Towards better characterization and quantification of emulsification of silicone oil in vitro
Abstract
Purpose
Emulsification is related to complications arising from silicone oil (SO) tamponade. Currently, there is no widely accepted method for testing the propensity of SO to emulsify that are physiologically realistic and quantitative.
Methods
We compared different ways of inducing emulsification namely vortex mixing, sonication and homogenization. Silicone oil (SO) emulsification was quantitatively assessed using the Coulter counter and laser light scattering. The in vitro results are compared with the droplet size distribution profile of vitreous clinical washout. Conventional SO was compared with two novel SO blends with high-molecular-weight (HMW) additives (SOHMW2000 and SOHMW5000).
Results
Of the three methods for inducing emulsification, homogenization generated the most consistent emulsion samples with the smallest variance. The results from the Coulter counter measurement correlated strongly with the laser light scattering measurement within the range of 1 to 30 µm. The droplet size distribution profiles from human eyes were similar to that of emulsions generated in vitro by homogenization. The human size distribution profile was within the range of values obtained by the in vitro experiment. Compared to the conventional SO, the emulsion droplet counts for the new SO blends were significantly lower (SOHMW2000 and SOHMW5000 were 79% (±17%) and 49% (±18%) of the SO2000 and SO5000, respectively; p = 0.03 and p = 0.002).
Conclusion
Emulsion generated in vitro by homogenization has similar droplet size profile as human eyes filled with SO. Using this method to induce emulsion, SO blends with HMW additives demonstrated less propensity to emulsification with lower droplet counts compared to conventional SO with similar shear viscosity.
Introduction
Silicone oil (SO) is widely accepted as a long-term tamponade, despite many known complications such as glaucoma (Honavar et al. 1999), inflammation (Theelen et al. 2004), peri-oil proliferation (Lewis et al. 1988) and redetachment (Falkner et al. 2001) after oil removal. Some of these complications are thought to be directly related to emulsification (Baino 2011). In recent years, attempts have been made to design new SO that is more resistant to emulsification (Williams et al. 2010). The concept is that adding a small amount of high molecular weight (2.5 million cSt, 423 kD) to conventional SO (1000 cSt, 37 kD) will increase not only the shear viscosity, but also importantly the extensional viscosity, thereby reducing the tendency to emulsify (Williams et al. 2010). Clinical comparison of the resistance between SO is fraught with difficulties. There are many patient-dependent factors such as the degree of inflammation and the extent of blood–ocular barrier breakdown that may influence the availability of surfactants in the eye. Emulsification depends on shear stresses applied on the SO bubble generated by eye movement (Chan et al. 2011). Using a model eye chamber simulating saccadic movements, we showed that surgical confounding factors such as the extent of fill and the presence of scleral indents might all influence SO emulsification (Chan et al. 2014a). Individual patient and surgical confounding factors combine to make it impractical to carry out randomized clinical trials on every new proposed silicone oil. Potentially, in the near future, there might be many silicone oils being introduced. If adding high-molecular-weight (HMW) components was effective in reducing emulsification, then different conventional SO could be combined with different concentrations of HMW components to produce new oils with clinically useful properties. Clearly, all other silicone-based oils might also benefit from this modification and this would include fluorinated SO and the heavy SO (Chan et al. 2014b) which are mixtures of SO with semi-fluorinated alkanes or alkenes. Consequently, in our opinion, there is an important role for a standardized in vitro test for the emulsification to screen new oils before proceeding to clinical evaluations (Scott et al. 2005).
Previous experiments used various mechanical agitations to induce emulsification that might not be physiological (Savion et al. 1996; de Silva et al. 2005). The mechanical force causing emulsification in the eye is mainly shear stress related to eye movements. Shear stress acting on the liquid is the product of the viscosity of the liquid and the shear rate. However, the mechanical agitation of both sonication and vortex mixing might not provide constant shear rate to the silicone oil to cause emulsification. There is a need to look for another method of mechanical agitation with constant shear rate for the purpose of studying SO emulsification that better mimics the conditions in the human eye. In the past, we have used a SO-filled model eye chamber to study the effect of eye movements and we estimated the maximum shear rate from stereotypical saccades (Chan et al. 2011). If a standardized method of testing emulsification was to be developed; ideally, it should take into account the physiological conditions that cause emulsification to occur in the human eye (Chan et al. 2015a).
We have recently used a Coulter counter to quantify emulsions removed from patients (Chan et al. 2015b). The majority of the droplets were too small to be observed even with slit-lamp biomicroscopy. The Coulter counter could accurately measure particles down to 1 μm. The peak of the particle size distribution profile could, however, not be obtained. We therefore did not know how many droplets there were smaller than 1 μm. There is still a need to find a way to characterize the size distribution profile more completely.
This study aims to achieve two objectives: firstly, to find a consistent method of mimicking emulsification in human eyes and secondly, to achieve a more comprehensive quantification of SO droplets size and number.
Materials and Methods
Materials
Five types of SO were used in this study. The shear viscosities of the SO are listed in Table 1. SO1300 was used to characterize the reproducibility of oil-in-water emulsions generated using different agitation methods. SOHMW2000 and SOHMW5000 were blends of SO 1000 cSt with 5% and 10% of the high-molecular-weight (HMW) additive (423kD polydimethylsiloxane), respectively. The shear viscosities of SOHMW2000 and SOHMW5000 were around 2000 and 5000 cSt, respectively. Conventional SO 1000 and 5000 cSt were blended (at a ratio of 55% to 45%) to make a SO of 2000 cSt. We named this blend SO2000. SO2000 was compared with SOHMW2000. Similarly, conventional 5000 cSt oil (SO5000) was used as the control oil and compared with SOHMW5000. SO1300 (Arciolane 1300) was purchased from ARCAD, France. Apart from SO1300, all the other SO samples were kindly donated by Fluoron GmbH, Germany.
| Silicone oil (SO) | Shear viscosity at 25°C/ (cSt) |
|---|---|
| SO1300 | 1300 |
| SO2000 | 2141 |
| SOHMW2000 | 2189 |
| SO5000 | 4910 |
| SOHMW5000 | 5090 |
Homogenization
The homogenizer (T10 Basic Ultra Turrax®, IKA®-Werke GmbH & Co. KG, Staufen, Germany) along with the dispersing element was used to disperse the five types of SO (SO1300 SO2000, SOHMW2000, SO5000 and SOHMW5000) and generate oil-in-water emulsion under a controlled shear rate for one minute. Two per cent Pluronic® F68 (Life Technologies, Carlsbad, CA, USA) in phosphate-buffered saline (PBS) was used as the aqueous phase (Caramoy et al. 2010, 2011, 2015; Williams et al. 2010). The volume ratio of SO1300 to aqueous was 1:99. The small volume of SO to aqueous was intended to make sure that the SO phase was exhaustively dispersed to allow the fair comparison between various SO agents. The sample size of the emulsions for each SO agent was 6.
Vortex mixing
The SO1300 sample and the same volume of 2% Pluronic® F68 in PBS were added into a glass syringe. The oil/aqueous ratio (1:1) was accordance with a previously published study (Savion et al. 1996). The glass syringes were mounted on a vortex machine (Vortex-Genie 2, Scientific Industries, Inc., Bohemia, NY, USA). The syringes were subjected to the highest speed of vortex mixing for three hours. The sample size of the emulsions for each SO agent was eight.
Sonication
The SO1300 sample and the same volume of 2% Pluronic® F68 in phosphate buffered saline (PBS) were added into a glass syringe. The oil/aqueous ratio (1:1) was in accordance with previously published studies (Caramoy et al. 2011, 2015). The glass syringe was immersed in an ultrasound water bath (2510DTH, Bransonic, Danbury, CT, USA) and subjected to sonication for one minute. The sample size of the emulsions for SO1300 was 8.
Human washout samples
We have previously published the results of using the Coulter counter to measure the size distribution profile of washings from patients collected during removal of oil (Chan et al. 2015b). Briefly, after silicone oil was removed, a fluid air exchange was carried out and the fluid collected for analysis. There were eight patients studied, five had 5000 cSt oil and four had 1300 cSt oil. These data were used to compare with that of emulsification generated by in vitro methods. The ethical committee from the Royal Liverpool Hospital granted us permission to study the washings from the patients.
Particle measurement by Coulter counter
The emulsion samples generated by various methods of agitation from different SO were analysed using the Coulter counter (Multisizer® 4, Beckman Coulter, Brea, CA, USA). In this study, the measuring probe with a 50 μm aperture hole was used to provide a measurement range from 1 μm to 30 μm. The Coulter counter adopts the electrical zone sensing method of Coulter's principle (Edmundson 1966), which measures the size of non-conducting particles suspended in a fluid. The particle counter provided both number and size of particles suspended in the tested sample. The particle count of each sample presented herein was a mean value of 10 consecutive measurements.
Particle measurement by laser light scattering method
The four SO emulsion samples (including SO2000, SOHMW2000, SO5000 and SOHMW5000) generated by the homogenizer were also analysed using the laser light scattering method (Mastersizer 2000, Malvern, UK). The method adopts the principle of laser light scattering and measures the size of particles in the suspension using laser diffraction (Sperazza et al. 2004). It covers the measurement range for the particle analysis from 0.02 to 2000 μm in diameter and provides information on the particle size distribution within the suspension. The size distribution measurement for each sample presented herein was a mean value of 10 consecutive measurements.
Estimation of the size distribution of the in vitro SO emulsified droplets by extrapolating the measurements from Coulter counter to that of the laser light scattering method
The Coulter counter measurement provided the absolute number count of the SO droplets in the emulsion samples in the size range 1 and 30 μm in diameter. The measurement using the laser light scattering method gave relative numbers and provided an overall percentage size distribution profile of the SO droplets in the emulsion samples in the size range 0.02–2000 μm in diameter. The two measurement methods overlapped in the 1 and 30 μm size range. The agreement of the two methods within this range was analysed.
Statistical method
The Coulter counting method gave absolute numbers of droplets for every size interval, whilst the laser light scattering gave relative numbers. The outputs from both the measurements were a droplet size distribution frequency table. The frequency was then expressed as a percentage of the total for a given size interval. (The number of droplets within a given size range divided by total number of droplets.) We used the frequency tables to calculate variance and standard deviation in order to measure the spread of the data.
Using the Weibull nonlinear regression model as the mathematical model, the outputs from the Coulter counter and laser light scattering were correlated between 1 and 30 μm, in terms of relative numbers. After the best fitting model was found, we used the model to examine the correlations and then used it to extrapolate the values from Coulter counter (in %) for ranges 0 to 1 μm. All calculations were performed in software Minitab 17, (Minitab, Inc., State College, PA, USA) and the models were fitted with Gauss–Newton optimization algorithm.
Statistical significance between the differences of the emulsification of SO was assessed using the Mann–Whitney statistical test. The p-value <0.05 was considered to be statistically significant.
Results
Methods of generating SO emulsion as measured by the Coulter counter
Homogenization yielded consistent results between the six samples. In contrast, both vortex mixing and sonication generated oil-in-water emulsion without such consistent result in terms of droplet count. Sonication in particular yielded very variable results (Table 2).
| Samples | Vortex | Sonication | Homogenization |
|---|---|---|---|
| 1 | 2493 | 3126 | 10 191 |
| 2 | 5794 | 11 859 | 9990 |
| 3 | 3621 | 211 817 | 9776 |
| 4 | 5855 | 35 400 | 9709 |
| 5 | 7817 | 19 830 | 8013 |
| 6 | 8456 | 87 057 | 7701 |
| 7 | 1271 | 908 | / |
| 8 | 11 072 | 80 930 | / |
| Mean ± SD | 5797 ± 3276 | 56 366 ± 71 089 | 9230 ± 1081 |
- Standard Deviation = SD.
Size distribution of SO droplets generated by homogenization measured by Coulter counting and laser light scattering methods
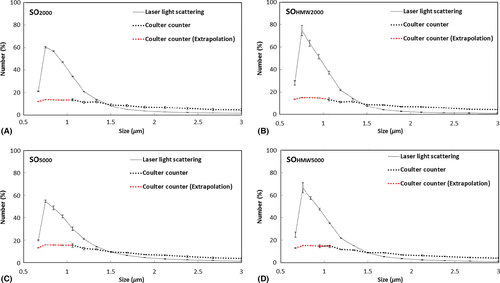
In Coulter counting measurement, the droplet size distribution profiles were similar for emulsions from SO2000 and SOHMW2000 (Fig. 1A) and from SOHMW5000 and SO5000 (Fig. 1B). Similarly, in laser light scattering measurement, the droplet size distribution profiles of emulsions were similar between SO2000 and SOHMW2000 (Fig. 2A) as well as between emulsions from SOHMW5000 and SO5000 (Fig. 2B). Besides the similarity of the size distribution profiles, using laser light scattering methods, it was found that the smallest size of the droplets detected was between 0.63 and 0.71 μm with a peak located in the range between 0.71 and 0.80 μm (Fig. 2).
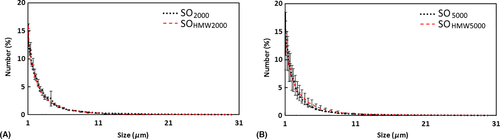
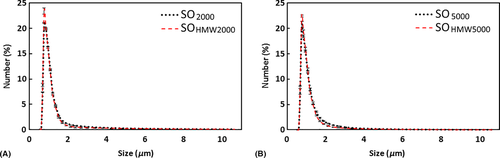
Statistical modelling
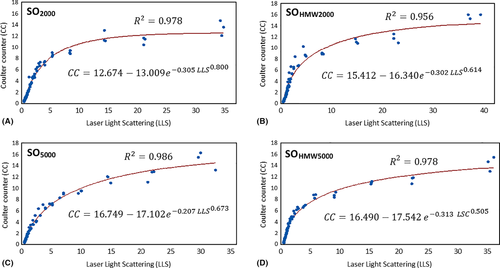
We plotted the percentages of one method against the other and explored their relationships with Weibull nonlinear regression model (Fig. 3). It was found that the two results were highly correlated with each other under this model (as indicated by the red lines in Fig. 3, R2 = 0.978, 0.956, 0.986 and 0.978, respectively).
In vivo emulsion vs in vitro emulsion
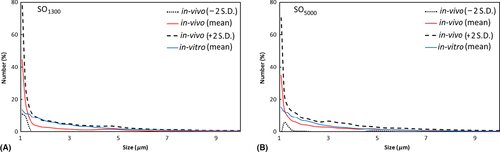
It was found that the droplet size distribution profile of in vitro emulsion generated by homogenization was positioned as an outer envelope (Blue line) around the profile of the in vivo emulsion (Fig. 4). This situation was present in both the SO groups (SO1300 and SO5000). In other words, the individual in vitro profiles coincided with the maximum values of the individual in vivo profiles for the range of 1.5 μm and greater. All in all, the profiles of the in vitro samples were within the 2 SD of the profiles of the in vivo samples.
Estimated total droplets number by extrapolation (combining the results from the Coulter counter and laser light scattering)
As the two methods strongly correlated to one another based on Weibull relationship, it was justifiable to extrapolate the data in order to determine the number of droplets smaller than 1 µm. The number of droplets between 0.5 and 1 µm was determined for the different oils using the Weibull model. It can be seen that the distribution profiles of the four different oils tested were similar (Fig. 5).
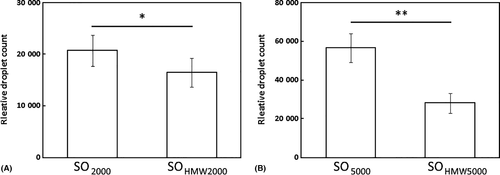
Comparing conventional silicone oil with those with HMW additives (Coulter counting)
Two pairs of SO (SO2000 and SOHMW2000; SO5000 and SOHMW5000) with similar shear viscosities were tested. The emulsion generated from SOHMW2000 (16 485 ± 2806) had a significantly lower droplet count than the emulsion from SO2000 (20 777 ± 3028; p = 0.03; Fig. 6A). The total droplet count from emulsion SOHMW2000 was 79% (±17%) of that of SO2000. Likewise, SOHMW5000 (28 054 ± 5168) had a significantly lower droplet count than SO5000 (58 536 ± 7473; p = 0.002; Fig. 6B). The total droplet count for SOHMW5000 was 49% (±18%) of that for SO5000.
Discussion
Until recently, the only way of preventing emulsification is to choose highly purified SO with higher viscosities (Nakamura et al. 1990). The advent of small gauge surgery in recent years has provided impetus for choosing less viscous SO, simply because they are easier to inject and remove through smaller cannulas. New SO with HMW additives claimed to be both more emulsification resistant and easier to inject and remove (Williams et al. 2010), and this assertion was corroborated in vitro (Caramoy et al. 2011). Nonetheless, others have observed early emulsification in vivo when these new SO were used (Maier et al. 2011). There is therefore justification to look again into whether the claims for emulsification resistance could be substantiated.
The current evidence of laboratory-based experiments might be flawed. There are several aspects to consider. Firstly, the method of generating emulsification has to be valid. Mixing SO with water could potentially generate either oil-in-water or water-in-oil emulsion. In the published images of a previous study using sonication, the droplets were in the bottom of the oil phase, suggesting that the droplets might be water-in-oil (Caramoy et al. 2010). This result might be irrelevant clinically because the droplets seen in patients are oil-in-water droplets. Secondly, the methods of generating emulsification should yield consistent results. Our results showed that the methods of vortex mixing and sonication were inconsistent (Table 2). The same SO produced widely varying results in terms of droplet numbers under identical conditions. Thirdly, the SO should be dispersed totally in the aqueous phase. Whether the method of generating emulsion is exhaustive will certainly affect the total number and the size of the SO droplets. Silicone oil (SO) with a higher resistance to emulsification in theory should produce droplets that are larger in size and fewer in number and vice versa. We believe that the results using vortex mixing and sonication were inconsistent mainly because too much oil was used (oil to water at the ratio of 1:1 in accordance with previous publications). At the end of the agitation, there were clearly two phases seen with SO on top and aqueous below (Caramoy et al. 2011). In other words, not all the oil was dispersed. From one experiment to another, the results using sonication and vortex might therefore reflect how much oil was successfully dispersed by the mechanical methods rather than measuring the resistance or readiness of the oil to emulsify. Homogenization on the other hand is an established scientific method for generating emulsions (Maa & Hsu 1996). The use of oil to water in the ratio of 1 to 99 using homogenization produced more consistent results. All the SO in each experiment was exhaustively emulsified leaving no bulk oil. We believe that this is the best way to make fair comparison the propensity of different SO to emulsify. The size distribution profiles of these in vitro emulsions generated by homogenization were similar to that of patients.
Emulsification is related to the presence of surfactants. In the eye, there are many surfactants present in the intraocular fluid which includes but not limited to different kinds of proteins, lipids and phospholipids (Savion et al. 1996). We opted to use Pluronic® F68 as the model surfactant in this study because it is widely used as a standard in emulsification science (Caramoy et al. 2010, 2011, 2015; Williams et al. 2010). Pluronic® F68 lowers the interfacial tension between SO and aqueous phase as effective as any surfactant that are present in the eye. It is important when comparing like with like that the ability to emulsify be standardized in concentration and effectiveness.
To date, the published results on resistance of the new SO with HMW additives relied on observation with the naked eye (Caramoy et al. 2011, 2015). We recently published quantification of washings from patients’ eyes that had oil in situ for months using the Coulter counter (Chan et al. 2015a,b). The majority of droplets were between 1 and 2 μm. With slit-lamp biomicroscopy, we would not see discrete droplets or cells less than 5 μm, observing instead the scattering of light by these droplets as ‘flare’. In this study, we also used the Coulter counter for droplet counting and sizing. Undiluted, the concentration of emulsification in the aqueous might be high enough to be detected by the laser light scattering method. However, the washings collected during SO removal procedures were diluted by infusion fluids. Currently, it is technically difficult to collect undiluted samples from the eye in sufficient quantity that would allow laser light scattering to yield meaningful quantitative results.
Our study chose two methods to quantify droplet number. The Coulter counter allows a precise particle counting of droplets. However, the Coulter counter has a lower limit of measurement of 1 μm in diameter. In our study, we could not determine the peak of the size distribution profile of the emulsion droplets (Fig. 1), showing that there must be droplets smaller than 1 μm in diameter. Laser light scattering provides a very broad range of sensitivity. The machine we used in the study (Mastersizer 2000) covered the measurement range from 0.02 to 2000 μm in diameter. This allowed us to obtain a more complete size distribution profile of the emulsified droplets (Fig. 2). Laser light scattering, however, only gives relative not absolute numbers of droplets. The two methods of measurements are based on different principles. The relationships have not been previously explored mathematically. We were able to exploit the overlap in the range of sizes (i.e. between 1 and 30 μm) to analyse the same sample. The graphs showed that the correlation between the two methods to be very strong based on the Weibull regression model (Fig. 3). We could therefore justify extrapolating beyond the measurement range of the Coulter counter based on the assumption that the Weibull relationship we found would still hold if the Coulter counter was measurable in the range between 0 and 1 μm. We were particularly interested in the peak frequency of size distributions. Without the full characterization of droplet distribution, we cannot confidently know if one emulsion compared to another had more or fewer droplets (Chan et al. 2015a,b). After extrapolating, we could detect that the most numerous droplets were between 0.5 and 1 µm (Fig. 3). There was a high degree of consistency between all SO tested as all plots have similar shapes. Using the statistical model to extrapolate below 1 µm revealed an important difference between the two methods. Figure 5 showed proportionately fewer droplets estimated by the calculation than the distribution indicated by laser light scattering method. This needs an explanation. We believe that this is due to one of the limitations of the Coulter Counter, which relies on the solutions to be suitably diluted. Otherwise, two or more droplets could pass through the aperture simultaneously and be counted as a single larger droplet. This known error is known as ‘coincidence’. It seems that emulsification in vitro and in patients has many more small droplets. Coincidence is the likely explanation of why the Coulter Counter underestimated the number of small droplets. Coulter Counter is accurate over a narrower range, whilst laser scatter is widely acknowledged for being fast, accurate over a wider range.
The high degree of correlation between the two methods suggested that using the Coulter counter alone without using the laser scatter method might be sufficient to reflect the overall propensity of an to emulsify. Significantly, although all four SO tested had very similar size distribution profiles, the absolute numbers of droplets were different. Because the total volume oil emulsified was the same, the difference could only be accounted for by the more resistant oils having a few larger droplets and thus fewer smaller droplets. This is in line with what was predicted if SO with HMW additives were more resistant to emulsification (Fig. 6).
There are limitations to our study. Firstly, with the use of homogenizer, we could only control the shear rate but not the shear stress. We know that the shear stress is a product of shear rate and shear viscosity. Therefore, with our experimental set-up, we could have been applying up to five times more force to 5000 than to 1000 cSt. We were therefore not able to compare SO with different shear viscosity using our experimental set-up as direct comparison between the two would not be valid. Secondly, the viscosity of SO is temperature dependent (Romano et al. 2016). We have not measured the viscosity difference in between in vitro and in vivo environments although published figures indicate that the difference is small. Our in vitro experiments were carried out at room temperature, whilst in vivo emulsification occurs at body temperature. However, we were careful to only compare the propensity of SO to emulsification in vitro. Therefore, the slight change of viscosity due to the temperature difference may not be so relevant. No direct comparison was made between in vitro and in vivo emulsification beyond the basic observation that the distribution profiles were similar.
Conclusion
In this study, we showed that homogenization provided a consistent way to generate oil-in-water emulsion in vitro. We stressed the importance of exhaustively emulsifying the oils, ideally using principally only shear forces as this might be more physiological and yield size distribution similar to that in patients. Although the Coulter counter could only count droplets within a narrow size range, the results correlated well with laser light scattering which has a widened range. The most numerous droplets seemed to be between 0.5 and 1 µm in diameter. We also showed that SO with HMW additive produced fewer emulsified droplets than conventional SO with similar shear viscosities.




