Anatomical compatibility of a novel total artificial heart: An in-silico study
Abstract
Background
ShuttlePump is a novel total artificial heart (TAH) recently introduced to potentially overcome the limitations associated with the current state-of-the-art mechanical circulatory support devices intended for adults. In this study, we adapted the outflow cannulation of the previously established ShuttlePump TAH and evaluated the anatomical compatibility using the virtual implantation technique.
Methods
We retrospectively assessed the anatomical compatibility of the ShuttlePump using virtual implantation techniques within 3D-reconstructed anatomies of adult heart failure patients. Additionally, we examined the impact of outflow cannula modification on the hemocompatibility of the ShuttlePump through computational fluid dynamic simulations.
Results
A successful virtual implantation in 9/11 patients was achieved. However, in 2 patients, pump interaction with the thoracic cage was observed and considered unsuccessful virtual implantation. A strong correlation (r <−0.78) observed between the measured anatomical parameters and the ShuttlePump volume exceeding pericardium highlights the importance of these measurements apart from body surface area. The numerical simulation revealed that the angled outflow cannulation resulted in a maximum pressure drop of 1.8 mmHg higher than that of the straight outflow cannulation. With comparable hemolysis index, the shear stress thresholds of angled outflow differ marginally (<5%) from the established pump model. Similar washout behavior between the pump models indicate that the curvature did not introduce stagnation zone.
Conclusion
This study demonstrates the anatomic compatibility of the ShuttlePump in patients with biventricular failure, which was achieved by optimizing the outflow cannulation without compromising hemocompatibility. Nevertheless, clinical validation is critical to ensure the clinical applicability of these findings.
1 INTRODUCTION
Total artificial hearts (TAHs) and biventricular assist devices (BiVAD) are the current treatment strategies for severe biventricular failure or right ventricular dysfunction after left ventricular assist device (LVAD) implantation. Biventricular heart failure poses a challenge among LVAD patients; approximately 11.6% of overall LVAD recipients between 2016 and 2020 suffered from severe right ventricular dysfunction.1 However, challenges arise from the off-label employment of LVADs for right ventricular support and the coordination of more than one controller, which limits the efficacy of BiVADs.2 A 12-month mortality rate of 41.5% has been reported in BiVAD patients.3 On the other hand, TAHs are specifically designed to support patients with biventricular failure by replacing the failing ventricles and, therefore, may provide long-term, durable support for these patients.
Available TAH devices are of limited durability and/or hemocompatibility, leading to infections, ischemic stroke, and mediastinal and gastrointestinal bleeding.4 Further, the current pumping principles of available and new TAH concepts utilize either pneumatic, hydraulic, or electromechanically driven pulsatile flow displacement pumps or electromagnetically driven continuous flow rotary blood pumps (RBPs).5 Pulsatile flow pumps suffer majorly from durability and technical complexities with multiple sensors, while RBPs generate higher shear stresses, resulting in hemocompatibility related adverse events.5 Despite extensive research, only two TAH devices are clinically available for bridge-to-transplantation therapy,5 with a suitable TAH for destination therapy yet to be realized. Consequently, the need for long-term, durable options poses a persistent challenge in treating these patients.
To address the limitations of current pumping principles of TAHs and the unmet medical need in patients with biventricular failure, a novel valveless pulsatile TAH system—the ShuttlePump—has recently been introduced.6 The innovative pumping concept utilizes a single moving component to deliver pulsatile flow to systemic and pulmonary circulation (Figure 1). The ShuttlePump consists of two main components: a cylindrical housing with two in−/outflow cannulas and a hydrodynamically levitated piston that integrates a continuous rotation with a synchronized linear motion within the housing. The design of the piston, together with the rotational motion, ensures that the in−/outflow cannulas open and close, while the linear motion induces periodic changes in the chamber volume, displacing the blood and resulting in pulsatile flow. This pumping principle may be a promising way to overcome the disadvantages associated with the current mechanical circulatory support (MCS) devices. The technical feasibility of the ShuttlePump in terms of hydraulic performance and hemocompatibility was demonstrated previously.6 The computer-aided design (CAD) model of the ShuttlePump and its working principle are depicted in Figure 1.
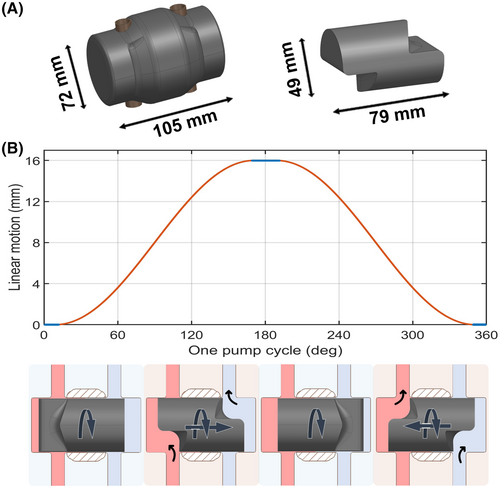
Beyond assessing hydraulic properties and hemocompatibility, early development of a novel TAH system requires the exploration of anatomical constraints to ensure optimal anatomical fit. The size and shape of the ShuttlePump must allow for implantation in the human body without compressing surrounding anatomical structures and ensure proper device-vessel alignment. Improper fit may result in intra-thoracic tissue compression, atrial and vessel (artery/venous) kinking, improper filling of pump chambers, and reduced hemodynamic performance. Therefore, the efficacy of the ShuttlePump depends on its successful anatomical compatibility. Virtual implantation is an emerging tool for assessing the anatomical compatibility of novel TAH devices and identifying the appropriate patient cohorts for the device. This method has gained prominence in recent years.7-11
This study aimed to optimize the anatomic fit of the ShuttlePump in the human anatomy using virtual implantation techniques without deteriorating hemocompatibility. The primary focus of the analysis was on device-to-vessel connectivity to determine the orientation and angulation of the pump's outflow cannula, followed by an assessment of anatomical compatibility and identification of predictors for pump fit.
2 MATERIALS AND METHODS
This study employed a dual-methodological approach, combining virtual implantation techniques with computational fluid dynamic (CFD) simulations to comprehensively explore the ShuttlePump's suitability for anatomical compatibility and its resulting hydraulic characteristics and hemocompatibility. In the first step, 3D reconstruction of pseudonymized computed tomography (CT) image data from adult heart failure patients who received a TAH/BiVAD was performed. In the next step, the pump's outflow cannula was modified with a manual iterative approach. Anatomical compatibility was assessed both qualitatively and quantitatively. Subsequently, CFD simulations were performed to assess the impact of outflow cannula modification on hemocompatibility.
2.1 In-silico anatomical compatibility
2.1.1 Patient selection
This exploratory study, conducted at a single center, was approved by the University's review committee (Ek. Nr. 1856/2021). Retrospectively collected pseudonymized preoperative CT image data of adult patients (>18 years) who suffered from congestive heart failure and received a TAH or a BiVAD were considered for this study. Patients with missing demographic data (age, sex, height, and weight) and poor CT imaging quality were excluded. Screening resulted in 11 adult patients’ CT image data, which were further utilized for virtual pump implantation.
2.1.2 3D reconstruction
The digital imaging and communication in medicine (DICOM) data from the CT images of the patients were imported into Materialize Mimics 23.0 (Materialize, Leuven, Belgium). The anatomical regions of interest (ROIs) to be segmented included the heart (atria, ventricles, aorta, pulmonary artery, pulmonary vein, vena cava, and pericardium), lungs, rib cage, and diaphragm. Segmentation of these regions was performed with a semi-automated thresholding tool by selecting the grayscale values of the pixels in the 2D CT images. The reconstructed 3D anatomical structures were further processed using a region-growing algorithm to separate and extract ROI from surrounding tissue. The segmented structures were further processed using manual tools to select the pixels of anatomical structures that were not separated by the preceding algorithms. Subsequently, the individual structures were imported into 3matic 15.0 (Materialize, Leuven, Belgium) as a stereolithography (.stl) file for additional post-processing to resolve inconsistencies in the 3D anatomical surface model.
2.1.3 Virtual implantation
The 3D CAD model of the ShuttlePump was imported into 3matic 15.0 together with the patient's segmented 3D anatomical surface model. The first step involved virtually removing the native ventricles of the heart. Next, the aorta was dissected above the sinotubular junction, and the pulmonary trunk was cut proximal to the pulmonary valve. The ShuttlePump was positioned in place of the ventricles using local translational and rotational movements, ensuring alignment of the inflow cannula with the atrioventricular (AV) valves. The optimal position, including in−/outflow alignment, was achieved through a manual iterative process. This process started with positioning the ShuttlePump at the location of the ventricles, ensuring alignment of the inflow cannulas with the AV valves. To modify the outflow cannula of the pump, a spline curve was created between the device outflow and the vessels. A virtual surface graft model was created based on this spline. The interaction between pump, graft, and anatomy was qualitatively analyzed. If necessary, the pump was repositioned, and the process was repeated until an optimal connection with better alignment with AV valves and minimal pump-graft-anatomy interaction was achieved. This manual iterative process was performed for all 11 patients. The average angulation and orientation of the final spline from all patients, together with the vessel diameter, served as a reference to modify of the outflow cannula. With the modified ShuttlePump outflow design, the final implantation was performed and exported to Materialize Mimics for qualitative and quantitative analysis. Figure 2 demonstrates the workflow involved in the modification of the outflow cannula.

Anatomical measurements tailored to the unique geometric characteristics of the ShuttlePump were recorded, together with various measurements such as the distance between the 10th thoracic bone to the sternum T10 distance, intrathoracic circumference, and sternum-dorsal (S-D) pericardium distance specified in the literature.7 In addition, the pump volume exceeding the pericardium was measured through local Boolean operators, providing an indirect measure of intrathoracic tissue compression. Based on the exceeding pump volume, the results of the virtual implantation were categorized into three classes: a successful fit with no interference of the pump volume with the surrounding tissue, a likely fit with interference of the pump volume <100 mL with the intrathoracic structures, and an unsuccessful fit with interference of the pump volume with the thoracic cage. Spearman's rank correlation coefficient was calculated between the exceeding pump volume and the measured anatomical parameters to establish predictors for a successful ShuttlePump implantation and to assess the appropriateness of virtual ShuttlePump implantation within the selected patient population.
2.2 Numerical hydraulic and hemocompatibility evaluation
The numerical evaluation was performed in a StarCCM+ (Siemens, Munich, Germany) solver. In this study, the computational mesh and the numerical setup utilized were identical to our previous study of the ShuttlePump.6 Since more than 75% of the volume of the computational domain experiences a shear rate greater than 100 s−1, blood was modeled as a Newtonian fluid with a 1,050 kg/m3 density and a dynamic viscosity of 3.5 mPas.12 To accurately predict the complex flow pattern and to capture the viscous sublayer, unsteady Reynolds averaged Navier stroke simulation was performed with the k-ω shear stress transport model13 for turbulence modeling. An overset mesh was utilized to discretize the domain and realize the piston's motion. In line with the established model,6 the piston motion was set to a 3 Hz rotational frequency, and a modified sinusoidal motion pattern was prescribed for the linear motion (Figure 1B). The operating conditions have been selected based on the stroke volume of the pump (30 mL) to achieve a cardiac output of 5 L/min. The mesh quality and the mesh independence were thoroughly validated together with the time step independence in an earlier study.6 Briefly, 100% of the elements in the computational domain satisfied the mesh quality metrics specified in the solver's user manual14 monitored throughout the simulation. An average Wall Y+ of 0.6 was achieved, with 99.999% of the near-wall surface lying within the viscous sublayer. Furthermore, with less than 5% deviation in the metrics such as wall shear stress on housing and piston, force and torque acting on the piston, and volume of blood exposed to shear >9 Pa between fine and medium mesh validated the usage of medium mesh for this study.6
To assess the impact of the change in the outflow cannulation on the hemocompatibility metrics of ShuttlePump, we conducted CFD simulations for pumps with both a straight outlet and an angled outlet. A two-element Windkessel model15 was implemented as the outlet boundary conditions for both pump models for a realistic representation of vascular properties. For a comparable outlet pressure boundary, the outlet diameter of the two pump configurations was adapted to the same diameter (17 mm). The compliance for the arterial and pulmonary circulation was set to 0.8 and 2.5 mL/mmHg,16 while the resistance was set to 1.2 and 0.1 mmHg-s/mL,6 respectively. A total pressure inlet boundary was applied at both left and right atria with 11 and 6 mmHg, respectively, based on results from a previous study.6
For comparing both pump models, the widely recognized metrics, such as the volume of blood exposed to shear >9 and >50 Pa, were calculated, representing the von Willebrand degradation and platelet activation, respectively.19, 20 Further, a washout study using a passive scalar transport equation quantified the pump's thrombogenic potential.
3 RESULTS
3.1 In-silico anatomical compatibility
The virtual implantation study included 11 patients (9 male and 2 female) with a median age of 59 (IQR 46.5–61) years and a median body surface area (BSA) of 2.15 (IQR 1.865–2.3) m2. Table 1 provides detailed patient demographic information and presents an overview of the virtual implantation results.
| Demographic data | Device fit | |||||
|---|---|---|---|---|---|---|
| Patient | Age | Sex | BSA (m2) | Body mass index (BMI) (kg/m2) | Exceeding pump volume (mL) | Compressed tissue type |
| 1 | 43 | Female | 1.76 | 24 | 42.4 | Lungs and ribcage |
| 2 | 66 | Male | 2.24 | 30.9 | 0 | – |
| 3 | 61 | Male | 1.89 | 25.4 | 0 | – |
| 4 | 69 | Male | 2.02 | 28.7 | 0 | – |
| 5 | 59 | Male | 2.36 | 37.6 | 0 | – |
| 6 | 59 | Male | 2.46 | 36.2 | 0 | – |
| 7 | 38 | Male | 1.84 | 22.9 | 81 | Lungs and diaphragm |
| 8 | 49 | Male | 2.45 | 43.9 | 4.1 | Lungs |
| 9 | 51 | Male | 2.15 | 26.3 | 58.7 | Ribcage |
| 10 | 61 | Male | 2.18 | 29.3 | 19.1 | Lungs and diaphragm |
| 11 | 44 | Female | 1.71 | 25.8 | 29.2 | Diaphragm |
| Median | 59 | [−] | 2.15 | 28.7 | 4.1 | [−] |
| IQR | (46.5–61) | [−] | (1.865–2.3) | (25.6–33.55) | (0–35.8) | [−] |
The result of the outflow cannula design of the ShuttlePump that ensures optimal positioning and enhanced compatibility with the cardiovascular system is shown in Figure 2. This involved rotating the aortic outlet by 32° in the anticlockwise direction (towards the pulmonary outlet) and the pulmonary artery outlet by 20° in the clockwise direction. Additionally, the outlet diameter was expanded from 12 mm to 17 mm to ensure proper graft connectivity between the outflow cannula and the vessels.
In five patients (Patients 2–6), the volume of the ShuttlePump does not exceed the pericardium, indicating no interaction with surrounding tissues. In four patients (Patients 7, 8, 10, and 11), the pump volume interfered with the lungs and/or diaphragm, with a slight to moderate exceeding pump volume ranging from 4.1 to 81 mL. Two patients (Patient 1 and 9) exhibited poor fit: In Patient 1, the pulmonary outflow cannula extended beyond the ribcage, and additional interferences were found between the pump and the left lung. Patient 9 experienced substantial interference of the pump volume with the rib cage. Figure 3 visualizes the virtual implantation of ShuttlePump in patients with successful fit, likely fit, and unsuccessful fit.
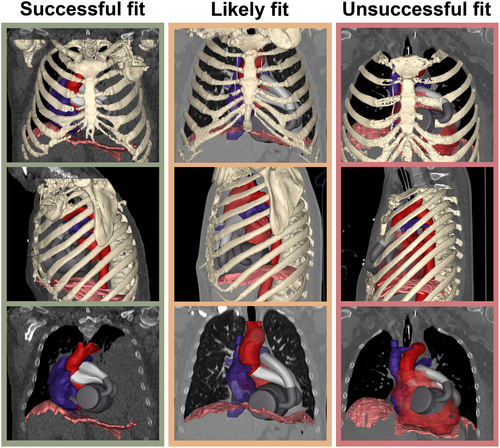
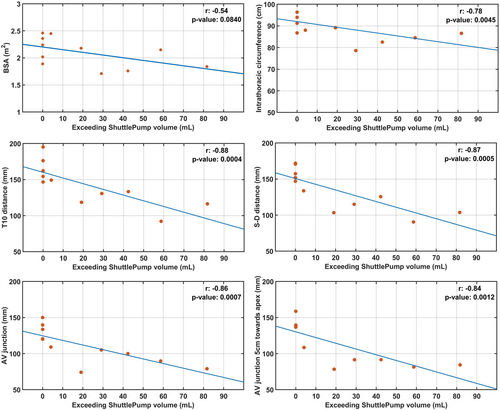
Figure 4 illustrates the correlation between the measured anatomical parameters and the exceeding pump volume, and the descriptive data were presented in Table S1. Exceeding pump volume did not correlate significantly with BSA (r = −0.5435, p = 0.08). Strong correlations (r <−0.78, p < 0.05) were observed between the exceeding pump volume and parameters such as the T10 distance (143 ± 29 mm), intrathoracic circumference (892 ± 68 mm), S-D pericardium distance (134 ± 28 mm), AV junction (111 ± 25 mm), and AV junction 5 cm toward apex (113 ± 29 mm).
3.2 Numerical hydraulic and hemocompatibility evaluation
A converged afterload pressure curve was obtained after 10 pump cycles. The subsequent analysis of hydraulic performance and hemocompatibility were derived under the conditions of converged afterload pressure.
The simulated pressure and flows are presented in Figure 5. With both outlet configurations, the ShuttlePump delivered an average flow rate of 4.9 L/min to the systemic circulation and 5.5 L/min to the pulmonary circulation. The total pressure losses in the outflow cannula were calculated by monitoring pressure at the outlet boundary and immediately after the pump outlet over one pump cycle. An average loss of 3.2 and 4.3 mmHg was observed in the ShuttlePump with angled outflow in systemic and pulmonary circulation, respectively, while for the straight outflow configuration, a 2.5 mmHg loss in both circulations was evident.

Of note, outlet pressures were different between the pump with straight and angled outlet configurations on the systemic side (91.2 vs. 87.5 mm Hg) and pulmonary side (17.9 vs. 16.4 mm Hg), respectively.
The HI for the ShuttlePump with angled outlet configuration was 0.0059%, while the straight outlet configuration exhibited an HI of 0.0065%, indicating comparable levels of hemolysis. In addition, the volume of blood exposed to shear >9 and >50 Pa with respect to the pump volume was 2.17% and 0.0077% for the pump with a straight outlet and 2.18% and 0.0081% for the pump with a curved outlet, respectively. Thrombogenicity assessment through the washout study revealed that the straight outlet configuration took 1.43 s for 95% washout, whereas the curved outlet configuration achieved 95% washout in 1.42 s (Figure 6).

4 DISCUSSION
Evaluating anatomical compatibility and optimizing novel TAHs during their early development stages are essential for addressing a large patient population. This study focused on assessing the anatomical compatibility of the ShuttlePump TAH through virtual implantation techniques, focusing on the pump outflow cannula to improve device-to-vessel connectivity. In addition, CFD simulations were employed to evaluate the impact of outflow cannula modification on the hydraulic and hemocompatibility performance.
To enhance anatomical compatibility, the ShuttlePump outflow cannulation was iteratively adapted within a virtual framework, focusing on the orientation and angulation appropriate for the selected patient cohort. This process also aimed to continuously refine the device-to-vessel connection to prevent graft kinking while maintaining the inflow cannula aligned with the AV junction to ensure adequate pump chamber filling.
Successful virtual implantation of the ShuttlePump, defined as no pump volume exceeding the pericardial space, was observed in five patients. Minimal interference (≤81 mL) of the pump with the intrathoracic structures was observed in four patients, and unsuccessful virtual implantation (interaction with the thoracic cage) was observed in two patients. Of note, implantation of state-of-the-art intrapericardial LVADs (HeartMate 3, Abbott Inc., Chicago, USA) with a device volume of 80 mL21 is feasible even in smaller patients (≤1.7 m2),22 indicating that the pericardial space may accommodate such an additional volume in adult patients. Therefore, successful implantation can be considered for patients with an exceeding pump volume of ≤81 mL.
Although BSA is a commonly used predictor for pump fit, our study revealed that BSA may not accurately predict the suitability of ShuttlePump implantation in the thoracic cavity. Instead, we found that the mediastinal space is a promising predictor of pump fit. For instance, in Patient 9, with a BSA of 2.15 m2, unsuccessful virtual implantation of the ShuttlePump was observed due to smaller mediastinal space relative to other patient cohorts of this study. Conversely, a successful fit was achieved in Patient 3 despite a BSA of 1.89 m2, attributable to an enlarged mediastinal space. This observation suggests that BSA calculations, based on patient height and weight, may not accurately reflect the mediastinal space. Individuals with considerable subcutaneous fat accumulation or different body types may exhibit an increased BSA, but their mediastinal space may not be proportionally enlarged.
The observation that BSA is a poor predictor for ShuttlePump fit aligns with previous literature on other TAH implantation,8, 9, 11, 23, 24 highlighting the importance of anatomical parameters. Among the measured anatomical parameters, the T10 distance, a well-established predictor for TAH implantation, showed a strong correlation with exceeding pump volume. A T10 distance greater than 116 mm led to a successful virtual implantation of the ShuttlePump. These T10 distances are similar to established TAH concepts such as SynCardia TAH (SynCardia systems LLC, Tucson, AZ, USA) with >100mm24 and BiVACOR TAH (BiVACOR, Inc., CA, USA) with >110 mm.23 Additionally, the introduction of pump-specific anatomical measurements, AV junction and AV junction 5 cm toward the apex revealed similar correlations and significance with exceeding pump volume compared to T10 distance, emphasizing their relevance as pump-specific anatomical parameters.
CFD simulation revealed that the ShuttlePump with angled outflow cannulation exhibits similar hemocompatibility metrics and acceptable hydraulic performance, comparable to the previously established ShuttlePump with straight outflow cannulation. The computed pressure loss across the modified outflow cannula indicated 0.7 and 1.8 mmHg higher total pressure losses in the systemic and pulmonary circulations, respectively, compared to the straight outlet configuration. These higher losses were attributable to the increased flow resistance in the curved geometry.
Despite the same pump flows, a higher afterload pressure of 4.2% and 9.6% was observed in the systemic and pulmonary circulation for the ShuttlePump with straight outflow compared to the pump with angled outflow cannulation. This discrepancy may be due to the coupling of the 3D flow simulation with the 0D two-element Windkessel model. The 0D model transmits transient pressure information to the outlet boundary based on the flow rate, and together with the specified inlet pressure boundary, the system lacks information regarding the velocity profile. Subsequently, the geometric differences in the outflow between the straight and modified pump models cause a variation in the velocity profile at the outflow boundaries, resulting in different kinetic energy levels at the outlet boundaries between the two pump configurations. This discrepancy results in different afterload pressures and consequently places the two pump configurations in different pressure regimes.
With comparable HI values between the ShuttlePump with straight and the angled outlet (0.0065% vs. 0.0059%), the hemocompatibility-related shear stress thresholds exhibited less than 5% variation. Additionally, thrombogenicity assessment through the washout study yielded similar results for both pump models, indicating that the curved geometry did not introduce substantial stagnation zones.
4.1 Study limitation
The study focused on patients eligible for TAH/BiVAD implantation and provided valuable insights into preliminary anatomical feasibility; it is essential to acknowledge certain limitations. A primary limitation is the relatively small study population of 11 patients, which may not fully encompass the heterogeneity within the biventricular failure patient population. Although turbulence model selection and blood properties were previously validated in RBPs,25 in vitro hydraulic and hemocompatibility assessments for ShuttlePump will add credibility to the CFD outcomes of this study. It is also necessary to acknowledge the limitations associated with the manual iterative design modification method, which were subjective in nature and based on qualitative assessment.
5 CONCLUSION
This study comprehensively evaluated the feasibility of ShuttlePump in human anatomy through virtual implantation techniques and CFD simulations. The modified outflow design enhances anatomical fit, with successful virtual implantations in 9 out of 11 cases. Notably, this design change does not compromise hemocompatibility. These results highlight the value of virtual implantation techniques in device optimization and its corresponding hemodynamic performance. Nevertheless, clinical validation is critical to ensure the clinical applicability of these findings.
AUTHOR CONTRIBUTIONS
Krishnaraj Narayanaswamy: participated in the research design, performed the research, collected and analyzed the data, and drafted the manuscript. Jakob Petz: participated in the research design, performed the research, collected and analyzed the data, and reviewed the manuscript. Tim Bierewirtz, Christian Loewe, Ulrich Kertzscher, and Daniel Zimpfer: participated in the research design and critically reviewed the manuscript. Marcus Granegger: participated in the research design, analyzed the data, secured the funding, and drafted/critically reviewed the manuscript.
ACKNOWLEDGMENTS
This work was supported by the SPARK-BIH program from the Berlin Institute of Health at Charité—Universitätsmedizin Berlin. The results of the computational fluid dynamics simulations were achieved using the Vienna Scientific Cluster (VSC).
FUNDING INFORMATION
This work was supported by the SPARK-BIH program from the Berlin Institute of Health at Charité—Universitätsmedizin Berlin.
CONFLICT OF INTEREST STATEMENT
Marcus Granegger received personal fees and research grants from BerlinHeart GmbH and research grants from 4Fontan AG. Daniel Zimpfer received personal fees from Abbott, Medtronic, Abiomed, Edwards, and Daiichi Sankyo and research grants from Abbott, Medtronic, Berlin Heart, Edwards, and Corcym.




