higher in patients with HLA-B27-associated uveitis than in patients with sarcoidosis and VKH disease. Additionally, the levels of CCL8 and CCL13 were significantly higher in nongranulomatous uveitis associated with BD and HLA-B27-related inflammation than those in granulomatous uveitis associated with sarcoidosis and VKH disease. The present study is the first to demonstrate the abundant expression of CCL8 and CCL13 in the ocular microenvironment of patients with nongranulomatous uveitis. Moreover, the levels of CCL8 and CCL13 were elevated 111-fold and 255-fold, respectively, in HLA-B27-associated uveitis and 49-fold and 135-fold, respectively, in BD. These data suggest that CCL8 and CCL13 are significant contributors to the immunopathogenesis of nongranulomatous uveitis, particularly HLA-B27-associated uveitis. It has been shown that the proinflammatory cytokines IFN-γ and IL-1β are good inducers of CCL8 (Van Coillie et al. 1999; Struyf et al. 2009). CCL8 has been implicated in the pathogenesis of rheumatoid arthritis (Pierer et al. 2004; Haringman et al. 2006; Galligan et al. 2007) and multiple sclerosis (McManus et al. 1998). Previous reports demonstrated that high expression of CCL8 in tuberculous pleural effusion induces recruitment of CD4+ T lymphocytes by activating its receptor CCR5 (Liu et al. 2013). In addition, high expression of CCL8 in mycoplasma-infected mice mediates recruitment and accumulation of CD4+ T-helper cells expressing CCR5 to the lung (Sun et al. 2006). CCL13 is a more recently identified CC chemokine from a human cDNA library and directs the migration of monocytes, T lymphocytes and eosinophils by binding to four different leukocyte surface receptors, CCR1, CCR2, CCR3 and CCR5 (Bachelerie et al. 2013; White et al. 2013). CCL13 expression was reported to be upregulated by stimulation with the proinflammatory cytokines IFN-γ and TNF-α in several types of cells (Stellato et al. 1997; Chakravorty et al. 2001; Iwamoto et al. 2007). CCL13 expression is upregulated at sites of inflammation in several inflammatory disorders, such as rheumatoid arthritis (Iwamoto et al. 2006, 2007), acute renal inflammation (Chakravorty et al. 2001) atherosclerosis (Breland et al. 2010), asthma (Lamkhioued et al. 2000; Kalayci et al. 2004) and atopic dermatitis (Taha et al. 2000).
CCL20 demonstrates chemotactic activity towards immature dendritic cells, B lymphocytes and activated and memory CD4+ and CD8+ T lymphocytes. CCR6 is presently the only known receptor for CCL20 (Schutyser et al. 2003). The proinflammatory cytokines IFN-γ, TNF-α, IL-1β, IL-6 and IL-17 stimulate the production of CCL20 by several cell lines (Matsui et al. 2001; Scapini et al. 2002; Inoue et al. 2006; Hirota et al. 2007; Kawashiri et al. 2009; Tanida et al. 2009). In the current study, our analysis identified significant positive correlations between AH levels of the proinflammatory cytokines IL-1β, TNF-α and IFN-γ and those of CCL8, CCL13 and CCL20. These findings are consistent with the above-mentioned previous in vitro studies that reported upregulation of CCL8 (Van Coillie et al. 1999; Struyf et al. 2009), CCL13 (Iwamoto et al. 2007; Chakravorty et al. 2001; Stellato et al. 1997) and CCL20 (Matsui et al. 2001; Scapini et al. 2002; Inoue et al. 2006; Hirota et al. 2007; Kawashiri et al. 2009; Tanida et al. 2009) expressions in several cell lines stimulated with proinflammatory cytokines.
The CCL20-CCR6 axis is implicated in several autoimmune diseases, such as rheumatoid arthritis (Matsui et al. 2001; Ruth et al. 2003; Hirota et al. 2007; Kawashiri et al. 2009; Tanida et al. 2009) and experimental autoimmune encephalomyelitis (Kohler et al. 2003; Liston et al. 2009). Upregulated production of CCL20 induces the recruitment of CCR6-expressing mononuclear cells to the inflamed joint in rheumatoid arthritis and its animal model (Hirota et al. 2007; Tanida et al. 2009). Administration of blocking anti-CCR6 monoclonal antibody inhibited inflammation in a mouse model of arthritis (Hirota et al. 2007). In experimental autoimmune encephalomyelitis, disease severity and accumulation of mononuclear cells were significantly reduced by administration of specific neutralizing anti-CCL20 and anti-CCR6 antibodies and in gene-targeted CCR6-deficient mice (Kohler et al. 2003; Liston et al. 2009).
Our subgroup analysis showed that CCL20 levels were found to be significantly higher in patients with HLA-B27-associated uveitis than in patients with BD, sarcoidosis and VKH disease. In addition, CCL20 levels were higher in patients with BD than in patients with VKH disease. In the present study, we confirmed our previous data (Abu El-Asrar et al. 2016) in a new set of AH samples with the use of another multiplex assay. Furthermore, the levels of CCL20 were elevated 465-fold and 148-fold in patients with HLA-B27-associated uveitis and BD, respectively, compared with controls. Our findings suggest that CCL20 is a potential mediator of inflammatory cell recruitment in the ocular inflammatory microenvironment of patients with acute nongranulomatous uveitis, particularly HLA-B27-associated uveitis. The pleiotropic properties of CCL20 and the specificity with which it acts suggest that the CCL20-CCR6 axis may serve as an excellent drug target for acute nongranulomatous uveitis associated with HLA-B27-related inflammation and BD.
In conclusion, we have shown that among the CC chemokines analysed, CCL8, CCL13 and CCL20 were the most strongly upregulated chemokines, particularly in patients with acute nongranulomatous uveitis associated with HLA-B27-related inflammation and BD. These data suggest that CCL8, CCL13 and CCL20 are significant contributors to the immunopathogenesis of nongranulomatous uveitis and that CCL8, CCL13 and CCL20 may serve as new targets for selective therapy of specific clinical entities of endogenous uveitis.




