Prognostic impact of chromosomal aberrations and GNAQ, GNA11 and BAP1 mutations in uveal melanoma
Abstract
Purpose
To evaluate clinico-pathological and molecular prognostic factors in a well-defined series of posterior uveal melanoma (UM) with focus on chromosomal aberrations and mutations in the GNAQ, GNA11 and BRCA1-associated protein 1 (BAP1) genes.
Methods
Formalin-fixed paraffin-embedded (FFPE) tissue samples were obtained from 50 consecutive eyes enucleated for UM between 1993 and 2005. The material was tested for loss of chromosome 3 and gain of chromosome 8q gene signatures by selective molecular gene markers using multiplex ligation-dependent probe amplification (MLPA), and for DNA mutations in the GNAQ, GNA11 and BAP1 genes.
Results
After a mean follow-up of 83 months (range, 8–205 months), 21 patients had died of metastatic UM and 16 patients of other causes. Tumour diameter, ciliary body involvement, mixed/epithelioid cell types, mitotic index, Ki-67 proliferation index, loss of chromosome 3 and gain of chromosome 8q showed statistically significant associations with metastatic disease. There were no significant differences in the prevalence of GNAQ and GNA11 mutations between patients with or without metastatic disease. Mutational analysis of the BAP1 gene was performed in 32 primary UM and in five UM liver metastases. Nine different BAP1 missense mutations were identified. BAP1 mutations were not more common in metastasizing than in nonmetastasizing UM.
Conclusion
The molecular gene markers showing loss of chromosome 3 and gain of 8q gene signatures were associated with an increased risk of metastatic disease. BRCA1-associated protein 1 (BAP1) gene mutation status had no prognostic significance. The frequency and spectrum of BAP1 mutations in UM may be more dependent on ethnicity and demographic variables than hitherto considered.
Introduction
Uveal melanoma (UM) arises from melanocytes in the uveal tract and accounts for approximately 5% of all melanomas (Chang et al. 1998). It is the most common primary malignant neoplasm of the adult eye, with a stable incidence over the past decades (Singh et al. 2011). Virgili et al. (2007) have reported an incidence of UM in Europe between less than two and more than eight per million population. They found that the incidence rate increased with latitude, supporting a protective role of ocular pigmentation. The annual age-adjusted incidence of UM in Norway is 8.5 for women and 8.9 for men (Krohn et al. 2008).
Over the years, there has been an evolution in prognostic assessment of UM, from clinical and histological features to the analysis of chromosomal abnormalities. Gains of 6p and 8q and losses of 1p and chromosome 3 are frequently observed in UM (Damato et al. 2011; Cassoux et al. 2014; Koopmans et al. 2014; Yavuzyigitoglu et al. 2016). Loss of 1p and chromosome 3 and gain of 8q lead to a higher risk of metastatic disease, whereas UM with gains of 6p are associated with a better prognosis (Ehlers & Harbour 2006; van den Bosch et al. 2010; Damato et al. 2010; de Lange et al. 2015). The GNAQ and GNA11 genes code for the alpha-subunit of the heterotrimeric GTP-binding protein that couples G-protein-coupled receptor signalling to the RAS-RAF-MEK-ERK (MAPK) pathway (Van Raamsdonk et al. 2009). Mutations in this pathway are considered to be an early event in the development of cancer (Onken et al. 2008). GNAQ mutations are present in about 50% of UM and may occur in all stages of the disease (Onken et al. 2008; Van Raamsdonk et al. 2009). GNA11 mutations are observed in 30–50% of UM at different stages of tumour progression (Van Raamsdonk et al. 2010). Codon Q209 is the most frequently mutated codon for both GNAQ and GNA11, and the two genes are found mutated in approximately 80–90% of UM.
The BAP1 gene is mapped to chromosome 3p21.1 and codes for a deubiquitinating enzyme located in the cell nucleus (Ventii et al. 2008). The enzyme regulates cell growth and seems to be important in the pathogenesis of cancer. Inactivating BAP1 mutations are found in up to 84% of metastasizing UM (Harbour et al. 2010; Field & Harbour 2014; Koopmans et al. 2014). Lack of nuclear BAP1 protein expression has lately been associated with metastatic disease (Kalirai et al. 2014).
The main objectives of this study were to evaluate molecular prognostic factors and to analyse the prevalence of GNAQ, GNA11 and BAP1 mutations in a population-based cohort of UM patients with long-term follow-up. We further wanted to test the MLPA method on aged FFPE archival material (van Dijk et al. 2005), as a signature screen for chromosomal gains and losses in UM.
Materials and Methods
Patients and specimens
A total of 50 eyes from 50 consecutive patients primarily enucleated for posterior UM during the time period from January 1993 to December 2005 were included in the study. In addition, liver metastases from five of these patients were analysed. Haukeland University Hospital is the regional hospital for the three Western counties of Norway (Rogaland, Hordaland, and Sogn og Fjordane), which have a total population of 1.1 million people (Statistics Norway 2015). All but one of the included patients were residents of this region. Nineteen benign dermal nevi and eight normal skin biopsies were used as a negative control/reference group. These randomly selected FFPE nevi and skin specimens were enrolled from 1993 through 2005, like the UM samples. DNA isolated from a FFPE UM tissue specimen with a known BAP1 mutation served as an internal experimental control. The study was registered and approved by the Regional Committee for Medical and Health Research Ethics, Western Norway and adhered to the tenets of the Declaration of Helsinki.
Clinico-pathological factors
Among the clinical data collected were patient age at time of enucleation, time from enucleation to occurrence of metastatic disease, time from enucleation to death due to malignant melanoma and vital status at the end of follow-up in February 2014. The cause of death was determined by analysing the hospital databases and other medical records. Relevant clinico-pathological and survival data were available for all the 50 patients.
The patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system (Sobin et al. 2009). Ciliary body involvement was determined by histopathologic examination of the anterior tumour margin. The UM were categorized as spindle cell, mixed cell or epithelioid cell type. The mitotic index was determined by counting the number of mitotic cells in 10 random and consecutive high-power fields (HPF; magnification ×400). Immunohistochemical staining for Ki-67 (clone MIB-1, dilution 1:250; Agilent Technologies/Dako, Glostrup, Denmark) was performed on an automated platform (Dako). The Ki-67 proliferation index was calculated as the average number of Ki-67-positive UM cells per HPF in randomly selected tumour areas. At least 100 Ki-67-positive cells or at least 10 consecutive HPF were analysed. Both the mitotic and the Ki-67 proliferation indexes were scored as high or low if they were above or below the median values, respectively.
DNA isolation
DNA was isolated from three 10 μm-thick FFPE tissue sections in which paraffin was removed using xylene and ethanol. The tissue was treated with ATL buffer and digested overnight with proteinase K (Qiagen GmbH, Hilden, Germany) at 56°C, as previously described (Berget et al. 2011). DNA was extracted using the DSP QS Mini Kit (Qiagen GmbH) on an automated Qiasymhony platform (Qiagen GmbH). The DNA concentration was measured on a spectrophotometer (NanoDrop, Minneapolis, MN, USA).
Multiplex ligation-dependent probe amplification (MLPA) assay
For the MLPA analysis, we used the SALSA kit P027-B1 for UM containing 26 single gene probes to test for abnormalities in chromosome 1, 3, 6 and 8, and also eight single gene reference probes (MRC Holland, Amsterdam, the Netherlands). In addition, eight single gene reference probes located to chromosomes 5p, 5q, 7p, 7q, 12q, 19p, 20p and 21q were analysed. The MLPA analysis was performed according to the kit protocol. Briefly, 75 ng DNA was used for the MLPA analysis and a nontemplate control was included in each run. All PCR reactions were performed using a 2720 Thermal Cycler (Thermo Fisher Scientific, Waltham, MA, USA). The resulting PCR/MLPA products were mixed with Hi-Di formamide (Thermo Fisher Scientific) and a 500-ROX size standard (Thermo Fisher Scientific), denatured at 95°C for 5 min and stored on ice until capillary electrophoresis was performed on a ABI-3130XL Genetic Analyzer (Thermo Fisher Scientific). The resulting data were preanalysed by the genemapper software (Thermo Fisher Scientific) and exported to Excel file format (Microsoft Corp., Redmond, WA, USA) for further analyses.
The relative gene probe area was calculated as the peak gene probe area in each sample divided by the mean peak sample area of all reference probes. The relative single gene probe area in all the UM specimens was then normalized to the relative single gene probe area in a pool of 12 benign nevi samples. The pooled mean value of all normalized gene probe data in a set of 10 nevi controls was 1.0, compatible with no gains or losses in gene signatures of chromosome 1, 3, 6 and 8 in the control nevi. Based on the distribution of MLPA results obtained in a cohort of 19 FFPE nevi, the gene content was scored as normal if the normalized peak area was between 0.70 and 1.30 in a single gene probe. A value below 0.70 was considered loss of a specific DNA gene locus, and a value above 1.30 was considered as gain. If more than half of the specific chromosomal gene probes showed a normalized relative peak area <0.70, a loss of chromosome gene signatures was registered, compatible with loss of that particular chromosome or chromosomal region. Similarly, if more than half of the specific chromosomal gene probes showed a normalized relative peak area above 1.30, a gain of that particular chromosome gene signature and corresponding chromosomal region was registered.
GNAQ, GNA11 and BAP1 mutation analysis
Mutation analysis of GNAQ exon 5 was performed using in-house designed primers provided by Dr. Hanne E. Puntervoll, CCBIO, Department of Clinical Medicine, University of Bergen, Norway. The primers used were (listed in a 5′-3′ direction): TAG TGC TAT ATT TAT GTT GAC (forward primer) and TTA CCT CAT TGT CTG AC (reverse primer). The annealing temperature (TA) for the primers was 50°C, yielding a 209-bp PCR product. Mutational analysis of GNAQ exon 4 and GNA11 exon 4 and 5 was performed using primers previously reported by Dono et al. (2014). We used a PCR reaction mixture consisting of 1× Multiplex Mastermix PCR kit (Qiagen GmbH), 0.5× Q-solution (Qiagen GmbH), 0.2 μm of each primer and ddH2O to a total volume of 23 μl. Two microlitres DNA was used in each reaction, which was carried out under the following PCR conditions: denaturation at 95°C for 15 min, 38 cycles of 95°C for 45 seconds, TA for 90 seconds and 72°C for 90 seconds, followed by final elongation at 72°C for 10 min and finally 4°C for ∞. The PCR products were controlled using a 3% agarose gel before they were enzymatically purified using Illustra ExoProStar 1-step (GE Healthcare, Buckinghamshire, UK) at 37°C for 15 min followed by an enzyme inactivation step at 80°C for 15 min. The purified PCR products were then sequenced using sequencing conditions as follows: 96°C for 1 min, 25 cycles of 96°C for 10 seconds, 50°C for 5 seconds and 60°C for 4 min, followed by 4°C for ∞. The PCR primers were used in combination with BigDye Terminator v1.1 Cycle Sequencing kit (Thermo Fisher Scientific) according to the standard protocol provided with the kit. Before analysed on a 3130XL Genetic Analyzer (Thermo Fisher Scientific), the products were purified using BigDye Xterminator kit (Thermo Fisher Scientific). The reading of the resulting DNA sequences was taken manually.
Analysis of BAP1 mutations was performed using the DNA PCR primers as described by Wiesner et al. (2011), and the same pre-PCR set up as described for the mutational analysis of GNAQ and GNA11 above. The PCR conditions were as follows: denaturation at 95°C for 10 min, 35 cycles of 95°C for 45 seconds, 57°C for 45 seconds and 45°C for 90 seconds, 72°C for 10 min and finally 4°C for ∞. Purification, sequencing and analysis of DNA were carried out essentially as described above. BAP1 exons 1–12, 15 and 16, thus comprising all the exons with BAP1 mutations reported by Harbour et al. (2010), were tested. A UM with known mutation in BAP1 exon 9 (c.724delG, p.Glu242Arg) served as experimental control.
Statistical analysis
Associations between variables were assessed by Pearson's chi-square test or Fisher's exact test. Continuous variables not following the normal distribution were compared between two or more groups using the Mann–Whitney U-test or Kruskal–Wallis test. Univariate survival analyses of time to metastasis and metastasis-related death were performed by the Kaplan–Meier method (log-rank test) with the date of enucleation as starting point. Patients who died of other causes than UM were treated as censored observations. Multivariate survival analysis was performed using the Cox proportional hazards method and likelihood ratio test. Model assumptions were examined by log-log plots, and no nonproportionality was found. The spss statistical package version 22.0 (SPSS Inc., Chicago, IL, USA) was used. All results were considered significant if p ≤ 0.05.
Results
Clinico-pathological findings
The study included 26 women and 24 men with a mean age at time of enucleation of 66 years (median, 71; range, 24–96 years). At the end of the clinical observation period, 13 patients (26%) were alive and 37 (74%) were dead. Twenty-one patients (57%) died from systemic metastasis of UM, while 16 patients (43%) died of causes other than UM.
The mean time to metastasis after enucleation was 36 months (median, 28 months; range, 5–121 months). The mean time to metastasis-related death after enucleation was 42 months (median, 34 months; range, 8–122 months). The mean follow-up period after enucleation was 83 months (median, 60 months; range, 8–205 months).
The median largest basal tumour diameter was 16.1 mm (range, 7.4–20.0 mm), and the median tumour height was 9.2 mm (range, 3.3–20.0 mm). The diameter was significantly larger in tumours from patients who developed metastases (metastasizing UM) than in tumours from those who did not (nonmetastasizing UM; p = 0.045). Sixteen tumours (32%) showed involvement of the ciliary body, which demonstrated an increased risk of metastatic disease (p = 0.009). Twenty-eighth UM (56%) were classified as pure spindle cell type and 21 (42%) as mixed or epithelioid cell types. Tumours with mixed or epithelioid morphology were significantly correlated with the group of UM leading to metastatic disease (p = 0.044). The median mitotic index was 1.5/10 HPF (range, <1–10/10 HPF), and the mitotic index was significantly higher in the metastasizing than in the nonmetastasizing UM (p = 0.005). The median Ki-67 proliferation index was 16.2/HPF (range, 2.0–126.0/HPF), and the proliferation index was significantly higher in metastasizing UM (p = 0.001). Tumour characteristics according to metastatic status are shown in Table 1.
| Variables | No metastasis (n = 29) | Metastasis (n = 21) | p | n* |
|---|---|---|---|---|
| Age at enucleation, years, mean, (median; range) | 65.7 (74; 24–96) | 66.4 (69; 41–85) | 0.23 | 50 |
| Female, n (%) | 13 (45) | 13 (62) | 0.23 | 50 |
| Right eye, n (%) | 12 (41) | 11 (52) | 0.44 | 50 |
| Largest tumour diameter, mm, median (range) | 15.0 (7.4–20.0) | 16.5 (12.4–20.0) | 0.045 | 50 |
| Tumour height, mm, median (range) | 9.0 (3.3–13.5) | 10.0 (4.8–20.0) | 0.39 | 50 |
| High T-stage (T3, T4), n (%) | 23 (79) | 20 (95) | 0.11 | 50 |
| Ciliary body involvement, n (%) | 5 (17) | 11 (52) | 0.009 | 50 |
| Mixed/epithelioid cell type, n (%) | 9 (31) | 12 (60) | 0.044 | 49 |
| Mitotic index, mean (range) | 2 (<1–7) | 5 (<1–10) | 0.005 | 49 |
| Ki-67 proliferation index, mean (range) | 13.9 (2.2–57.5) | 38.9 (2.0–126.0) | 0.001 | 49 |
| GNAQ mutation/wild-type, n/n | 15/14 | 5/16 | 0.06 | 50 |
| GNA11 mutation/wild-type, n/n | 8/19 | 10/11 | 0.20 | 48 |
| BAP1 mutation/wild-type, n/n | 5/13 | 4/10 | 0.96 | 32 |
| Chromosome 3 loss/no loss, n/n | 4/24 | 10/11 | 0.011 | 49 |
| Chromosome 8 gain/no gain, n/n | 9/19 | 16/5 | 0.002 | 49 |
- n* = number of analysed cases. Statistically significant p-values are shown in bold.
Chromosomal losses and gains in gene probe signature using MLPA
A total of 49 UM samples were successfully analysed by MLPA. The chromosomal gene probe signature was tested in 24 analyses of benign FFPE archival nevi by amplification of eight reference genes and 26 gene probes in chromosome 1, 3, 6 and 8. The mean normalized value of all reference genes and gene probes was 1.02 (SEM ±0.03) and 1.06 (SEM ±0.02), respectively, indicating that the DNA content in benign nevi was close to euploid by this method. Mean variation in the normalized single gene probe values within samples was 0.33 (SEM ±0.06) and 0.33 (SEM ±0.03) in nevi and in UM, respectively.
Gains and losses in chromosomal gene probe signature in chromosome 1p, 3, 6 and 8q were tested in 19 benign nevi, 28 nonmetastasizing UM and in 21 metastasizing UM (Table 2). A significantly higher frequency of loss of chromosome 3 gene signature was observed in UM compared to benign nevi (p = 0.038). Similarly, there was a significant gain of chromosome 8q gene signature in UM versus benign nevi (p = 0.008). The spread in mean chromosomal gene signature for chromosome 3 and 8q in all cases of benign nevi, nonmetastasizing UM and metastasizing UM are shown in Fig. 1.
| Variables | Benign dermal nevi (n = 19) | Uveal melanoma (n = 49) | pa | Nonmetastasizing uveal melanoma (n = 28) | Metastasizing uveal melanoma (n = 21) | pa |
|---|---|---|---|---|---|---|
| Chromosome 1p loss | 0 | 2 | 0.30 | 0 | 2 | 0.10 |
| Chromosome 3 loss | 1 | 14 | 0.038 | 4 | 10 | 0.011 |
| Chromosome 6 gain | 1 | 1 | 0.48 | 1 | 0 | 0.38 |
| Chromosome 8q gain | 3 | 25 | 0.008 | 9 | 16 | 0.002 |
- a p-values for loss and gains of chromosomal DNA content were determined in benign dermal nevi vs. uveal melanoma, and in nonmetastasizing uveal melanoma vs. metastasizing uveal melanoma, using Pearson's chi-square test for categorical data. Statistically significant p-values are shown in bold.
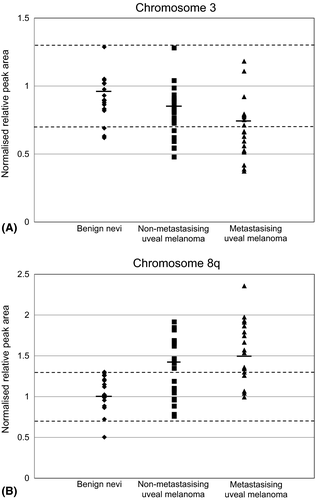
Of the 28 UM patients without metastasis, four (14%) had loss of chromosome 3 gene signature, whereas among the 21 patients with metastasis, 10 (48%) had loss of chromosome 3 gene signature (p = 0.011). Nine UM patients (32%) without metastasis and 16 patients (76%) with metastasis had gain of chromosome 8q gene signature (p = 0.002). For chromosome 1p and 6, there was no significant relation to metastatic disease (Table 2). Loss of chromosome 3 and gain of chromosome 8q gene signature were both significantly associated with mixed or epithelioid cell types and high Ki-67 proliferation index (Table 3).
| Variables | Tumour diametera | Location ciliary body | Mixed/epitheloid Morphology | Mitotic indexa | Ki-67 proliferation indexa |
|---|---|---|---|---|---|
| GNAQ mutation | 0.48 | 0.18 | 0.1 | 0.82 | 0.77 |
| GNA11 mutation | 1.0 | 0.88 | 0.09 | 0.83 | 0.63 |
| BAP1 mutation | 0.36 | 0.75 | 0.58 | 0.28 | 0.57 |
| Chromosome 3 loss | 0.07 | 0.34 | 0.02 | 0.79 | 0.02 |
| Chromosome 8 gain | 0.20 | 0.92 | 0.01 | 0.06 | 0.01 |
- a For association between molecular findings and selected clinico-pathological parameters, patients below and above median values were included in the analysis using Pearson's chi-square test. Median values for tumour diameter, mitotic index and proliferation index (Ki-67) were 16.1 mm, 1.5/10 high power fields (HPF) and 16.2/HPF, respectively. Statistically significant p-values are shown in bold.
GNAQ, GNA11 and BAP1 mutational analysis
Twenty patients (41%) with UM had a GNAQ Q209 mutation, whereas none showed mutation in GNAQ codon R183. In patients without metastatic disease, 15 patients (52%) had a GNAQ Q209 mutation as compared to five patients (24%) in the group of patients with metastasizing UM (p = 0.06). Seventeen patients (35%) had GNA11 Q209 mutations, and one patient (2%) had a GNA11 R183 mutation. Among the GNA11 mutations, eight (30%) were found in patients without and 10 (48%) were found in patients with metastatic disease (p = 0.20; Table 1). Altogether, 38 patients (76%) showed mutations in the GNAQ and GNA11 specific codons, and these mutations were mutually exclusive. No GNAQ or GNA11 mutations were found in the negative control group.
Sequencing for BAP1 mutations succeeded in 32 primary UM samples (including 14 metastasizing UM) and failed in the remaining 18 samples. Nine different missense mutations were identified in the BAP1 gene. The following mutations were observed in exon 9; R237C, P235S, A232T, D236N, in exon 10; T310I, A298T, G307D, E278K and in exon 12; V386I, respectively. The R237C mutation was found in two patients: one with and one without metastasizing UM. The other mutations were observed only once, in three patients with and in three patients without later metastatic disease. A double mutation (P235S and V386I) was found in one patient without metastatic disease, and another double mutation (A232T and D236N) was found in a patient with metastatic disease. One mutation, H274Y, in exon 10 has been reported as a SNP in the COSMIC database (cancer.sanger.ac.uk) and was not further considered a significant mutation or DNA sequence variant in this study. The BAP1 mutations were not more common in metastasizing than in nonmetastasizing UM (p = 0.96; Table 1). Liver metastases from five patients, one with loss of chromosome 3, were also analysed for BAP1 mutations. No mutations were found in the primary or metastatic tumours. No insertions, deletions or nonsense mutations were found in our material. Neither GNAQ, GNA11 nor BAP1 mutations showed any significant association with the clinico-pathological variables (Table 3).
Univariate survival analysis of uveal melanoma
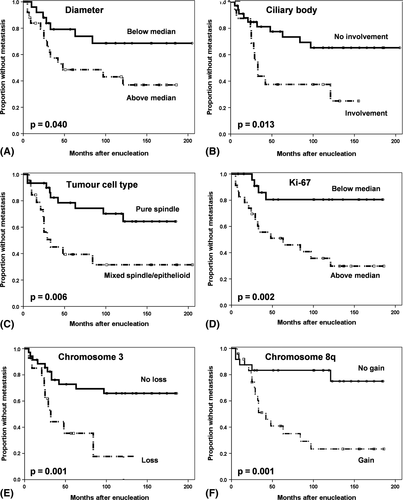
Clinico-pathological and molecular variables were tested for association with time to metastasis and metastasis-related death. Large tumour diameter (p = 0.040), involvement of the ciliary body (p = 0.013), mixed or epithelioid cell types (p = 0.006), high mitotic index (p = 0.009), high Ki-67 proliferation index (p = 0.002), and loss of chromosome 3 (p = 0.001) and gain of chromosome 8q (p = 0.001) gene signature were all significantly associated with shorter time to metastasis (Table 4, Fig. 2). Age, gender, T-stage, tumour height, GNAQ and GNA11 mutations, and aberrations of chromosome 1p and 6 had no significant impact on the occurrence of metastasis. Similar findings were obtained for time to metastasis-related death (data not shown).
| Variables | p |
|---|---|
| Age at enucleation | 0.74 |
| Gender | 0.07 |
| Low (T1, T2) vs. high (T3, T4) T-stage | 0.12 |
| Largest tumour diameter | 0.040 |
| Tumour height | 0.46 |
| Ciliary body involvement vs. not | 0.013 |
| Mixed/epithelioid vs. spindle cell type | 0.006 |
| Mitotic index | 0.009 |
| Ki-67 proliferation index | 0.002 |
| Chromosome 1p loss | Only two events |
| Chromosome 3 loss | 0.001 |
| Chromosome 6 gain | Only one event |
| Chromosome 8q gain | 0.001 |
| GNAQ mutation | 0.09 |
| GNA11 mutation | 0.11 |
- Statistically significant p-values are shown in bold.
Multivariate survival analysis of uveal melanoma
In the series of UM, a total of eight clinico-pathological and molecular variables with p-values <0.15 in the univariate survival analyses were included in the multivariate analysis. With time to metastasis (21 events) as endpoint, ciliary body involvement (HR, 4.9; 95% CI, 1.8–13.8; p = 0.002), high Ki-67 proliferation index (HR, 3.2; 95% CI, 1.1–9.9; p = 0.024) and gain of chromosome 8q (HR, 7.0; 95% CI, 2.0–24.5; p = 0.001) showed an independent prognostic effect. Factors that did not reach significance in the multivariate analysis were tumour diameter, mixed or epithelioid cell types, mitotic index, loss of chromosome 3 gene signature, and GNAQ and GNA11 mutations.
Discussion
Uveal melanoma is a clinically aggressive tumour, and once it has metastasized, the prognosis is very poor. A realistic evaluation of its metastatic potential is important for patient counselling and selection of optimal treatment, as well as for studies of adjuvant immuno- and chemotherapy to prevent or delay metastatic disease (Damato et al. 2011). Patients at high risk for metastasis should be monitored more closely and offered medical treatment at an early stage of the disease.
We searched for factors influencing survival in a population-based series of patients with posterior UM and long-term follow-up. As all patients were regularly screened for metastatic disease and because modern immunotherapy may prolong survival in patients with metastasizing UM (Triozzi & Singh 2015), we considered metastasis-free survival to be the most reliable clinical endpoint. Large tumour diameter, ciliary body involvement, mixed or epithelioid cell types, and high mitotic and Ki-67 proliferation indexes were all significant predictive factors for metastatic disease. These results are in accordance with previous studies reporting on the prognosis of UM (Kujala et al. 2003; Damato & Coupland 2009; Angi et al. 2011; Damato et al. 2011). Many of the clinico-pathological factors are closely interrelated, and in the multivariate analysis only ciliary body involvement and high Ki-67 proliferation index remained independent prognostic predictors for metastatic disease.
A number of genes and molecular markers predict the prognosis of UM patients (Ehlers & Harbour 2006). Yet, few studies have evaluated the long-term prognostic value of a genetic factor when clinical and histological information is included in multivariate models. In a study analysing frozen UM specimens by MLPA, Damato et al. (2009) found the method useful for estimating the risk of metastatic death, but that it requires multivariate analysis of chromosome 3 losses and 8q gains by taking into account other risk factors such as tumour diameter, cell type and mitotic index. Recently, Ewens et al. (2014) used a multivariate regression model to determine whether somatic mutations in various key genes, such as GNAQ, GNA11 and BAP1, were associated with the presence of UM metastases after taking into consideration well-known risk factors such as tumour size, tumour location and chromosome 3 copy number. Similar to our results, they found no statistically significant association between GNAQ and GNA11 mutations and risk of metastatic disease. On the contrary, GNAQ mutations in our study showed a tendency to be inversely associated with progression to metastatic disease, an observation also made by others (Dono et al. 2014). Consistent with previous reports, we found that the mutations in the GNAQ and GNA11 genes occurred in a mutually exclusive pattern (Van Raamsdonk et al. 2010; Dono et al. 2014; Ewens et al. 2014; Decatur et al. 2016).
In the present study, exons 1–12, 15 and 16 in the BAP1 gene, thus covering the exons with BAP1 mutations reported by Harbour et al. (2010), were tested. None of the mutations identified by Harbour and co-workers were observed in this study, but a total of nine other BAP1 missense mutations were found. None of these mutations have previously been reported in UM. The R237C mutation has been reported in cancers of the oesophagus and liver, whereas the G307D mutation has been reported in cancer of the large intestine (cancer.sanger.ac.uk; Forbes et al. 2015). All BAP1 mutations in this study, apart from the one in exon 12, were located in exon 9 and exon 10, a region of the BAP1 gene involved in the functional deubiquitination activity of the protein. To determine the significance of these mutations in relation to BAP1 function and pathogenesis of UM would require functional studies and was beyond the scope of this study. BAP1 mutations are common in UM, especially in association with chromosome 3 monosomy (Dono et al. 2014; Ewens et al. 2014; Koopmans et al. 2014). In the present study, seven of the BAP1-tested UM had loss of chromosome 3 and two of these had BAP1 mutations (data not shown). As heterogeneity in the primary tumour could affect detection of a BAP1 mutation (Maat et al. 2007), five liver metastasis, one with loss of chromosome 3, were analysed for BAP1 mutations. No mutations were found in these tumours. In our study, BAP1 mutations were not associated with metastatic disease. This is in contrast to the results reported by Harbour et al. (2010) in which 84% of metastasizing UM carried BAP1 mutations. The number of patients analysed for BAP1 mutations in our study is low. It is, however, of interest that none of the BAP1 mutations identified in this study have been previously reported in UM. This has led us to speculate on the significance of BAP1 mutations in the pathogenesis of UM and to what extent these mutations may reflect differences in the genetic background due to ethnic and demographic variations.
The MLPA assay was evaluated with respect to selective gene losses of chromosome 1p, 3p and 3q and potential gains of chromosome 6p, 6q and 8q in a set of UM and nevi. The cut-off value for loss of single gene signature was set to 0.7 as the mean probed gene values for chromosome 1p and 3 in nevi was 0.98 and 0.94, respectively. Similarly, the cut-off value for gain of single gene signature in chromosome 6 and 8q was set to 1.3 as the mean probed gene values were 1.08 and 1.09, respectively. Thus, for aged archival material, we suggest stricter criteria for evaluation of gains and losses in probed genes in the MLPA assay than previously reported (van Dijk et al. 2005), and as also recommended by the manufacturer of the UM MLPA kit. More strict criteria for evaluation of gains and losses of single gene signature in formalin fixed material have also been used by others (Lake et al. 2012). There was no major adverse performance in the MLPA assay for tissue samples from the period 1993 to 1999 versus samples from 2000 to 2005 (data not shown). The main obstacle in using the MLPA assay to assess gain or loss of chromosome region(s) in archival material was the variation in normalized single gene probe values within samples. Partly to address this issue, no single gene signature, but only overweight (dominant) pooled single gene signatures, was used to assess gains and losses of chromosomes or chromosomal regions.
Although fresh tissue would be preferable for the molecular analysis, the FFPE archival material used in the present study was useful for the GNAQ and GNA11 mutational analysis. For the MLPA analysis, however, the control genes showed a higher degree of heterogeneity within the control and tumour samples than previously reported in formalin fixed tissue samples (van Dijk et al. 2005). For the BAP1 sequencing, we used PCR primers as previously reported by Wiesner et al. (2011). Although these primers seemed to work well in FFPE cutaneous melanomas (Wiesner et al. 2011), some of the UM in our study did not show sufficient DNA sequence quality. The reason for this is unclear, but the age of the FFPE samples and morphological features like the degree of pigmentation and necrosis could have specific implications with respect to DNA quality and sequencing of this particular gene. The success rate of the BAP1 sequencing did not differ significantly between tumour samples obtained during the first and second half of the study period (data not shown). A more comprehensive gene screening, such as whole genome sequencing, would be needed to further determine the mutational status of our cohort. In the present study, only primarily enucleated eyes were included. The study is therefore biased towards larger tumours (unsuitable for episcleral brachytherapy), which may have some influence on the chromosomal and genetic analyses due to a potential higher intra-tumour heterogeneity (Maat et al. 2007). Other limitations of the study include the relatively small number of samples and a lack of post-mortem examinations to determine the exact cause of death.
In conclusion, several clinico-pathological and molecular parameters have been evaluated with respect to long-term risk of metastatic disease in a population-based cohort of UM patients from Western Norway. Loss of chromosome 3 and gain of 8q gene signatures were associated with increased risk of metastasis. We identified several previously unreported BAP1 gene mutations, but found no significant association between these mutations and metastatic disease. Our study indicates that the frequency and spectrum of BAP1 mutations in UM are more dependent on ethnicity than hitherto considered, a finding that may have important implications for the prognostic assessment of the patients.




