Partial replacement of fishmeal by a protein-rich product from methanotrophic bacteria in juvenile red sea bream (Pagrus major)
Abstract
The optimum level of fishmeal (FM) protein replacement by bacterial protein meal (BPM) was determined in the diet of juvenile red sea bream, Pagrus major. Six isoproteic and isolipidic diets were formulated to replace 0 (control diet), 190 , 280, 370, 470 and 560 g of the FM protein kg-1 diet with BPM protein, and referred to as C, BP19, BP28, BP37, BP47 and BP56, respectively. Each diet was fed to triplicate groups of twenty fish (mean weight ~43 g) twice per day to apparent satiation. Fish fed diets C and BP19 did not show significant differences (p > .05) in growth, apparent digestibility or protein and lipid retention efficiency, but the daily feeding rate (DFR) was significantly lower under BP19 than under C. However, almost all growth parameters were significantly reduced when FM protein replacement with BPM was further increased from 280 to 560 g kg-1 diet (p < .05). The results of polynomial regression analysis (second-order) revealed significant negative correlations between dietary copper levels and final mean weight (R2 = .913), specific growth rate (R2 = .913) and DFR (R2 = .956). The results indicated that BPM could replace approximately 190 g of FM protein kg-1 diet in the juvenile red sea bream diet without compromising growth performance or feed efficiency.
1 INTRODUCTION
The demand for high-quality protein sources in the rapidly expanding aquaculture sector is growing, as the supply of highly preferable fishmeal (FM) is decreasing, while its price is increasing daily. Meals containing soybeans, cottonseed, rapeseed, corn, lupins, canola, barley, potato, insects, krill, squid and by-products have been used during the last couple of decades for different species (Alam et al., 2012; Barroso et al., 2014; Belghit et al., 2018, 2019; Biswas et al., 2007, 2017, 2019; Bu et al., 2017; Cheng et al., 2010; Cruz-Suárez et al., 2007; Dossou et al., 2018; Faudzi et al., 2018; Gatlin et al., 2007; Henry et al., 2015; Kader et al., 2010, 2012; Kissil et al., 2000; Lock et al., 2015; Salze et al., 2010; Sánchez-Muros et al., 2014; Silva-Carrillo et al., 2012; Takakuwa et al., 2020; Xu et al., 2017; Zhang et al., 2014). However, concerns over the future availability of some of these ingredients together with the existence of anti-nutritional factors, inferior quality and quantity of nutrient content suggest that alternative protein sources need to be continuously explored.
The major constituent of natural gas is methane, which has approximately 26 times the climate warming potential of carbon dioxide (Lelieveld et al., 1993). In recent years, however, bacterial protein meal (BPM) with high protein content (approximately 70%) has been produced from the bacterium Methylococcus capsulatus (Bath), which utilizes methane as a carbon and energy source. Reportedly, BPM has the potential to serve as an alternative protein source for different species, although its use has yielded some mixed results (Aas et al., 2006, 2007; Aas, Hatlen, et al., 2006; Berge et al., 2005; Biswas et al., 2020; Øverland et al., 2006, 2010; Perera et al., 1995; Storebakken et al., 2004). While successful replacement of 25% to 30% of the FM protein by BPM has been reported in the Atlantic salmon and Japanese yellowtail (Berge et al., 2005; Biswas et al., 2020; Storebakken et al., 2004), it can only effectively replace 9% of FM in the diet of halibut (Aas et al., 2007).
Red sea bream (Pagrus major) is believed to symbolize good fortune in Japan, and the versatility of this species makes it one of the most important fish in Japan. It is not only eaten raw as ‘sashimi’ but also presented in ceremonies, such as weddings, after grilling with salt. Therefore, the production of this species has steadily increased and ranked second only to yellowtail in Japan (Watanabe & Vassallo-Agius, 2003). Consequently, many studies have attempted to investigate the utility of different protein sources in red sea bream of different sizes (Kader et al., 2010, 2011, 2012; Aoki et al., 1998; Biswas et al., 2007, 2017, 2019; Takagi et al., 1999; Takagi, Shimeno, Hosokawa, & Ukawa, 2000, 2001; Uyan et al., 2007). However, investigations to determine the utility of BPM as an alternative to FM are lacking. Therefore, this study aimed to determine the safety and optimal inclusion levels of BPM as an alternative protein source in the diet of red sea bream.
2 MATERIALS AND METHODS
2.1 Processing of BPM
The BPM used in this study was supplied by Marubeni Nisshin Feed Co., Ltd. (Tokyo, Japan). Methane was used as the sole carbon and energy source by the bacterial biomass of BPM. The process of growing methane-oxidizing bacterial cultures was performed in simple mineral media in continuous mode at a temperature of 42–44°C and pH level of 5.4–5.6 with vigorous mixing and aeration with the release of heat and metabolic products. In BPM, M. capsulatus comprised 95% of the total biomass, and the remaining 5% was composed of additional strains such as Cupriavidus gilardii, Stenotrophomonas acidaminiphila and Klebsiella pneumoniae. The bacterial biomass was packaged as a finished product (crude protein, 75.0%; crude lipid, 1.6%) after it was centrifuged, heat inactivated, spray dried and granulized.
2.2 Diets and proximate composition
Table 1 shows the formula and proximate composition of the experimental diets. The control diet (C) contains FM as the sole protein source without supplementation of BPM. Approximately 190, 280, 370, 470 and 560 g of FM protein kg-1 diet in diet C were replaced by BPM protein, and these diets are hereafter referred to as BP19, BP28, BP37, BP47 and BP56, respectively. To determine the apparent digestibility coefficient (ADC), chromic oxide (Cr2O3) was added to each diet as an inert marker. The ingredients were mixed properly and pelletized after adding water. The pellets were dried and stored in a freezer (−20°C) until use.
| Diets | C | BP19 | BP28 | BP37 | BP47 | BP56 |
|---|---|---|---|---|---|---|
| Fishmeala | 700.0 | 560.0 | 490.0 | 420.0 | 350.0 | 280.0 |
| Methanotroph mealb | 120.0 | 180.0 | 240.0 | 300.0 | 360.0 | |
| Wheat flourc | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
| Fish oild | 30.0 | 41.0 | 46.0 | 51.0 | 56.0 | 61.0 |
| Mineral mixe | 20.0 | 21.0 | 22.0 | 23.0 | 24.0 | 25.0 |
| Vitamin mixe | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Calcium phosphate | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Taurine | 1.0 | 1.2 | 1.4 | 1.8 | 2.1 | |
| L-Lysine HCl | 3.0 | 5.0 | 7.0 | 9.0 | 11.0 | |
| Cellulose | 15.0 | 19.0 | 20.8 | 22.6 | 24.2 | 25.9 |
| Chromic oxide | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Proximate composition | ||||||
| Crude protein | 543.2 | 535.8 | 529.8 | 550.0 | 546.2 | 530.6 |
| Crude lipid | 112.5 | 110.1 | 113.1 | 114.7 | 123.1 | 122.4 |
| Crude ash | 182.0 | 163.7 | 154.5 | 143.2 | 134.5 | 125.6 |
| Copper (mg/kg diet) | 14.8 | 59.5 | 121.0 | 123.0 | 148.0 | 189.0 |
- a TASA, Lima, Peru (crude protein, 68.3%; crude lipid, 8.3%).
- b Marubeni Nisshin Feed Co., Ltd., Tokyo, Japan (crude protein, 75.0%; crude lipid, 1.6%).
- c Nisshin Flour Milling Inc., Tokyo, Japan (crude protein, 18.0%; crude lipid, 4.3%).
- d Tsuji Oil Co. Ltd., Tokyo, Japan.
- e Marubeni Nisshin Feed Co., Ltd. (Tokyo, Japan) formula.
Dietary levels of crude protein and crude lipid ranged from 529.8 g kg−1 to 550.0 g kg−1 diet and 110.1 g kg−1 to 123.1 g kg−1 diet, respectively (Table 1). Table 2 shows the amino acid (AA) compositions, AA indices and taurine contents of the experimental diets. Although there were marginal differences in the contents of some indispensable (IAAs) and dispensable AAs (DAAs) between the BPM diets and the C diet, the total of both IAAs and DAAs was reduced in all BPM-based experimental diets than the C diet. The IAA index ranged from 92.8 to 96.1 in BPM-based diets and decreased with increasing levels of FM replacement.
| Diets | C | BP19 | BP28 | BP37 | BP47 | BP56 |
|---|---|---|---|---|---|---|
| Indispensable amino acids (IAA) | ||||||
| Arginine | 28.7 (13.3)a | 27.7 (13.6) | 27.3 (13.6) | 28.0 (13.8) | 27.2 (13.7) | 27.9 (13.8) |
| Histidine | 15.7 (7.3) | 13.7 (6.7) | 12.8 (6.4) | 12.5 (6.2) | 11.6 (5.9) | 11.5 (5.7) |
| Isoleucine | 20.7 (9.6) | 19.4 (9.5) | 19.1 (9.5) | 19.2 (9.5) | 18.9 (9.5) | 19.2 (9.5) |
| Leucine | 36.8 (17.1) | 34.8 (17.1) | 34.2 (17.1) | 34.1 (16.8) | 33.7 (17.0) | 34.0 (16.8) |
| Lysine | 37.4 (17.3) | 35.3 (17.3) | 35.5 (17.7) | 36.3 (17.9) | 36.0 (18.1) | 36.4 (18.0) |
| Methionine | 10.1 (4.7) | 9.2 (4.5) | 9.1 (4.5) | 9.1 (4.5) | 8.9 (4.5) | 9.2 (4.5) |
| Phenylalanine | 21.0 (9.7) | 20.0 (9.8) | 19.7 (9.8) | 19.8 (9.8) | 19.6 (9.9) | 20.1 (9.9) |
| Threonine | 20.7 (9.6) | 19.9 (9.8) | 19.5 (9.7) | 19.8 (9.8) | 19.2 (9.7) | 19.6 (9.7) |
| Valine | 24.7 (11.4) | 23.6 (11.6) | 23.5 (11.7) | 23.7 (11.7) | 23.5 (11.9) | 24.2 (12.0) |
| ΣIAA | 215.8 (100) | 203.6 (100) | 200.7 (100) | 202.5 (100) | 198.6 (100) | 202.1 (100) |
| IAA index | 96.1 | 94.2 | 94.3 | 93.4 | 92.8 | |
| Dispensable amino acids (DAA) | ||||||
| Alanine | 31.3 | 29.7 | 29.6 | 29.5 | 29.0 | 29.4 |
| Aspartic acid | 43.4 | 40.4 | 39.3 | 39.4 | 37.8 | 38.0 |
| Cystine | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 |
| Glutamic acid | 69.0 | 63.2 | 60.8 | 59.9 | 57.6 | 56.9 |
| Glycine | 31.3 | 28.5 | 27.4 | 26.7 | 25.4 | 25.2 |
| Proline | 24.2 | 22.5 | 21.9 | 21.7 | 21.2 | 21.0 |
| Serine | 18.9 | 17.8 | 17.1 | 17.1 | 16.5 | 16.5 |
| Tyrosine | 16.6 | 16.0 | 15.9 | 15.9 | 15.8 | 16.3 |
| ΣDAA | 235.0 | 218.2 | 212.2 | 210.4 | 203.4 | 203.5 |
| Taurine | 5.1 | 4.8 | 4.5 | 4.2 | 3.9 | 3.9 |
- a Data in parenthesis indicate contribution of each amino acid to total IAA in diets.
Dietary fatty acid (FA) content (g kg−1 diet) is provided in Table 3. Palmitic acid (C16:0) increased with increasing levels of BPM in diets, which caused a similar trend in total saturated fatty acid (SFA) content among the treatments. Similarly, the total monounsaturated fatty acid (MUFA) content increased gradually with increasing levels of BPM in diets due to a gradual increase in the palmitoleic acid (C16:1) content. However, the total polyunsaturated fatty acid (PUFA) content decreased as dietary BPM supplementation levels increased. In particular, the total n-3 PUFA content markedly varied among the experimental diets. Although eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) contents decreased with increasing BPM supplementation, the DHA/EPA ratio remained similar among the treatments.
| Fatty acids | C | BP19 | BP28 | BP37 | BP47 | BP56 |
|---|---|---|---|---|---|---|
| C14:0 | 5.4 | 4.5 | 4.4 | 4.0 | 3.9 | 3.9 |
| C15:0 | 0.4 | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 |
| C16:0 | 17.1 | 18.0 | 19.6 | 20.5 | 21.6 | 23.9 |
| C18:0 | 3.4 | 2.7 | 2.6 | 2.3 | 2.3 | 2.2 |
| ΣSFA | 26.3 | 25.6 | 27.0 | 27.1 | 28.1 | 30.3 |
| C16:1 | 6.6 | 10.2 | 12.6 | 14.7 | 16.1 | 19.0 |
| C18:1n-9 | 11.4 | 10.0 | 10.4 | 9.8 | 10.3 | 10.2 |
| C18:1n-7 | 3.7 | 3.6 | 3.7 | 3.8 | 3.9 | 4.1 |
| C20:1 | 1.8 | 2.1 | 2.4 | 2.5 | 2.7 | 2.9 |
| C20:1n-9 | 1.8 | 1.7 | 1.9 | 1.9 | 2.1 | 2.1 |
| ΣMUFA | 25.3 | 27.6 | 31.0 | 32.7 | 35.1 | 38.3 |
| PUFA | ||||||
| C18:2n-6 | 4.3 | 3.6 | 3.5 | 3.2 | 3.4 | 3.2 |
| C20:3n-6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| C20:4n-6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| C22:5n-6 (DPA) | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 |
| Σn-6 | 4.7 | 4.1 | 3.9 | 3.5 | 3.7 | 3.5 |
| C18:3n-3 | 0.8 | 0.7 | 0.7 | 0.6 | 0.6 | 0.6 |
| C18:4n-3 | 2.0 | 1.7 | 1.6 | 1.5 | 1.5 | 1.5 |
| C20:3n-3 | 0.5 | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 |
| C20:4n-3 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| C20:5n-3 (EPA) | 10.9 | 8.9 | 8.8 | 8.0 | 8.0 | 7.8 |
| C22:5n-3 | 1.1 | 0.9 | 0.9 | 0.8 | 0.8 | 0.7 |
| C22:6n-3 (DHA) | 12.5 | 9.5 | 8.9 | 7.8 | 7.4 | 7.0 |
| Σn-3 | 28.3 | 22.5 | 21.7 | 19.4 | 19.0 | 18.3 |
| ΣPUFA | 33.0 | 26.6 | 25.6 | 22.9 | 22.7 | 21.8 |
| n-3/n-6 | 6.0 | 5.5 | 5.6 | 5.5 | 5.1 | 5.2 |
| DHA/EPA | 1.1 | 1.1 | 1.0 | 1.0 | 0.9 | 0.9 |
- Abbreviations: MUFA, monounsaturated fatty acid; PUFA, poly unsaturated fatty acid; SFA, saturated fatty acid.
2.3 Fish husbandry and sampling
The red sea bream juvenile test fish were raised at the Aquaculture Technology and Production Center, Kindai University, Wakayama, Japan, after collecting eggs from the broodstocks maintained at the same facilities. After transferring the test fish to the indoor rearing facilities at Uragami Station, Aquaculture Research Institute, Kindai University, red sea bream were stocked into two 3000-L tanks for a 2-week acclimation and fed a commercial diet (crude protein, ~500 g kg-1 diet, Marubeni Nisshin Feed Co. Ltd., Tokyo, Japan). During the acclimation period, photoperiod was set to a 12-h light:12-h dark cycle (07:00–19:00). UV-treated filtered seawater was supplied to each tank at 10 L min−1, and the oxygen level was maintained at approximately 100% saturation through aeration.
After the acclimation period, red sea bream juveniles were fasted for 24 h before distribution to the experimental tanks. A total of 360 juveniles (mean weight of ~43 g) were equally distributed into each of the 18 circular tanks of volume 500 L. The experiment was set up in triplicate for each treatment, and during the 8-week rearing period, fish were fed to apparent satiation, twice per day at 09:00 and 15:00. The water supply in each tank was maintained at 7 L min−1, and the oxygen saturation level was maintained at approximately 100%. During an 8-week feeding trial, the average values of temperature and dissolved oxygen were 24.9 ± 1.1°C and 7.6 ± 0.5 mg L−1, respectively. Tanks were cleaned daily, and dead fish were collected and weighed. Fish were fasted for 24 h before weighing in a pool biweekly and at the end of the feeding trial. Fish were anesthetized with 300 ppm 2-phenoxyethanol (Wako Pure Chemical Corp., Tokyo, Japan) before each weighing. Twenty fish were sampled at the beginning, and a group of five fish from each tank was sampled at the end of the feeding trial to determine proximate composition. Another three fish from each tank were anesthetized with 300 ppm 2-phenoxyethanol, and their blood was collected from the caudal vein using a heparinized syringe, centrifuged at 10,000 g for 5 min at 4°C to separate the plasma and stored at −80°C until analysis. Relative organ weights were determined from the same fish used for blood collection. All fish for dissection and proximate analyses were frozen at −20°C until use.
2.4 Faecal collection for digestibility analysis
For the digestibility trial, a total of 30 fish were pooled from three tanks for each treatment and stocked into a 350-L faecal collection tank. Each group of fish was fed with Cr2O3 mixed experimental diets, and fish were acclimated to the faecal collection tank for one week before faecal collection, maintaining the rearing conditions similar to those used in the growth trial. To avoid faecal contamination, tank walls and faecal collectors were thoroughly cleaned at the end of the last feeding of the previous day, and faeces were collected prior to the first feeding on the following day. The collected faeces were centrifuged at 2000 g for 30 min at 4°C to separate the excess water, following which the samples were stored at −80°C. Faeces were freeze-dried prior to analysis.
2.5 Biochemical analysis
The proximate composition samples were analysed using standard methods (Association of Official Analytical Chemists International, 1995). The method reported by Teshima et al. (1986) was used to analyse the AA of experimental diets with an automatic AA analyser (HPLC-GL7700, GL-Science Inc., Tokyo, Japan). For FA analysis, lipid was extracted according to the method described by Folch et al. (1957). FAs were then analysed with a gas chromatograph (GC-4000; GL-Science Inc., Tokyo, Japan) after the formation of FA methyl esters using methanolic 2 M NaOH and 2 M HCl solutions according to the method described by Yoshinaka and Satoh (1989).
Dietary copper (Cu) contents were determined using atomic absorption spectrophotometry (ZA-3300, Hitachi High-Tech Science Corporation, Tokyo, Japan) at the Japan Food Research Laboratory (JFRL, Osaka, Japan). A wet-acid digestion method (Furukawa & Tsukahara, 1966) was used to determine the Cr2O3 content in the diets and faeces. An automatic analyser (Fuji Dri-Chem 7000; Fujifilm) was used to determine the levels of total protein (TP), glucose (GLU), triglyceride (TG), total cholesterol (TCHO), glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) in the plasma.
2.6 Statistical analysis
One-way analysis of variance (ANOVA) was used to compare the mean data among treatments for different variables followed by Tukey's multiple comparison test with a 95% significance level. For the digestibility trials, faeces were collected for 9 consecutive days to obtain sufficient samples. Faeces from each 3-day period were pooled together to obtain three data points from each tank and were considered one set of data for each treatment for comparisons using post hoc tests. Second-order polynomial regression analysis was applied to determine the relationship between dietary Cu levels and some growth parameters. The SPSS program for Windows (v. 10.0) was used to perform all statistical analyses.
3 RESULTS
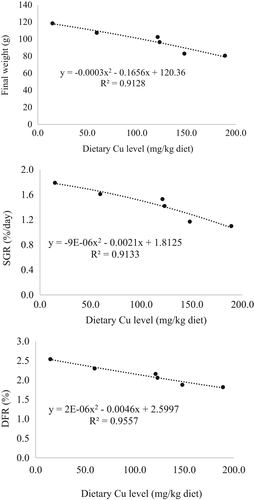
Table 4 summarizes the growth performance in the feeding trial. The mean weight at the end of this study showed a decreasing trend with increasing levels of BPM in diets. Fish fed with diets BP28, BP37, BP47 and BP56 showed significantly lower mean weights than the control group (p < .05). However, the mean weight was not significantly different between fish fed diets C and BP19 (p > .05). Total weight gain and specific growth rate (SGR) also showed a similar trend among the treatments. All BPM-based diets showed a significantly lower daily feeding rate (DFR) than the control group (p < .05). However, there were no significant differences in feed efficiency (FE), condition factor (CF) and survival rate among the treatments (p > .05). Figure 1 shows the second-order polynomial regression analyses between dietary Cu levels and final mean weight, SGR and DFR. The results showed a significant linear reduction in final weight (r2 = .913), SGR (r2 = .913) and DFR (r2 = .956) with increasing levels of dietary Cu.
| Diets | C | BP19 | BP28 | BP37 | BP47 | BP56 |
|---|---|---|---|---|---|---|
| Initial weight (g) | 43.4 ± 0.0 | 43.4 ± 0.0 | 43.4 ± 0.0 | 43.5 ± 0.0 | 43.4 ± 0.0 | 43.4 ± 0.0 |
| Final weight (g) | 118.4 ± 3.9a | 107.3 ± 0.1ab | 102.4 ± 4.4b | 96.4 ± 6.2bc | 82.9 ± 5.8cd | 80.5 ± 2.3d |
| Weight gain (g) | 1449.6 ± 77.3a | 1241.1 ± 50.1ab | 1109.3 ± 51.9b | 991.5 ± 110.9bc | 783.8 ± 140.8c | 688.9 ± 95.7c |
| SGR (% day−1)a | 1.8 ± 0.1a | 1.6 ± 0.1ab | 1.5 ± 0.1b | 1.4 ± 0.1b | 1.2 ± 0.1c | 1.1 ± 0.1c |
| DFR (%)b | 2.5 ± 0.1a | 2.3 ± 0.1b | 2.2 ± 0.1bc | 2.1 ± 0.1cd | 1.9 ± 0.1de | 1.8 ± 0.0e |
| FE (%)c | 65.2 ± 1.9 | 64.6 ± 3.6 | 63.9 ± 0.7 | 62.3 ± 3.1 | 58.5 ± 5.8 | 55.4 ± 5.8 |
| CFd | 39.4 ± 2.0 | 39.4 ± 1.8 | 38.5 ± 2.6 | 36.5 ± 3.3 | 35.9 ± 2.3 | 34.5 ± 3.6 |
| Survival rate (%)e | 100.0 ± 0.0 | 98.3 ± 2.4 | 96.7 ± 2.4 | 96.7 ± 4.7 | 98.3 ± 2.4 | 96.7 ± 4.7 |
Note
- Values in a row with different superscripts are significantly different (p < .05, Tukey's test).
- a SGR (specific growth rate, % day−1) = 100 × (lnW2-lnW1)/time (days), where W1 and W2 indicate the initial and final mean weight (g), respectively.
- b DFR (daily feeding rate, %BW day−1) = 100 × total feed intake / [(average of initial and final body weight × average of initial and final number of fish) / rearing period], where BW indicates body weight.
- c FE (feed efficiency, %) = 100 ×[wet weight gain (g) / dry feed intake (g)].
- d CF = 1000 × (W / L3), where, W = wet body weight (g) and L = body length (cm).
- e Survival rate (%) =100 × final fish number / initial fish number.
In comparisons of relative organ weights among fish fed different diets (Table 5), the viscerosomatic, hepatosomatic, stomatosomatic and intestinosomatic indices did not show significant differences among the treatments (p > .05). For whole-body proximate composition (Table 6), there were no significant differences in moisture, crude protein and crude ash contents among the treatments (p > .05); however, the final whole-body crude lipid contents in red sea bream fed diets BP47 and BP56 were significantly lower than those in red sea bream fed diet C (p < .05).
| Diets | C | BP19 | BP28 | BP37 | BP47 | BP56 |
|---|---|---|---|---|---|---|
| VSI | 8.9 ± 1.4 | 8.8 ± 1.2 | 8.7 ± 0.7 | 8.8 ± 1.4 | 8.2 ± 1.5 | 7.7 ± 1.4 |
| HSI | 1.7 ± 0.1 | 1.9 ± 0.4 | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.3 | 1.4 ± 0.3 |
| SSI | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| ISI | 0.7 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.3 | 1.0 ± 0.2 |
Note
- VSI, HSI, SSI and ISI (%) = 100 × (weight of viscera, liver, stomach and intestine) / whole body weight.
- Abbreviations: HSI, hepatosomatic index; ISI, intestinosomatic index; SSI, stomatosomatic index; VSI, viscerosomatic index.
| Initial | Final | ||||||
|---|---|---|---|---|---|---|---|
| C | BP19 | BP28 | BP37 | BP47 | BP56 | ||
| Moisture | 689.2 | 640.2 ± 2.0 | 647.7 ± 7.0 | 657.7 ± 1.0 | 654.0 ± 13.0 | 667.3 ± 21.0 | 671.5 ± 18.0 |
| Crude protein | 172.1 | 187.3 ± 6.0 | 187.3 ± 4.0 | 182.4 ± 5.0 | 184.8 ± 3.0 | 183.4 ± 5.0 | 182.0 ± 7.0 |
| Crude lipid | 75.4 | 120.8 ± 12.0a | 111.2 ± 16.0ab | 109.8 ± 11.2ab | 106.7 ± 23.1ab | 89.3 ± 22.3b | 91.1 ± 19.2b |
| Crude ash | 58.2 | 47.2 ± 1.0 | 46.3 ± 5.0 | 47.6 ± 6.0 | 48.5 ± 7.0 | 52.6 ± 9.0 | 50.2 ± 4.0 |
Note
- Values in a row with different superscripts are significantly different (p < .05, Tukey's test).
The final whole-body FA compositions are shown in Table 7. FA contents in the SFA group did not show significant differences among the treatments (p > .05). Among the MUFAs, C16:1 content increased, whereas C18:1n-9 content decreased as the levels of BPM in the diets increased (p < .05). Although EPA content was gradually and significantly decreased in BPM-based diets (p < .05), there were no significant differences in other n-6 and n-3 PUFA contents among the treatments (p > .05).
| C | BP19 | BP28 | BP37 | BP47 | BP56 | |
|---|---|---|---|---|---|---|
| C14:0 | 34.8 ± 1.0 | 34.0 ± 0.6 | 33.0 ± 0.7 | 32.9 ± 1.9 | 33.7 ± 2.8 | 33.1 ± 2.4 |
| C15:0 | 3.1 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.2 | 3.5 ± 0.3 | 3.5 ± 0.2 |
| C16:0 | 235.4 ± 3.1 | 241.9 ± 3.2 | 241.5 ± 2.4 | 246.0 ± 0.9 | 243.5 ± 5.0 | 250.6 ± 6.1 |
| C18:0 | 85.2 ± 2.3 | 78.9 ± 0.4 | 78.6 ± 1.2 | 76.3 ± 4.1 | 75.3 ± 3.9 | 75.8 ± 2.2 |
| ΣSFA | 358.6 | 358.1 | 356.4 | 358.5 | 356.0 | 363.0 |
| C16:1 | 60.1 ± 0.1a | 86.5 ± 0.07bc | 95.6 ± 1.0cd | 111.2 ± 1.5d | 114.3 ± 8.8d | 116.5 ± 11.2d |
| C18:1n-9 | 243.8 ± 8.0a | 220.0 ± 0.2bc | 217.1 ± 4.0cd | 204.7 ± 5.1d | 200.1 ± 6.1d | 200.1 ± 3.2d |
| C18:1n-7 | 39.7 ± 0.0 | 43.4 ± 0.9 | 43.8 ± 0.5 | 45.4 ± 0.6 | 45.7 ± 0.8 | 45.7 ± 0.9 |
| C20:1 | 14.8 ± 0.1 | 18.9 ± 0.9 | 19.7 ± 1.2 | 21.2 ± 0.5 | 21.5 ± 1.2 | 20.2 ± 1.4 |
| C20:1n-9 | 20.3 ± 0.0 | 20.1 ± 0.4 | 20.6 ± 0.7 | 20.2 ± 0.6 | 20.3 ± 1.2 | 20.9 ± 0.9 |
| ΣMUFA | 378.6 | 388.8 | 396.7 | 402.6 | 401.9 | 403.5 |
| PUFA | ||||||
| C18:2n-6 | 57.7 ± 2.0 | 59.2 ± 0.10 | 55.6 ± 0.7 | 55.5 ± 1.0 | 54.0 ± 1.2 | 51.4 ± 0.8 |
| C20:3n-6 | 1.3 ± 0.0 | 1.2 ± 0.01 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| C20:4n-6 | 1.1 ± 0.1 | 1.2 ± 0.00 | 1.2 ± 0.0 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.2 |
| C22:5n-6 (DPA) | 1.9 ± 0.1 | 1.7 ± 0.00 | 1.8 ± 0.1 | 1.6 ± 0.1 | 1.8 ± 0.4 | 1.9 ± 0.4 |
| Σn-6 | 62.0 | 63.2 | 59.7 | 59.3 | 58.0 | 55.3 |
| C18:3n-3 | 7.7 ± 0.4 | 8.0 ± 0.2 | 7.9 ± 0.1 | 7.8 ± 0.4 | 7.9 ± 0.4 | 7.7 ± 0.3 |
| C18:4n-3 | 1.9 ± 0.0 | 1.8 ± 1.0 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| C20:3n-3 | 4.3 ± 0.3 | 4.2 ± 0.0 | 4.1 ± 0.1 | 3.9 ± 0.3 | 4.3 ± 0.7 | 4.1 ± 0.7 |
| C20:4n-3 | 6.4 ± 0.3 | 6.4 ± 0.1 | 6.4 ± 0.3 | 6.2 ± 0.3 | 6.2 ± 0.0 | 6.0 ± 0.1 |
| C20:5n-3 (EPA) | 47.8 ± 2.8a | 42.9 ± 0.4ab | 41.3 ± 1.2b | 39.4 ± 0.8bc | 38.2 ± 1.2bc | 35.8 ± 0.6c |
| C22:5n-3 | 20.9 ± 1.1 | 19.6 ± 0.3 | 18.9 ± 1.1 | 18.1 ± 0.7 | 18.7 ± 1.3 | 18.0 ± 1.4 |
| C22:6n-3 (DHA) | 89.3 ± 4.1 | 83.0 ± 1.8 | 81.5 ± 2.9 | 76.6 ± 3.6 | 81.2 ± 11.0 | 80.0 ± 9.4 |
| Σn-3 | 178.2 | 166.0 | 161.6 | 153.4 | 157.9 | 152.9 |
| ΣPUFA | 240.2 | 229.2 | 221.3 | 212.7 | 215.9 | 208.2 |
| n-3/n-6 | 2.9 | 2.6 | 2.7 | 2.6 | 2.7 | 2.8 |
| DHA/EPA | 1.9 | 1.9 | 2.0 | 1.9 | 2.1 | 2.2 |
Note
- Values in a row with different superscripts are significantly different (p < .05, Tukey's test).
- Abbreviations: MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
The apparent digestibility of protein, lipid, AA and taurine at the end of the rearing trial is shown in Table 8. For the ADC of proteins and lipids, diets BP47 and BP56 showed significantly lower values than diets C and BP19 (p < .05). Except for lysine, the ADC of all IAAs and DAAs tended to decrease significantly as the BPM content increased in diets. However, there was no significant difference in the ADC of taurine among the treatments (p > .05).
| Diets | C | BP19 | BP28 | BP37 | BP47 | BP56 |
|---|---|---|---|---|---|---|
| Protein | 91.9 ± 0.5a | 90.3 ± 0.7a | 88.7 ± 1.2ab | 89.0 ± 0.6ab | 88.4 ± 0.4b | 87.3 ± 0.5b |
| Lipid | 92.8 ± 0.3a | 92.2 ± 0.7a | 89.5 ± 0.5ab | 87.5 ± 0.5b | 86.2 ± 0.6b | 87.7 ± 0.4b |
| Indispensable amino acids | ||||||
| Arginine | 97.0 ± 0.2a | 95.5 ± 0.1ab | 94.8 ± 0.3ab | 94.1 ± 0.1ab | 93.6 ± 0.3b | 93.3 ± 0.2b |
| Histidine | 95.8 ± 0.3a | 94.2 ± 0.1ab | 93.0 ± 0.0b | 92.1 ± 0.0b | 91.2 ± 0.3bc | 90.4 ± 0.3c |
| Isoleucine | 95.9 ± 0.1a | 93.9 ± 0.4ab | 92.5 ± 0.2ab | 91.1 ± 0.1bc | 90.4 ± 0.2bc | 89.6 ± 0.7b |
| Leucine | 96.3 ± 0.2a | 94.1 ± 0.1b | 92.6 ± 0.1c | 91.0 ± 0.1cd | 90.1 ± 0.3cd | 89.2 ± 0.5d |
| Lysine | 97.4 ± 0.2 | 96.9 ± 0.1 | 96.6 ± 0.1 | 96.2 ± 0.1 | 96.2 ± 0.1 | 96.2 ± 0.1 |
| Methionine | 97.8 ± 0.4a | 96.5 ± 0.1ab | 95.3 ± 0.4ab | 93.8 ± 0.2b | 93.1 ± 0.6bc | 92.3 ± 0.1c |
| Phenylalanine | 94.0 ± 0.3a | 90.9 ± 0.2b | 88.6 ± 0.1bc | 86.8 ± 0.0c | 85.4 ± 0.3cd | 84.4 ± 0.7d |
| Threonine | 95.1 ± 0.4a | 92.9 ± 0.0ab | 91.2 ± 0.1bc | 90.0 ± 0.1bc | 89.0 ± 0.5bc | 88.2 ± 0.1c |
| Valine | 94.2 ± 0.4a | 91.8 ± 0.1ab | 90.3 ± 0.4b | 89.3 ± 0.1bc | 88.2 ± 0.5bc | 87.7 ± 0.3c |
| Dispensable amino acids | ||||||
| Alanine | 95.5 ± 0.3a | 93.4 ± 0.2ab | 92.0 ± 0.3ab | 91.0 ± 0.0b | 90.5 ± 0.3bc | 89.7 ± 0.2c |
| Aspartic acid | 94.1 ± 0.4a | 92.9 ± 0.0ab | 92.0 ± 0.1ab | 91.2 ± 0.1ab | 90.8 ± 0.4bc | 90.2 ± 0.0c |
| Cystine | 97.4 ± 1.8a | 95.1 ± 1.2ab | 92.4 ± 0.3b | 92.5 ± 2.4ab | 89.9 ± 0.7bc | 87.0 ± 2.2c |
| Glutamic acid | 96.5 ± 0.2a | 95.5 ± 0.1ab | 94.5 ± 0.0ab | 93.8 ± 0.1ab | 93.5 ± 0.2ab | 93.1 ± 0.1b |
| Glycine | 93.3 ± 0.4a | 91.0 ± 0.4ab | 89.6 ± 0.7ab | 89.6 ± 0.2ab | 89.4 ± 0.3ab | 89.0 ± 0.2b |
| Proline | 94.8 ± 0.2a | 92.9 ± 0.0ab | 91.5 ± 0.5ab | 91.0 ± 0.2ab | 90.8 ± 0.4ab | 90.3 ± 0.1b |
| Serine | 94.2 ± 0.5a | 92.2 ± 0.1ab | 90.6 ± 0.2b | 89.6 ± 0.1bc | 88.9 ± 0.5bc | 87.8 ± 0.3c |
| Tyrosine | 96.6 ± 0.4a | 94.0 ± 0.1ab | 92.4 ± 0.3b | 90.4 ± 0.0bc | 89.4 ± 0.6bc | 88.4 ± 0.4c |
| Taurine | 93.7 ± 0.4 | 94.1 ± 0.0 | 93.6 ± 0.1 | 93.4 ± 0.1 | 93.7 ± 0.2 | 93.0 ± 0.1 |
Note
- Values in a row with different superscripts are significantly different (p < .05, Tukey's test). ADC of protein, lipid, AA or taurine (%) = 100 × [1 − {(dietary Cr2O3/faecal Cr2O3) x (faecal protein, lipid, AA or taurine / dietary protein, lipid, AA or taurine)}].
Figure 2 shows the apparent retention efficiency of proteins and lipids. Although protein retention decreased with increasing levels of BPM, it was not significantly different among the treatments (p > .05). Lipid retention efficiency values were significantly lower in diets BP47 and BP56 than in diets C and BP19 (p < .05).
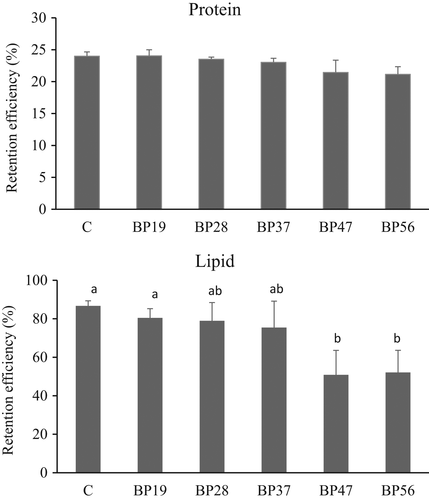
Table 9 shows the plasma parameters in fish from the experimental diets. Although measurements of the TP, GLU, TG, GOT and GPT contents revealed no significant differences among the treatments (p > .05), TCHO content tended to decrease as the BPM content increased, and diets BP37, BP47 and BP56 showed significantly lower TCHO levels than the control group (p < .05).
| Diets | C | BP19 | BP28 | BP37 | BP47 | BP56 |
|---|---|---|---|---|---|---|
| TP (mg dl−1) | 4.4 ± 0.3 | 4.2 ± 0.5 | 4.3 ± 1.0 | 3.9 ± 0.7 | 3.8 ± 0.9 | 3.8 ± 0.9 |
| GLU (mg dl−1) | 64.9 ± 2.9 | 71.4 ± 3.7 | 69.7 ± 7.1 | 73.3 ± 7.7 | 71.2 ± 6.5 | 77.1 ± 10.1 |
| TG (mg dl−1) | 251.3 ± 19.8 | 283.1 ± 43.2 | 297.6 ± 51.1 | 267.6 ± 39.8 | 288.8 ± 45.1 | 281.9 ± 33.3 |
| TCHO (mg dl−1) | 287.3 ± 23.9a | 233.7 ± 43.1ab | 251.3 ± 37.9ab | 227.7 ± 27.4b | 197.1 ± 15.9bc | 167.3 ± 43.1c |
| GOT (IU L−1) | 67.6 ± 19.7 | 76.2 ± 17.5 | 81.2 ± 23.1 | 69.9 ± 20.1 | 79.6 ± 19.9 | 97.2 ± 29.9 |
| GPT (IU L−1) | 15.2 ± 2.9 | 22.2 ± 5.1 | 16.6 ± 3.1 | 20.2 ± 3.3 | 25.1 ± 4.2 | 19.9 ± 4.1 |
Note
- Values in a row with different superscripts are significantly different (p < .05, Tukey's test).
- Abbreviations: GLU, glucose; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; TCHO, total cholesterol; TG, triglyceride; TP, total protein.
4 DISCUSSION
Although the DFR was significantly reduced in fish fed with diet BP19 (approximately 190 g of FM protein kg-1 diet replaced by BPM protein) compared with the control group, there were no significant differences in final mean weight and SGR between these two groups. However, the growth parameters in the BP28, BP37, BP47 and BP56 treatments were significantly reduced compared with those under diet C, which suggests that approximately 190 g of FM protein kg-1 diet can be replaced by BPM protein in red sea bream. This replacement level is lower than that of yellowtail (300 g protein replacement kg-1 diet), which is another important marine species in Japan (Biswas et al., 2020). It is also marginally lower than the results of 250 g protein kg-1 diet successful replacement demonstrated in rainbow trout (Perera et al., 1995) and Atlantic salmon reared in freshwater (Storebakken et al., 2004) and saltwater (Berge et al., 2005). However, the replacement level in this study was higher than that of the 90 g kg-1 diet reported in halibut (Aas et al., 2007). Therefore, the variation in the BPM utilization rate among different species may be related to species-specific sensitivity for this protein source or differences in the bacterial species composing different BPMs, experimental design and fish size among different studies.
Compared to the control group, a significant reduction in DFR was observed under all BPM-based diets (irrespective of replacement levels), suggesting that dietary palatability was reduced by replacing FM with BPM. It is considered that the unfavourable taste of plant protein-based diets reduces feed palatability and DFR, which is related to impaired growth performance in carnivorous fish (Nagel et al., 2012). Although single-cell protein sources did not affect the feed intake even at higher substitution levels in Japanese yellowtail (Biswas et al., 2020) and Atlantic salmon (Aas, Grisdale-Helland, et al., 2006; Berge et al., 2005; Storebakken et al., 2004), the results from this study were consistent with the findings for Atlantic halibut (Romarheim, 2002), where a high negative correlation between dietary single-cell protein level and feed intake was reported. The conflicting information regarding the palatability of BPM-based diets based on DFR data could be related to the fish species studied, the distinct members of the bacterial species found in different BPM products, age of the fish, the chemical forms of Cu used and other unknown dietary factors.
In general, BPM products contain high levels of Cu compared with FM because Cu is a required element for the methane-oxidizing enzymes of the bacteria (Murrell et al., 2000). In this study, although measurements of FE revealed no significant differences among the treatments, there was a significant linear reduction in the DFR with increasing levels of dietary Cu that resulted in lower growth performance with increasing levels of BPM. Although Cu is essential for normal growth and development in fish (Mohseni et al., 2014; Sabatini et al., 2009; Tan et al., 2011), Cu requirements differ between species and even among the different life stages of a single species (Berntssen et al., 1999; Clearwater et al., 2002; Gatlin & Wilson, 1986; Lorentzen et al., 1998; Ogino & Yang, 1980). While growth performance, including the DFR, was improved in red sea bream juveniles at 3.48 to 5.03 mg Cu kg-1 diet when nano-Cu was used, growth parameters, including the DFR, were reduced at >7 mg Cu kg-1 diet (El Basuini et al., 2016). Since the sources of Cu, feed formula, and fish size are different, it may not be fair to directly compare the results of this study with those reported by El Basuini et al. (2016). Nevertheless, the higher Cu levels in BPM-based diets in this study may be one of the reasons that affected the DFR. Moreover, Cu toxicity thresholds (as daily doses/kg body weight) differ between species as follows: >1 mg for channel catfish, 1–15 mg for Atlantic salmon depending on life stage and 44 mg for rainbow trout (Clearwater et al., 2002). The daily doses of Cu in this study were 3.8, 13.7, 26.1, 25.3, 27.8 and 34.4 mg kg body weight−1 day−1 in diets C, BP19, BP28, BP37, BP47 and BP56, respectively, when calculated from dietary Cu levels and DFR. Although these values are within the range provided for the different species mentioned above, the level of dietary Cu that is tolerable is dependent on copper bioavailability and waterborne copper concentrations, and Cu tolerance levels vary between aquatic species as well as between fish of the same species of different sizes and growth stages (Clearwater et al., 2002). However, the threshold for Cu toxicity from dietary intake still needs to be elucidated for red sea bream. It is also assumed that nucleic acid content is higher in BPM than in FM (Aas, Grisdale-Helland, et al., 2006). Irrespective of the beneficial role of nucleic acids in fish (Li & Gatlin, 2006), high nucleic acid levels (10 g kg-1 diet) may have negative effects on feed intake and growth performance (Tacon & Cooke, 1980). Although this study did not elucidate nucleic acid contents of products and diets, the lower DFRs in fish fed BPM-based diets may be due to the combined effects of Cu, nucleic acids or other unknown factors, which warrant further study for clarification.
It has been reported that the EPA and DHA dietary requirements for red sea bream are approximately 10 and 5 g kg-1 diet, respectively (Takeuchi et al., 1990). While the minimum dietary content of DHA (7 g kg-1 diet) in diet BP56 fulfils the requirement for this species, the control group (10.9 g kg-1 diet) only met the requirement level for dietary EPA content. However, the dietary contents of EPA in BPM-based diets ranged from 7.8 to 8.9 g kg-1 diet and marginally missed the requirement level. Moreover, Takeuchi et al. (1992) investigated the n-3 PUFA requirement levels for juvenile red sea bream fed on diets with varying lipid contents (100, 150 and 200 g kg-1 diet), and they found that the suitable levels ranged from 12 to 22 and 27 to 32 g kg-1 diet at dietary lipid levels of 100 and 150 g kg-1 diet, respectively. When these n-3 PUFA levels in the diet are expressed as a percentage of dietary lipids, they reach 120 to 220 and 180 to 210 g kg-1 deit at levels of 100 and 150 g lipid kg-1 diet, respectively (Takeuchi et al., 1992). The dietary lipid levels in this study ranged from 110.1 to 123.1 g kg-1 diet, while the percentages of n-3 PUFA ranged from 150 to 251 g kg-1 diet, which are within the range reported by Takeuchi et al. (1992). Therefore, the levels of the important and essential FAs in the diets are unlikely to be the reason for reduced growth in juvenile red sea bream fed diets with increasing levels of BPM.
When fed single-cell protein-containing diets, reduced growth in tilapia may have resulted from AA imbalance (Davies & Wareham, 1988). It has also been reported that methionine supplementation improves the performance of rainbow trout fed with this protein source (Murray & Marchant, 1986). Although the levels of almost all AAs decreased in BPM-based diets of red sea bream, the lowest levels of arginine (27.2 g kg-1 in diet BP47), lysine (35.3 g kg-1 in diet BP19) and valine (23.5 g kg-1 in diets BP28 and BP47) were higher than their reported respective requirements of 23.7 g kg-1 diet (Rahimnejad & Lee, 2014), 25 g kg-1 diet (Forster & Ogata, 1998) and 9 g kg-1 diet (Rahimnejad & Lee, 2013) for this species. If other AA requirements are compared with the values estimated by Forster and Ogata (1998) based on the lysine requirement of juvenile red sea bream, almost all IAAs in our diets, except the combined amount of methionine and cysteine, exceeded the estimated requirement levels. Moreover, the IAA index was more than 92% in all BPM-based diets compared with the FM-based diet. A previous study reported that the growth performance of juvenile red sea bream was not affected when the IAA index was approximately 88% in a diet containing soy protein concentrate as a protein source derived from soymilk (Biswas et al., 2019). Therefore, lower dietary IAA levels are likely not the reason for poor growth under high BPM diets. Although the levels of dietary alanine, arginine, glutamic acid, lysine and valine, which have been reported as feeding stimulants for red sea bream (Fuke et al., 1981), were higher than the reported requirement levels for this species, this study did not measure the free AA contents in the different diets. It may be necessary to elucidate whether free AA content affects the DFR under diets with higher BPM content.
Although 280 g kg-1 diet replacement of FM protein by BPM did not significantly affect protein and lipid digestibility in this study, and protein retention was not significantly different between the treatments, diets with more than 280 g kg-1 diet replacement levels significantly reduced the growth performance. This is attributed to the significantly decreasing trend in DFR with the increasing level of FM replacement, as described earlier. This means that the fish attempted to utilize an ingested diet through proper digestion and retention into the body until a certain level of replacement was achieved. However, the ingested diet may have taken a long time to digest properly, resulting in lower feed intake. Therefore, the variation in growth performance is directly related to the variation in DFR. However, the tendency towards poorer digestibility of nutrients in this study when substituting more FM with increasing amounts of BPM concurs with the results of previous studies on Atlantic salmon (Aas, Grisdale-Helland, et al., 2006; Berge et al., 2005; Storebakken et al., 2004) and rainbow trout (Aas, Hatlen, et al., 2006). As reported for rainbow trout (Kiessling & Askbrandt, 1993; Rumsey et al., 1991), the inferior nutrient digestibility of BPM-based diets compared with high-quality FM diets may be ascribed to bacterial cell walls and membranes that are resistant to enzymatic digestion. The retention efficiency observed in this study is also consistent with the results observed in Atlantic salmon (Storebakken et al., 2004). In contrast, Aas et al. (2007) reported a marked reduction in protein/nitrogen retention in halibuts fed an 180 g kg-1 BPM diet.
Similar to that of proteins, the ADC of all IAAs and DAAs, except lysine, tended to significantly decrease as the level of BPM in the treatment diet increased. However, even in treatments with high levels of dietary BPM, the ADC of almost all IAAs and DAAs showed markedly higher values compared with those of protein. Since protein was calculated from nitrogen content, which contains some non-protein nitrogen, the higher ADCs indicate that AAs are digested more efficiently than the nitrogen from non-protein fraction, which is in agreement with the results for Atlantic salmon (Aas, Grisdale-Helland, et al., 2006; Skrede et al., 1998). However, another study in rainbow trout did not find a significant difference in the digestibility of nitrogen and AAs (Øverland et al., 2006). This discrepancy may be related to species-specific differences in either their response to different protein sources or their digestion efficiencies (Skrede et al., 1980). Like other studies using similar protein source to replace FM, the protein content was determined from nitrogen content through indirect method (Kjeldahl's method) with subsequent conversion using a nitrogen-to-protein conversion factor of 6.25. This factor leads to an overestimation of protein content (Imafidon & Sosulski, 1990; López et al., 2010; Mæhre et al., 2018; Mariotti et al., 2008), which may lead to a variation between the ADC of protein and AAs. Therefore, it may be necessary to revise the method of protein determination to calculate a true level of protein utility from BPM in future endeavour.
There were no major variations in almost all blood parameters, which are considered important indicators of general health in fish, except at the plasma cholesterol level. Among blood parameters, GOT and GPT are often used to evaluate liver function, and the deficiency of IAAs in diet increased blood levels of these two enzymes. Therefore, it is difficult to assume that BPM affects the health conditions of fish used in this study, as there were no significant differences in either parameter. However, a significantly decreasing trend in TCHO levels with increasing levels of BPM is a matter of concern. TCHO is important for maintaining bile acid levels in enterohepatic circulation, and bile acids are very important in lipid digestion and absorption. Bile acids are synthesized from TCHO in the liver and secreted into the duodenum as a major component of bile but are then reabsorbed in the small intestine, and 95% of these acids are returned to the liver and re-synthesized into bile acids (Konishi & Nabeya, 2013). It has been reported that soy protein has the effect of inhibiting the digestion and absorption of lipids by binding to bile acids (Watanabe et al., 2013). When the reabsorption of bile acids in the intestinal tract is inhibited, it results in excessive mobilization of TCHO to bile acid synthesis, leading to lower plasma TCHO levels. The BPM may somehow interrupt the enterohepatic circulation to inhibit the reabsorption of bile acids in the intestinal tract of red sea bream at higher replacement levels, resulting in a lower TCHO level in plasma. This, in turn, could have affected the digestion of lipids more than that of protein in diets with higher BPM content in this study, as indicated in the results.
In conclusion, the results suggest that, at best, 190 g of FM protein kg-1 diet can be replaced by the BPM used in this study without affecting growth and feed efficiency. The inferior growth in diets with more than 280 g kg-1 diet replacement levels of FM protein by BPM may be mainly related to the significantly low DFR. Therefore, the results indicate the need for additional studies to elucidate whether the utility of BPM products can be further increased by improving the product quality, reducing the Cu content in the product or supplementing the diet with palatability enhancers.
ACKNOWLEDGEMENT
The expenses of this study were partly defrayed by Marubeni Nisshin Feed Co., Ltd. (Tokyo, Japan) through a collaborative research project between Aquaculture Research Institute, Kindai University and Nisshin Marubeni Feed Co., Ltd.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




