Lutein supplementation leads to decreased soluble complement membrane attack complex sC5b-9 plasma levels
Abstract
Purpose
The purpose of this study was to investigate the effect of lutein on systemic complement activation in elderly individuals.
Methods
Seventy patients with signs of early age-related macular degeneration (AMD) were included in this study. All subjects were randomly assigned to receive a 10 mg daily dose of lutein or a placebo for a time period of 1 year. EDTA blood was collected before and at various time-points during the study (0, 4, 8 and 12 months). The plasma level of the soluble complement membrane attack complex sC5b-9 was measured by ELISA.
Results
We found a significant 1.1 ng/ml monthly decrease in the plasma sC5b-9 concentration in the lutein group (p < 0.001), resulting in a decrease from 60.3 ng/ml at baseline to 46.3 ng/ml at 12 months. For the placebo group, we found a significant 0.6 ng/ml monthly increase in plasma sC5b-9 concentration (p = 0.001), resulting in an increase from 51.6 ng/ml at baseline to 58.4 ng/ml at 12 months.
Conclusions
Lutein supplementation inhibits the systemic activation of the complement system, which provides further functional evidence for the reported beneficial effects of this carotenoid in the management of AMD.
Introduction
Age-related macular degeneration (AMD) is the most frequent cause of blindness in the elderly population in the Western world (Jager et al. 2008). It is a complex, degenerative and progressive disease, which involves multiple genetic and environmental factors (Fine et al. 2000; Bok 2005), such as ageing and cigarette smoking (Smith et al. 2001; Thornton et al. 2005). The molecular mechanisms causing AMD remain unknown although inflammatory processes have been implicated as evidenced by the identification of susceptibility genes encoding complement factors (Tuo et al. 2004; Klein et al. 2005; Chen et al. 2010; Khandhadia et al. 2012) and the presence of complement proteins in drusen (Hageman et al. 2001). These findings were supported by studies showing an increase in the level of various complement activation products in the circulation of AMD patients (Donoso et al. 2006; Scholl et al. 2008; Reynolds et al. 2009; Hecker et al. 2010) and provided further evidence for a systemic inflammatory component to the disease pathogenesis (Parmeggiani et al. 2012). The exact relationship between AMD and the innate immune system, however, remains to be clarified.
Dietary factors have also been implicated in the pathogenesis of AMD, and intervention trials have shown that certain supplements can halt the progression of AMD (Age-Related Eye Disease Study Research 2001; Coleman & Chew 2007; Chew et al. 2013). These supplements contain a variety of antioxidants underlining the role of oxidative stress in the pathogenesis of AMD (Beatty et al. 2000). How these antioxidants exactly influence the outcome of AMD is not yet known and was the subject of the study described here. As a model, we studied the effect of the antioxidant lutein, which is an important component of the supplements considered to affect the outcome of AMD (Age-Related Eye Disease Study Research 2001) Individuals with signs of early AMD received either a lutein supplement or a placebo. In this study, we hypothesize that antioxidants may not only play a beneficial role in the eye itself, but may also affect systemic inflammation. As a marker of systemic inflammation, we chose to study circulating blood levels of proteins of the complement system. We recently reported that lutein supplementation over a time period of 12 months led to a significant decrease in the complement factor D level and also C5a and C3d (Tian et al. 2013). In present study, we focused on the blood level of sC5b-9, which is known as the membrane attack complex (MAC) of the complement system (Muller-Eberhard 1988). Our study shows that taking a daily 10 mg lutein supplement over a time period of 12 months, resulted in a significant decrease in the circulating sC5b-9 level.
Methods
Design
This study was a separate extension of a randomized, double-blind, placebo-controlled multicenter study undertaken to investigate and compare the effect of a 10 mg daily lutein supplement on visual performance in early AMD patients from two countries: United Kingdom and the Netherlands. The study, registered as a clinical trial NCT01042860 at clinicaltrials.gov, is in accordance with the Declaration of Helsinki and approved by the South Manchester Regional Ethical Committee and Medical Ethical Committee at the University Hospital of Maastricht. All the study participants provided written informed consent. From August 11, 2007 to June 16, 2009, early AMD subjects were randomly assigned to receive lutein or a placebo and were followed for 1 year. Primary outcome and results were recently published elsewhere. Blood samples had been collected from the participants for future analysis of markers of inflammation, although this was not the initial primary goal of the study.
Subjects
Subjects aged between 50 and 80 were included if they met the following inclusion criteria: AMD grade 0 to 4 in one eye according to the Rotterdam criteria, best-corrected visual acuity LogMAR of 0.5 or better and minimal cataract without any ophthalmic disorder other than early signs of AMD. Individuals had to have a BMI lower than 30. They were excluded if they showed any ophthalmic disorder other than early signs of AMD, especially, diabetic retinopathy, optic atrophy and pigmentary abnormalities considered by a supervising ophthalmologist to be less typical of AMD. Subjects were also excluded if they had a history of glaucoma, or if they had taken dietary supplements that contained lutein, zeaxanthin or meso-zeaxanthin at least 12 months before the study.
Eligible individuals took a daily 10 mg lutein supplement or a placebo over a 12-month time period. Both the lutein supplement and placebo were manufactured by Cognis GmbH, Rheinpromenade, 140789 Monheim, Germany, in accordance with Good Manufacturing Practice. According to the manufacturer's guidelines, the supplementation products were stored safely at temperatures below 25°Celsius.
The following randomization procedure was used. A randomization code list was made by Cognis, and supplement or placebo capsules were distributed according to this list. Each participant was assigned a specific treatment number, and the numbers were allocated in ascending order using the next available consecutive number. In case a discontinued patient was replaced, we used the next available treatment number. Participants and personnel were unaware to which treatment group an individual was allocated. The person performing the ELISA for sC5b-9 was unaware, of which participant was assigned to which group.
Whole blood was collected into commercially available EDTA-treated tubes at each time-point of the scheduled visit (0, 4, 8 and 12 months). Plasma samples were obtained by centrifugation and stored in aliquots at −80°Celsius.
ELISA measurement
The level of human complement factor sC5b-9 was detected using a multiplex sandwich ELISA according to the manufacturer's instructions (Human sC5b-9 ELISA Set; BD Biosciences, San Diego, CA, USA). Plasma samples were tested for sC5b-9 at a 1/10 dilution. The sensitivity range of the assay is 0.5 to 15 ng/ml.
Statistical analysis
Statistical analysis was performed using spss 21.0. Data are presented as mean ± 1 SD. Levels of sC5b-9 were normally distributed as shown by the Kolmogorov–Smirnov test. The Pearson chi-square test was used to test the differences in gender distribution over the experimental groups, and a Student's t-test was used to evaluate the differences in age and plasma concentrations between groups. To study the influence of supplementation on serum concentration, a linear mixed models (LMM) analysis was performed with subject ID and supplementation and time and their interaction as covariates. Significance was accepted at p < 0.05.
Results
Seventy subjects were randomly assigned to the two intervention groups. An extensive flow diagram of the included volunteers is shown in our paper describing the effects of lutein supplementation on macular pigment and visual function (Murray et al. 2013). Sample size showed a small decrease during the study but remained comparable between the groups at the different time-points of the study. In the lutein group, the sample size gradually decreased from 34 to 30 participants during the 12-month study period and for the placebo group from 36 to 32.
Table 1 shows the baseline characteristics of the 70 subjects, of which the plasma sC5b-9 concentration was determined. Age and gender were not statistically different between the two groups. At baseline, the concentration of sC5b-9 in lutein group was 60.3 ng/ml, which was significantly higher (p = 0.013) compared with the 51.6 ng/ml level in the placebo group.
| Lutein | Placebo | p-Value | |
|---|---|---|---|
| Gender (m/f) | 15/19 | 14/22 | 0.42 |
| Age (years) | 72 ± 9 | 69 ± 9 | 0.14 |
| C5b-9 (ng/ml) | 60.4 ± 16.3 | 51.6 ± 11.8 | 0.013 |
Following supplementation with a daily dose of 10 mg lutein, the plasma sC5b-9 concentration decreased over time in the lutein group (Table 2). The relative concentration in the lutein group showed a rapid decrease at the first 4 month time-point and then stabilized over time and increased slightly in the placebo group (Fig. 1).
| Baseline | 4 months | 8 months | 12 months | |
|---|---|---|---|---|
| sC5b-9 (ng/ml) | ||||
| Lutein | 60.4 ± 16.3 | 49.9 ± 14.6 | 48.9 ± 13.7 | 46.1 ± 15.8 |
| Placebo | 51.6 ± 11.8 | 49.8 ± 10.7 | 57.2 ± 15.3 | 58.4 ± 19.0 |
| p-Value | 0.013 | 0.97 | 0.028 | 0.008 |
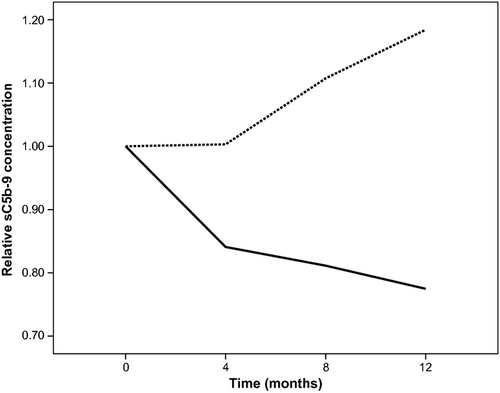
A LMM analysis revealed a significant effect for the interaction between supplementation and time (p < 0.001) on sC5b-9 plasma levels, indicating that the change over time differed significantly between the two groups. Stratifying for supplementation, the LLM analysis yielded a significant 1.1 ng/ml monthly decrease in plasma sC5b-9 concentration in the lutein group (p < 0.001), resulting in a decrease from 60.3 ng/ml at baseline to 46.3 ng/ml at 12 months. For the placebo group, we found a slight but statistically significant 0.6 ng/ml monthly increase in plasma sC5b-9 concentration (p = 0.001), resulting in an increase from 51.6 ng/ml at baseline to 58.4 ng/ml at 12 months. We incorporated gender as covariate into the LMM analysis, but did not find a significant effect of gender on the effect of supplementation on plasma concentrations of sC5b-9.
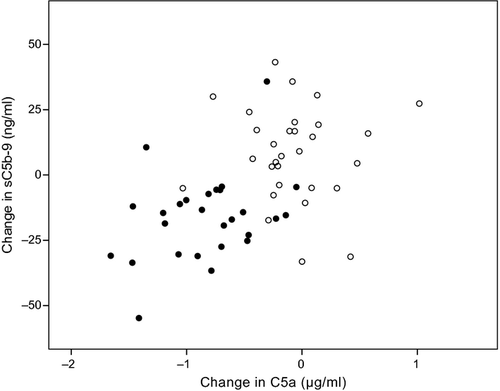
An earlier analysis of blood samples from this trial showed that lutein supplementation led to a significant decrease in the plasma C5a levels (Tian et al. 2013). Analysis of the change of C5a and sC5b-9 over the 1-year intervention period showed a significant correlation (Fig. 2; r = 0.48, p < 0.001). In this graph, most individuals who were on the lutein supplement were in the lower left quadrant, whereas those in the placebo group were in the upper right quadrant.
Discussion
This study is the first to show that an increased dietary intake of lutein reduces the plasma level of sC5b-9. This finding confirms and extends our previous work showing that daily supplementation with lutein affects systemic complement activation as shown by the decrease of circulating levels of the complement factors CFD, C3d and C5a (Tian et al. 2013).The study provides further support for a role of lutein in controlling systemic inflammatory responses that are implicated in the pathogenesis of AMD (Kijlstra et al. 2012). The main limitation of the present study is that baseline sC5b-9 concentration differed between the treatment and placebo groups. We believe this to be an unfortunate chance finding, despite the double-blind character of the trial. A larger sample size might have circumvented this problem.
The pathogenesis of AMD is complicated and is considered to be multifactorial (Beatty et al. 2000; Zarbin 2004; Ding et al. 2009). The complement system, which plays a central role in hosting defence against infection and in the modulation of antigen-specific immune and inflammatory responses, has been suggested to play an important role in the aetiology of AMD (Johnson et al. 2001; Klein et al. 2005; Donoso et al. 2006). Evidence from immunocytochemical and genetic studies suggested that local activation of the complement system by drusen in combination with a dysfunctional regulation might contribute to the process of AMD (Tuo et al. 2004). The fact that several groups have shown that blood of AMD patients contains raised levels of various complement factors, such as C5a, C3d and CFD, suggests that systemic complement activation is associated with AMD (Scholl et al. 2008; Reynolds et al. 2009; Anderson et al. 2010). The exact reason for an enhanced activation of the complement system has not yet been identified although it has been hypothesized that it may be due to the association with atherosclerosis (Mullins et al. 2000; Klein et al. 2004; Machalinska et al. 2012). Increasing evidence is becoming available to show that atherosclerotic plaques can activate the complement system (Speidl et al. 2011).
Recent experimental and observational data suggest that micronutrients with antioxidant capabilities may retard the development of AMD (Age-Related Eye Disease Study Research 2001; Coleman & Chew 2007). The mechanisms whereby lutein affects the development of AMD is not yet known, but our study suggests that the control of complement activation that is associated with lutein supplementation may offer an explanation. A number of clinical trials have been proposed to control activation of the complement system in AMD. These include trials using intravitreal monoclonal anti-C5 and anti-FD therapies (Gehrs et al. 2010; Zarbin & Rosenfeld 2010; Ambati et al. 2013). The anti-C5 trials have not yet appeared to be successful, whereas unpublished data of a phase 2 study using intravitreal lampalizumab (anti-factor D, Roche) slowed the progression of geographic atrophy in patients with advanced dry AMD.
As AMD is a process that takes many years to develop, we would like to argue that a long-term control of complement activation via the diet or with supplements might be beneficial to those at risk for AMD. A prospective trial may shed more light on this issue. Most studies on complement and AMD have focused on the alternative pathway. In the current study, we investigated the soluble factor sC5b-9, which is the final product of the three complement activation pathways (the classic pathway, the alternative pathway and the lectin pathway), also known as MAC. The relative contribution of the three pathways to sC5b-9 in plasma is not yet known in AMD patients nor is it clear from our data whether lutein has a differential effect on the three activation pathways mentioned. To date, we have circumstantial evidence that lutein may affect release of CFD from adipocytes, thereby controlling the alternative pathway. Whether lutein has an effect on the classical or lectin pathway necessitates further study.
The sC5b-9 levels found in our study are somewhat lower than that reported earlier (Hugo et al. 1987; Reynolds et al. 2009). We found a range of 56.0–128 ng/ml for the sC5b-9 plasma concentration in this study. Using a different kit, other studies found levels in a range of 100–600 ng/ml (Hugo et al. 1987) and a similar range of 164–716 ng/ml for AMD subjects (Reynolds et al. 2009). Whether the subjects of the latter study were taking lutein-containing supplements was not mentioned and may have affected the level of circulating complement activation products. Difference between our study and earlier data on sC5b-9 would be sampling, sample storage and differences between populations.
A further limitation of our study is the fact that all participants were informed about the background of the trial, which may have changed their dietary habit inadvertently. Due to the strictly randomized double-blind placebo-controlled nature of the trial, this should have affected both groups. Medical ethical committees insist that subjects are adequately informed about the nature of clinical trials and one should be aware of the fact that this may introduce confounding circumstances. Our findings therefore need to be confirmed by further epidemiological and interventional studies as well as by experimental animal studies.
The dose of lutein used in our study is similar to that incorporated in many commercial supplements. Studies on nutritional modulation of AMD were recently reviewed and suggested that 10 mg of lutein confers the highest benefit to the health of the retina (Weikel et al. 2012). A dose–response intervention study may provide further evidence concerning the regulatory control of lutein on complement activation. We are currently investigating plasma complement levels in blood samples obtained from other intervention trials performed elsewhere by other groups.
In summary, we have shown that the plasma concentration of sC5b-9 can be modulated by lutein supplementation. Due to the important role of the complement system as a mediator of inflammation and its role in the pathogenesis of AMD, lutein supplementation can be considered as a potential prevention and treatment for AMD.