Predictors of visual outcome in patients operated for craniopharyngioma – a Danish national study
Abstract
Purpose
Craniopharyngioma often causes visual loss due to the close relation to the anterior visual pathways. This study investigates the incidence and predictors of visual outcomes in patients with craniopharyngioma.
Methods
Data from sixty-six patients who underwent surgery for craniopharyngioma from 2009 to 2013 in Denmark were reviewed. Primary outcomes were visual acuity (VA) and visual field (VF) defects from pre-and postoperative visits. Secondary outcomes were optic nerve atrophy (OA) and papilledema.
Results
Fifty-eight patients were included. The VA of the patients 1-year after surgery improved by −0.16 log(MAR) (95%CI: −0.30 to −0.02; p = 0.0266). Visual field (VF) defects worsened in 17 eyes (30%), remained stable in 21 eyes (37%) and improved in 19 eyes (33%). The presence of papilledema and the absence of OA were significantly correlated with an improvement in VA postoperatively (p = 0.011 and p = 0.011, respectively). Patients undergoing surgery within a week or less after their first ophthalmological examination had a significant improvement in VA (−0.36; 95%CI: −0.62 to −0.09; p = 0.0099). Patients undergoing surgery using a subfrontal approach also showed improvement in VA (p = 0.048). Tumour recurrence had a significantly worse VA outcome (p = 0.0074).
Conclusion
Patients show a slight improvement in VA 1-year after operation for craniopharyngioma. The presence of papilledema and early surgical intervention is associated with a significant improvement in VA. Early involvement of a dedicated ophthalmologist is recommended to secure an early detection of a visual decline and potential tumour recurrence.
Introduction
Visual decline is one of the most common symptoms in patients with craniopharyngioma (Prieto et al. 2015). Visual loss caused by craniopharyngioma is often rapid and progressive and can result in permanent blindness. Therefore, visual decline is a prime indicator for surgical treatment. However, visual decline is also a potential complication to the surgical treatment, making the prediction of visual outcome even more challenging. As a result, reports on predictive factors are conflicting (Repka et al. 1989; Chen et al. 2003; Kim et al. 2012).
Craniopharyngioma is a benign slowly growing tumour most often occurring in the sellar and suprasellar region of the central nervous system. It represents 2% of all intracranial tumours and approximately 5% of intracranial tumours in children (Drimtzias et al. 2014). Age distribution at onset is bimodal with a peak incidence in children around 10–14 years and in adults around 50 years of age (Louis et al. 2007; Nielsen et al. 2011).
Visual loss is common due to the close relation to the anterior visual pathway, but symptoms are often nonspecific and highly variable (Garnett et al. 2007). Subjective visual symptoms associated with craniopharyngioma are blurry vision and VF defects. Ophthalmological findings include decreased VA, bitemporal depression of the VF, papilledema and OA due to chiasmal compression (Kennedy & Smith 1975; Sorva et al. 1988; Repka et al. 1989; Abrams & Repka 1997; Fisher et al. 1998; Rohrer et al. 2002; Chen et al. 2003; Campbell et al. 2010; Pekmezci et al. 2010; Gucev et al. 2011; Lee & Hwang 2012). Postoperative visual disturbances continue to be a factor affecting the patient's quality of life (Prieto et al. 2015).
In recent years, there has been a shift in the treatment of craniopharyngioma patients, changing from a standardized approach to a more individually tailored treatment approach, taking the anatomy of the tumour, tumour extension, relation to critical structures and direction of tumour growth into account (Alshail & Salma 2016). Additionally, there has been a tendency towards a more centralized organizational structure in the surgical treatment of craniopharyngioma in Denmark. These changes in surgical approach make it relevant for a renewed investigation of visual outcomes and predictors, to minimize postoperative visual disturbances.
In a previous study by Nielsen and colleagues, the incidence and epidemiology of craniopharyngioma patients in a large Danish cohort is examined (Nielsen et al. 2011). Our study specifically investigates the incidence and possible predictors of visual outcome to provide valid benchmarks for the future treatment of craniopharyngioma patients.
Materials and Methods
Study design
This study was designed as a cohort study with retrospectively collected longitudinal data. The aim was to include all patients diagnosed with craniopharyngioma in Denmark during a 5-year period (2009–2013). Permits from the Department of Data Supervision, the Danish National Pathology Registry (DNPR), and the Danish Health and Medicines Authority were obtained prior to the start of the study. We followed the STROBE guidelines for reporting observational studies.
Patients
We searched for all patients undergoing surgery for craniopharyngioma in Denmark from 2009 to 2013. Patients were identified through the DNPR, which is a database containing detailed nationwide records of all pathology specimens analysed in Denmark since 1997. Patients with no ophthalmic records were excluded from the study. From 2011 onwards, all patients were operated at the Department of Neurosurgery at Rigshospitalet, which has served as a national referral centre for the surgical treatment of craniopharyngioma since 2011. Patients undergoing surgery in 2009 and 2010 were operated at regional hospitals, including Odense, Aarhus and Aalborg University Hospital besides Rigshospitalet.
Surgical approach
The surgical approach for intended complete excision of craniopharyngiomas used for this cohort was chosen according to the size and location of the tumour, the anticipated position of the chiasm, hypothalamus and the age of the patient. The transnasal transsphenoidal route was used for intrasellar craniopharyngioma. The small number of transnasal operations was performed as microscopic or microscopic endoscope assisted procedures. None of the operations was performed entirely endoscopic. The subfrontal approach was used either with subchiasmal or terminal translaminal access. In cases of a small tumour and sufficient space, a supraorbital approach was used as a less invasive variation of the subfrontal approach. The frontotemporal (pterional or orbitozygomatic) approach was an option for most suprasellar tumours, especially with asymmetric growth of the tumour. Finally, the transpetrous transtentorial presigmoid approach was used for tumours with a posterior position and a large third ventricle component.
Data collection and validation of data
We compared the neurosurgical records of patients operated for craniopharyngioma from 2009 to 2013 to the patients identified through the DNPR in order to ensure that there were no discrepancies between the two registers.
Patients who did not have any ophthalmic hospital record were contacted by telephone in order to clarify whether they have had any follow-up examination, and where those examinations were performed, for example in private clinics. Respective hospital departments or private clinics were contacted and relevant patient records obtained when possible.
Ophthalmological data from pre-and postoperative visits were collected from the ophthalmic records that could be retrieved. The preoperative visit was defined as the first visit to an ophthalmologist. The postoperative follow-up was defined as a visit to an ophthalmologist 1 year after the surgery (Stark et al. 1999). If no postoperative visit 1 year after the surgery was available, the visit closest to this was accepted as follow-up.
Predictive factors of interest were age, gender, comorbidities, symptoms, ophthalmological findings, time until surgery (defined as number of days between first visit and surgery date), surgical approach, tumour size (measured by widest diameter on MRI), and tumour recurrence.
Primary outcomes were VA and VF defects. Visual acuity (VA), measured in Snellen fractions, was converted to log(MAR); this conversion was performed by calculating the negative logarithm to the decimal notation VA (Holladay 2004). Preoperative visual status was divided into three groups according to decimal VA at first visit: normal VA (VA ≥ 0.8), mild-to-moderate visual decline (VA between 0.79 and 0.1), and severe visual decline (VA ≤ 0.099). To assess changes in VF, all types of VF test were allowed; available tests included Goldmann, Kampimetry, Octopus DG Standard (Haag-Streit AG, Koeniz-Berne, Switzerland), and Humphrey, program 30-2 (Carl Zeiss Meditec, Dublin, CA). Pre- and postoperative VF-tests were compared, and the change in VF defects was classified into three predefined categories: 1) better, 2) status quo or, 3) worse. An ophthalmologist with expertise in VF defects (KRN) evaluated all VF-tests included in the data set blinded. We also extracted mean deviation and mean defect from quantifiable VF-tests (Humphrey's and Octopus) pre- and/or postoperatively and compared the changes. Visual field (VF)-tests were considered unreliable and excluded in cases of fixation loss >33% and/or false positives >15%. Visual field (VF)-tests using Octopus were excluded in cases where the reliability factor (RF) was >15%. Secondary outcomes were OA and papilledema.
Extraction of ophthalmological data was performed by two individuals (MFJ and AST). Two neurosurgeons performed the data extraction for neurosurgical data (KF and LP).
Statistics
The mean change of log(MAR) from preoperatively to 1 year postoperatively was analysed in multivariable multilevel linear regression models where nested person and eye random effects accounted for repeated observations on the same person and eye. The overall change was assessed as well as the change within categories of various baseline characteristics, and whether the change was different between these categories. The analyses were adjusted for sex, age, hypertension, diabetes, time from first ophthalmological visit to surgery, and tumour size.
The difference in change in VF of various baseline characteristics was analysed in two-part logistic regression models where the first part investigates the probability for worse VF relative to the status quo and the second part investigates the probability for better VF relative to the status quo. For the patients that underwent VF testing using Humphrey's and Octopus, the change in mean deviation and mean defect was analysed in a multilevel linear regression model similar to the one used for log(MAR).
The changes in prevalence of the secondary outcomes from preoperatively to 1 year postoperatively were analysed in logistic regression models where generalized estimating equation (GEE) methods accounted for repeated observations on the same person and eye. Due to limited data, the analysis of the secondary outcomes and VF was performed unadjusted.
Results
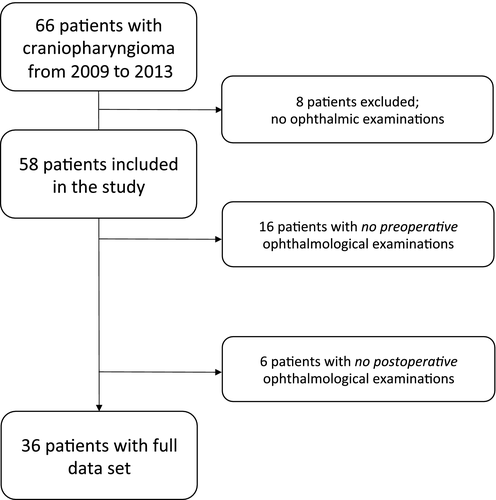
Sixty-six patients who underwent surgery for craniopharyngioma from 2009 to 2013 were identified. For eight patients, no ophthalmic examinations were found and they were excluded from the study. As a result, 58 patients were included in the study (Fig. 1). The excluded patients were represented in all age groups. See Table 1 for an overview of patient demographics and comorbidities for the included cohort of patients. Age distribution showed a bimodal peak from 0 to 19 years and again from 40 to 59 years.
| n (%) | |
|---|---|
| Overall | 58 (100) |
| Age | |
| 0–19 years | 14 (42) |
| 20–39 years | 9 (16) |
| 40–59 years | 21 (36) |
| 60 + years | 14 (24) |
| Sex | |
| Women | 28 (48) |
| Men | 30 (52) |
| Diabetes | |
| No | 56 (97) |
| Yes | 2 (3) |
| Hypertension | |
| No | 54 (93) |
| Yes | 4 (7) |
The duration of symptoms prior to first preoperative visit ranged from 2 days to 2 years, with a median duration of 3 months. Fifty-two percent of patients had a normal VA at first visit (mean VA 1.0), 29% had mild-to-moderate VA decline (mean VA 0.43), and 19% had severe decline in VA at first visit (mean VA 0.05). The most common VF defect observed were bitemporal VF depression of the temporal quadrants (Fig. 1).
Baseline characteristics related to change in VA
Patients were examined on average 288 days postoperatively (range: 22–518 days) with one outlier at 805 days. Overall, the VA of the patients (116 eyes) from preoperative to postoperative visit improved by −0.16 log(MAR) (95%CI: −0.30 to −0.02; p = 0.0266; Table 2). In the cohort of patients with preoperative VA data, five patients (14.3%) were visually impaired with a best-corrected visual acuity (BCVA) of <6/18 on both eyes (decimal VA <0.33), compared to five patients (11.6%) in the cohort with postoperative VA data. Three patients (21%) with severe preoperative VA decline had OA. Papilledema was present in 10% of patients with normal preoperative VA, and in 25% and 27% of patients with mild-to-moderate and severe preoperative visual decline, respectively. The presence of papilledema and the absence of OA had a significant correlation with an improvement in VA postoperatively (p = 0.011 and p = 0.011, respectively).
| No. of patients | No. of eyes (%) | Δlog(MAR) (95%CI)a | p-value | p-valueb | |
|---|---|---|---|---|---|
| Overall | 58 | 116 (100) | −0.16 (−0.30 to −0.02) | 0.0266* | |
| Preoperative visual acuity (mean decimal VA)c | 35 | 70 (100) | 0.0499* | ||
| Normal (1.0) | 39 (56) | 0.04 (−0.04 to 0.11) | 0.395 | ||
| Mild-to-moderate (0.43) | 17 (24) | −0.12 (−0.24 to 0.00) | 0.058 | ||
| Severe (0.05) | 14 (20) | −0.17 (−0.36 to 0.03) | 0.093 | ||
| Secondary outcomesd OA | 34 | 68 (100) | 0.291 | ||
| No | 54 (79) | −0.23 (−0.41 to −0.06) | 0.011* | ||
| Yes | 14 (21) | −0.03 (−0.37 to 0.30) | 0.839 | ||
| Papilledema | 0.059 | ||||
| No | 62 (91) | −0.13 (−0.28 to 0.03) | 0.110 | ||
| Yes | 6 (9) | −0.56 (−0.99 to −0.14) | 0.011* | ||
| VF measured with Humphrey or Octopus | 58 | 116 (100) | 0.888 | ||
| No | 48 (41) | −0.18 (−0.45 to 0.09) | 0.198 | ||
| Yes | 68 (59) | −0.15 (−0.32 to 0.01) | 0.065 |
- Bold letters indicates a significant P-value on a 0.05 significance level.
- Estimates are based on a multivariable multilevel linear regression model controlling for repeated observations on the same person and eye.
- a Mean change in log(MAR) from surgery to 1 year postoperative, adjusted for sex, age, hypertension, diabetes, time from first ophthalmologist visit to surgery and tumour size.
- b p-value of a test whether the log(MAR) changes are different between the categories of the corresponding variable.
- c missing for 46 eyes.
- d missing for 48 eyes.
- OA = Optic nerve atrophy.
Surgical outcomes related to change in VA
Time from first ophthalmological visit to surgery was on average 175 days, with three outliers above 600 days (mean: 91 days without outliers; Table 3). There was no statistically significant difference in log(MAR) change between the different time categories. However, patients (20 eyes) undergoing surgery within a week after first ophthalmological visit showed a significant improvement in VA: −0.36 (95%CI: −0.62 to −0.09; p = 0.0099). Mean preoperative decimal VA for this group was 0.51. None of the patients in this group had OA while 29% had papilledema. In the other groups, the percentage of patients with OA and papilledema was 25% and 0% for patients operated from 1 week to 1 month (VA 0.59), 17% and 8% for patients operated from 1 month to 1 year (VA 0.77), and finally 40% and 0% for patients operated more than 1 year after their first ophthalmological visit (VA 0.76).
| No. of patients (%) | No. of eyes | Δlog(MAR) (95%CI)a | p-value | p-valueb | |
|---|---|---|---|---|---|
| Time from first ophthalmological examination to surgeryc | 42 (100) | 84 | 0.335 | ||
| 1 week or less | 10 (24) | −0.36 (−0.62 to −0.09) | 0.0099* | ||
| 1 month to 1 week | 8 (19) | −0.18 (−0.55 to 0.15) | 0.265 | ||
| 1 year to 1 month | 17 (40) | −0.07 (−0.28 to 0.14) | 0.499 | ||
| More than 1 year | 7 (17) | −0.04 (−0.36 to 0.28) | 0.794 | ||
| Surgical approachd | 57 (100) | 114 | 0.367 | ||
| Frontotemporal | 24 (42) | −0.03 (−0.27 to 0.21) | 0.782 | ||
| Subfrontal | 15 (26) | −0.26 (−0.52 to −0.00) | 0.048* | ||
| Supraorbital | 2 (4) | 0.19 (−0.30 to 0.68) | 0.437 | ||
| Transsphenoidal | 15 (26) | −0.27 (−0.56 to 0.02) | 0.066 | ||
| Transtentorial | 1 (2) | −0.26 (−0.95 to 0.43) | 0.452 | ||
| Tumour size | 58 (100) | 116 | 0.256 | ||
| Less than 25 mm | 25 (43) | −0.04 (−0.27 to 0.18) | 0.718 | ||
| 25–35 mm | 15 (26) | −0.30 (−0.53 to −0.07) | 0.011* | ||
| More than 35 mm | 18 (31) | −0.10 (−0.37 to 0.16) | 0.442 | ||
| Recurrence | 58 (100) | 116 | 0.0074* | ||
| No | 47 (81%) | −0.24 (−0.39 to −0.10) | 0.0015* | ||
| Yes | 11 (19%) | 0.25 (−0.07 to 0.58) | 0.124 |
- Bold letters indicates a significant P-value on a 0.05 significance level.
- Estimates are based on a multivariable multilevel linear regression model controlling for repeated observations on the same person and eye.
- a Mean change in log(MAR) from surgery to 1 year postoperative, adjusted for sex, age, hypertension, diabetes, time from first ophthalmologist visit to surgery and tumour size.
- b P-value of a test whether the log(MAR) changes are different between the categories of the corresponding variable.
- c Indicated for each patient; missing for 16 patients.
- d Indicated for each patient; missing for one patient.
Patients (30 eyes) undergoing surgery using a subfrontal approach had a significant improvement in VA (p = 0.048). However, there was no statistically significant difference in log(MAR) change between the applied surgical approaches (p = 0.367).
Mean tumour size was 29 mm. Only tumours between 25 and 35 mm (30 eyes) had a statistically significant improvement in log(MAR) from preoperative to postoperative visit (Table 3; Fig. 2).
Tumour recurrence related to change in VA
Eleven patients (19%) were operated for a recurring tumour. The median age of patients with tumour recurrence was 23.5 years compared to 44.5 years for patients without tumour recurrence. Time to tumour recurrence ranged from 1 month to 15 years with a median duration of 4 years. Patients with tumour recurrence had a significantly worse VA outcome (p = 0.0074). Patients without tumour recurrence (47 patients) had a significant improvement in VA of −0.24 (95%CI: −0.39 to −0.10; p = 0.0015).
Visual fields
For 57 eyes, both pre- and postoperative visual field data (all VF methods included) were available and were analysed qualitatively in a blinded manner by an expert in visual fields defects. Visual field (VF) defects worsened in 17 eyes (30%), remained stable in 21 eyes (37%) and improved in 19 eyes (33%) when comparing the first visit to postoperative state (adjusted for both time and tumour size). No statistically significant correlation was found between changes in VF defects and any of the investigated variables, including reported symptoms, time until surgery (defined as number of days between first visit and surgery date), surgical approach, tumour size (measured by widest diameter on MRI), surgical approach, or tumour recurrence.
The distribution of various VF testing methods used pre-/postoperatively was as follows: 36%/41% with Humphrey's program 30-2 (program 24-2 was used for one patient), 25%/36% with Octopus DG standard, 3%/6% with kampimetry, 3%/3% with Goldmann, and 33%/14% of the patients had no VF test performed.
In total, autoperimetry (Humphrey's or Octopus) was available and reliable for quantitative analysis in 90 eyes; specifically for 39 eyes preoperatively and for 51 eyes postoperatively. As can be seen from Table 4, the patients who had Humphrey autoperimetry done did not have a significant change in mean deviation (but a trend toward improvement) while the patients who had Octopus autoperimetry done had a significant smaller mean defect postoperatively. Overall this corresponds well with our blinded examiners evaluation, that a significant number of patients had improvement of the visual fields postoperatively. There was no difference in change of VA from pre-to postoperatively for the group of patients examined with automated perimetry methods compared to the remaining patients (Table 2).
| Pre-op | Post-op | ||||||
|---|---|---|---|---|---|---|---|
| VF-test method | n pre/post | Mean MD (SD) | Median (IQR) | Mean MD (SD) | Median (IQR) | ΔMD (95%CI) | p-value |
| Humphrey (30-2) | 26/28 | −8.81 (8.37) | 6.09 (4.05; 8.99) | −12.48 (9.75) | 10.44 (3.63; 19.59) | −1.77 (−0.88 to 4.42) | p = 0.1834 |
| Octopus (DG Standard) | 13/23 | 9.89 (8.18) | 6.7 (2.9;14.7) | 8.41 (6.24) | 6.8 (2.4; 13.3) | −3.95 (−7.15 to −0.74) | p = 0.0249* |
- Estimates are based on a multivariable multilevel linear regression model controlling for repeated observations on the same person and eye.
Discussion
The objective of this study was to investigate the incidence and possible predictors of VA and VF decline in craniopharyngioma patients in a Danish cohort. We found that craniopharyngioma patients in general show a slight improvement in VA 1 year postoperatively and that the presence of papilledema and early surgical intervention is associated with a significant improvement in VA. Tumour recurrence is a strong predictor of decline in VA.
Our finding of a general improvement in VA is similar to Prieto et al. (2015), who found that 75% of patients experienced an improvement in VA. However, other groups have reported no overall improvement in VA from initial examination to follow-up (2.8 and 10 years) (Chen et al. 2003, Repka et al. 1989). Additionally, Stark et al. (1999) reported that VA deficits rarely improve. Our finding of an improved VA for patients included in this study may be due to advancements in the surgical procedure. However, the use of different definitions of VA improvement as an outcome measure from study to study may also have an impact. In our study, we have used the numerical value as a measure of VA, enabling us to detect more subtle changes in VA. In comparison, some previous studies have applied different definitions of VA changes, which may contribute to the different findings.
Preoperative visual status as a variable was defined as: normal (VA ≥ 0.8), mild-to-moderate visual decline (VA between 0.79 and 0.1), or severe visual decline (VA ≤ 0.099) prior to surgery. As expected, patients with normal preoperative VA (mean decimal VA 1.0) did not show a significant change in VA postoperatively (0.04; 95%CI: −0.04 to 0.11; p = 0.395), which corresponds to the findings of Prieto et al. (2015). There was a trend towards a greater improvement in postoperative VA for patients with a lower preoperative VA, and the difference in VA change between the different VA baseline categories was slightly significant (p = 0.0499). There was a higher proportion of patients with OA and papilledema in the groups with preoperative mild-to-moderate and severe decline in VA. The presence of papilledema and the absence of OA were associated with a significant improvement in postoperative VA (p = 0.011 and p = 0.011, respectively). This finding is expected as a result of papilledema being reversible and OA irreversible.
Patients undergoing surgery within a week after their first visit had a significant improvement in VA (−0.36; 95%CI: −0.62 to −0.09; p = 0.0099), and the improvement became smaller the longer duration before surgery. A higher proportion of patients in this group had papilledema (and a smaller proportion had OA) compared to the groups operated at a later stage, which may partially explain this finding. Nevertheless, the finding indicates that duration of compression on the optic chiasm may have an influence on VA in craniopharyngioma patients and is similar to the findings of Galal et al. (2010) that duration of compression in suprasellar meningioma patients had a significant impact on visual outcome (Galal et al. 2010). If the primary examination revealed no significant visual decline or other significant symptoms, there was no medical indication for acute surgery and a MR scan should optimally be performed prior to the surgical intervention. This may have resulted in a longer period between diagnosis and surgical intervention compared to cases with acute symptoms.
When investigating the correlation of surgical approach and primary outcomes, we found that patients undergoing surgery using a subfrontal approach had a significant improvement in VA (p = 0.048). However, there was no statistically significant difference in log(MAR) change between the different surgical approaches (p = 0.367). Assignments to the different surgical approaches are inherently based on tumour size and location and therefore represent a selection bias. Consequently, no conclusions can be made regarding a superior surgical access compared to others in regard to visual outcome. Alshail & Salma (2016) stated that the choice of selecting one surgical approach over another should be made on an individual basis, taking into account the microsurgical anatomy of the location, tumour extension, the tumour's relationship to critical structures, directions of tumour growth, the distribution of calcification, the number of cystic formations, and the relationship of these formations to the solid component. This individually tailored approach, which may be addressed as state-of-the-art, has been used in the surgical treatment of the included patients.
Our study also suggests that tumour recurrence is a predictor of poor VA. Patients that developed tumour recurrence had a significantly worse VA outcome (p = 0.0074). This is consistent with the findings of Kim et al. (2012).
Regarding changes in VF from pre-to postoperative examination, we found that VF defects remained unaltered in 37%, improved in 33%, and worsened in 30% of the patients. This finding is also comparable to previous study findings (Chen et al. 2003). We found no significant change in mean deviation from pre-to postoperative in the subgroup of patients that underwent VF examination using Humphrey's. We found a statistically significant change in mean defect in the subgroup of patients that underwent VF examination using Octopus (p = 0.0249). However, due to limited data, this analysis may be underpowered and no final conclusions can be made.
Eight patients never had contact with an ophthalmologist throughout the process of diagnosis and treatment. Thirty-three per cent of the patients diagnosed with craniopharyngioma from 2009 to 2013 did only undergo an ophthalmological examination either pre- or postoperatively. This is a considerate limitation to our study findings; however, the advanced statistical method applied makes it possible to include also incomplete data sets in the analysis. A considerate amount of patients had VF examinations performed with nonquantifiable VF testing methods; this is the background for our categorization of change in VF defects – including all available VF testing methods – evaluated blinded by an ophthalmologist with expertise in VF defects. When comparing a possible bias caused by the substantial amount of patients not undergoing automated perimetry examination, we found no change in VA outcomes when comparing the group who completed automated perimetry with the group who did not undergo automated perimetry examinations. This indicates that our study findings may be generalizable for the entire cohort of patients. An additional, possible source of bias in our study is the fact that some patients with acute onset of visual decline underwent immediate surgery and therefore did not receive a full ophthalmological examination beforehand. As a result, there is a risk of underestimating the potential improvement in visual outcome in these patients.
Currently, there is no consensus on the ophthalmological evaluation of craniopharyngioma patients. In the future, early involvement of a dedicated ophthalmologist is recommended to secure an early detection of a visual decline and potential tumour recurrence. Our study provides useful benchmarks for the future treatment of craniopharyngioma patients.




