Dietary manganese levels influence growth, manganese bioaccumulation and expression of genes involved in antioxidant response of swimming crab (Portunus trituberculatus)
Abstract
An 8-week feeding trial was conducted to investigate the effects of dietary manganese (Mn) levels on growth performance, Mn bioaccumulation in tissues and antioxidant capacity in juvenile swimming crab (Portunus trituberculatus). Six semipurified experimental diets were formulated to contain graded levels of Mn (manganese sulphate monohydrate as Mn source); the analysed Mn concentrations were 9.8, 20.2, 29.7, 38.2, 65.6 and 123.6 mg/kg. The percent weight gain (PWG) and specific growth rate (SGR) were significantly affected by dietary Mn levels; the highest PWG and SGR were found in crabs fed with the diet containing 38.2 mg/kg Mn. Crabs fed with the diet containing 123.6 mg/kg Mn had higher Mn concentration in muscle and carapace than those fed with the other diets. However, the highest Mn concentration in hepatopancreas was recorded in crabs fed with the diet containing 65.6 mg/kg Mn. Moreover, the lowest activities of manganese superoxide dismutase (Mn-SOD), catalase (CAT) and total antioxidant capacity (T-AOC) occurred at crabs fed with the dietary 9.8 mg/kg Mn. Crabs fed with the 9.8 mg/kg Mn diet exhibited lower relative expression of genes involved into antioxidant ability such as cytosolic manganese superoxide dismutase (cMnsod), glutathione peroxidase (gpx), peroxiredoxin (prx) and catalase (cat) in hepatopancreas than those fed with the other diets, and the highest expression were recorded in crabs fed with the diet containing 38.2 mg/kg Mn. Based on two slope broken-line and quadratic regression analysis of PWG against dietary Mn levels, the optimum dietary Mn requirement was estimated to be 48.02 and 53.30 mg/kg for juvenile Portunus trituberculatus. The present study provided further insight into the function of dietary Mn in crustacean.
1 INTRODUCTION
Trace elements, as cofactors and activators of a variety of hormones and enzymes, involved in multiple biochemical processes which are essential nutrients for organisms (Lall, 2002). As a vital trace element, manganese (Mn) is widely distributed in all tissues of bacteria, plants and animals (Hänsch & Mendel, 2009). It is essential for the normal growth, reproduction and skeletal development; it also performs some functions either as a cofactor in some enzyme systems, including numerous enzymes that form metal-enzyme complexes, or as an integral part of certain metalloenzymes in protein, lipid and carbohydrate metabolism (Aschner & Aschner, 2005; Keen et al., 1984). All of the oxidoreductases, transferases, lyases, hydrolases, isomerases and ligases belong to Mn-dependent enzyme families (Aschner & Aschner, 2005). Mn metalloenzymes included Mn superoxide dismutase (Mn-SOD), arginase, glutamine synthetase and phosphoenolpyruvate decarboxylase (Aschner & Aschner, 2005; Munder, 2009; Zafar & Khan, 2019). Dietary Mn deficiency signs exhibited reduced growth, poor feed intake, cataract, skeletal malformations, increased whole body lipid and decreased Mn-SOD activity (Zhang et al., 2016; Antony Jesu Prabhu et al., 2019; Zafar & Khan, 2019). Moreover, dietary Mn deficiencies were also reported to decrease the natural killer cell activity of leukocyte in rainbow trout Oncorhynchus mykiss, but that activity was recovered with Mn supplementation (NRC, 2011). Although Mn is essential for various physiological activities, excessive Mn accumulation in tissue can result in severe toxicity (Chen, 2018). Excessive Mn concentration in water can cause negative effects including impact on carbohydrate metabolism in freshwater perch, Colisa fasciatus (Nath & Kumar, 1987), suppressed fundamental immune mechanisms in Norway lobsters, Nephrops norvegicus (Hernroth et al., 2004), and negative effects on the growth and survival of mulloway, Argyrosomus japonicus (Partridge & Lymbery, 2009).
Generally, Mn concentration in seawater is very low (0.01 mg/L); Mn present in seawater is insufficient to satisfy the metabolic requirements of marine crustacean or fish; therefore, dietary Mn supplementation is necessary for marine crustacean and fish (NRC, 2011). Until now, dietary optimal Mn requirement for crustacean ranged from 16 and 80 mg/kg, such as 32 mg/kg for Pacific white shrimp Litopenaeus vannamei (Cai et al., 2016; Liu & Lawrence, 1997), 16 mg/kg for giant freshwater prawn Macrobrachium rosenbergii (Asaikkutti et al., 2016), 30.34 mg/kg for Chinese mitten crab Eriocheir sinensis (Zhao et al., 2017) and 17.59 mg/kg for oriental river prawn Macrobrachium nipponense (Ding et al., 2019). The results indicated that dietary optimal Mn could improve growth performance, enhance antioxidant enzymes activities and increase Mn accumulation in tissues (Liang et al., 2015; Nie et al., 2016).
As a commercially typical marine crab species, swimming crab is widely distributed in the Pacific and Indian oceans (Hamasaki et al., 2011). Due to the success of artificial propagation, intensive artificial aquaculture has become the main mode of culture in swimming crab (Jin et al., 2013; Luo et al., 2020). However, feeding with trash fish or shellfish for swimming crabs may result in environmental damage and spread of infectious diseases (Jin et al., 2013). Therefore, studies focusing on the nutritional requirement of swimming crab are indispensable to develop cost-effective and nutritionally balanced artificial feed for swimming crab (Jin et al., 2013). In the past decade, the optimal requirement for macronutrients including protein, amino acids, lipids and vitamins has been determined for juvenile swimming crab (Han et al., 2018; Jin et al., 2013, 2016; Sun et al., 2020; Wang et al., 2016). Nevertheless, few studies have focused on trace elements requirements of swimming crab. Therefore, the objectives of the present study were to determine the dietary optimal Mn requirement and to evaluate the effects of dietary Mn levels on growth performance, Mn bioaccumulation in tissue, antioxidant enzymes activities and relative expression of genes involved into antioxidant ability for juvenile swimming crab.
2 MATERIALS AND METHODS
2.1 Ethics statement
All experimental procedures complied with the Standard Operation Procedures (SOPs) of the Guide for Use of Experimental Animals of Ningbo University. The study was approved by the Ethics-Scientific Committee for Experiments on Animals of Ningbo University.
2.2 Diet preparation
Dietary protein and lipid levels can meet the normal growth requirements of swimming crab (Yuan et al., 2019). Six isonitrogenous (~450.0 g/kg crude protein) and isolipidic (~100.0 g/kg crude lipid) experimental diets were formulated to contain different Mn levels (MnSO4·H2O as Mn source, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China); the dietary Mn concentrations were analysed to be 9.8 (basal diet), 20.2, 29.7, 38.2, 65.6 and 123.6 mg/kg (Table 1). The experimental diets were produced according to the method described in detail previously (Luo et al., 2020). Briefly, the ground ingredients were blended in a Hobart-type mixer and cold-extruded pellets produced (F-26, Machine factory of South China University of Technology) with pellet strands cut into two uniform sizes (3- and 5-mm diameter pellets) (G-250, Machine factory of South China University of Technology). Pellets were heated for 30 min at 90°C and then air dried to ~10 100 g/kg moisture, sealed in vacuum-packed bags and stored at −20°C until used in the feeding trial.
| Ingredients | Dietary Mn levels (mg/kg) | |||||
|---|---|---|---|---|---|---|
| 9.8 | 20.2 | 29.7 | 38.2 | 65.6 | 123.6 | |
| Peru fish meal | 400.0 | 400.0 | 400.0 | 400.0 | 400.0 | 400.0 |
| Casein | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Krill meal | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Wheat flour | 282.0 | 282.0 | 282.0 | 282.0 | 282.0 | 282.0 |
| Fish oil | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soy oil | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean lecithin | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Vitamin premixa | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Mineral premixb | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Ca(H2PO4)2 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Choline chloride | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Sodium alginate | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| MnSO4·H2O (mg/kg) | 0.0 | 30.8 | 61.5 | 92.3 | 184.6 | 369.1 |
| Proximate composition (g/kg) | ||||||
| Dry matter | 928.1 | 916.5 | 915.7 | 913.3 | 920.7 | 923.8 |
| Crude lipid | 103.0 | 107.9 | 99.4 | 98.6 | 106.9 | 104.6 |
| Crude protein | 452.4 | 464.3 | 463.5 | 463.0 | 456.6 | 459.2 |
| Ash | 90.5 | 93.0 | 91.8 | 98.2 | 94.5 | 95.8 |
| Analysed Mn (mg/kg) | 9.8 | 20.2 | 29.7 | 38.2 | 65.6 | 123.6 |
- a Vitamin premix supplied the diet with (g/kg vitamin premix) retinyl acetate, 1.2121; cholecalciferol, 1.2000; all-rac-a-tocopherol, 20.0000; menadione, 9.0909; thiamine, 10.8696; riboflavin, 7.5000; ascorbic acid, 30.0000; pyridoxine hydrochloride, 12.1212; cyanocobalamin, 2.0000; folic acid, 40.0000; biotin, 12.5000; nicotinic acid, 40.4040; D-Ca pantothenate, 16.1290; inositol, 204.0816; and cellulose, 592.8915.
- b The mineral premix provided as follows (g/kg mineral premix): CuSO4·5H2O (99%), 6.61; ZnSO4·7H2O, 9.43; FeC6H5O7, 4.57; MgSO4·7H2O (99%), 238.97; KH2PO4, 233.2; NaH2PO4, 137.03; C6H10CaO 6·5H2O (98%), 34.09; and CoSO4·7H2O (99.5%), 1.60. The mineral premix does not supply Mn.
2.3 Feeding trial and experimental conditions
Swimming crab juveniles were obtained from Jia-Shun Aquatic Cooperative of Taizhou (Zhejiang, China). Prior to the feeding trial, the crabs were acclimated to (rear individually in single crab unit [33 × 22.5 × 25 cm]) a continuous flow-through water system and fed a commercial diet (450.0 g/kg crude protein, 80.0 g/kg crude lipid, Evergreen Feed Co. Ltd., Zhanjiang, China) for 2 weeks. A total of 180 juvenile crabs (initial weight, 9.00 ± 0.14 g) were randomly allocated to 180 single crab units, 10 crabs corresponding to triplicate replicates for each of the six dietary treatments. Crabs were hand-fed twice daily at 8:00 am and 6:00 pm for 8 weeks with a daily ration of 3%–6% of crab weight, and the daily ration was adjusted according to consumption and residual feed in order to maintain a level of apparent satiation. Faeces and residual feed were removed by siphon each morning. During the experimental period, temperature ranged from 26.0℃ to 29.9℃, salinity ranged from 26 to 28 mg/L, pH ranged from 7.7 to 8.0 and dissolved oxygen ranged from 6.5 to 7.0 mg/L.
2.4 Sample collection
At the end of feeding trial, all crabs were weighed individually and counted to obtain the survival, percent weight gain (PWG), specific growth rate (SGR), feed efficiency (FE) and moulting rate (MR). Haemolymph samples were taken from the pericardial cavity of six crabs per replicate by using 1-ml syringes, sorted into 1.5-ml centrifuge tubes and centrifuged at 4℃, 956 g for 10 min by centrifuge. The supernatant was collected and stored at −80℃ prior to further analysis. Hepatopancreas from the same six crabs per replicate were dissected and collected into two 1.5-ml centrifuge tubes immediately, frozen in liquid nitrogen and stored at −80℃ for analysis of enzyme activities and gene expression. Hepatopancreas, muscle and carapace from the same crabs were collected and stored at −80℃ to analyse Mn contents in tissues.
2.5 Determination of tissues composition
2.5.1 Proximate composition analysis
Moisture contents in diets, hepatopancreas and muscle samples were measured by drying the samples to a constant weight at 105℃ which were determined by standard methods of the Association of Official Analytical Chemists (AOAC, 2006). Crude lipid in diets, hepatopancreas and muscle samples was determined by the ether extraction method using Soxtec method (Soxtec System HT6, Tecator, Sweden) (AOAC, 2006). Crude protein in diets, hepatopancreas and muscle samples was determined according to the Dumas combustion method with a protein analyser (FP-528, Leco, USA), and ash content in diets, hepatopancreas and muscle samples was measured using a muffle furnace at 550℃ for 8 h (AOAC, 2006).
2.5.2 Mn concentration analysis
The hepatopancreas, muscle and carapace were weighed and freeze dried before acid digestion, where samples were digested in 68% HNO3 solution at 80℃, with the acid solution added drop-wise until complete digestion of organic matter. The digested solution was filtered through an aqueous phase syringe filter. Mn concentrations in diets and tissue were measured by inductively coupled plasma optical emission spectrometry (ICP-OES, PE2100DV, Perkin Elmer, USA; Luo et al., 2020).
2.6 Haemolymph biochemical analysis
The contents of glucose (GLU), cholesterol (CHO), low-density lipoprotein-CHO (LDL) and high-density lipoprotein-CHO (HDL) and the activity of gamma-glutamyl transpeptidase (GGT) were analysed using commercial kits (Biosino Bio-Technology and Science Inc., China) according to the manufacturer's instructions by an automatic biochemistry analyser (VITALAB SELECTRA Junior Pros, the Netherlands).
2.7 Enzymes activities analysis
Hepatopancreas samples were homogenized in nine volumes (w/v) of ice-cold physiological saline 0.89% (w/v). Samples were centrifuged at 956 g for 10 min at 4℃, and then the supernatant were collected for enzymes assays. Total antioxidant capacity (T-AOC), Mn-SOD, total superoxide dismutase (T-SOD), catalase (CAT) and malondialdehyde (MDA) in hepatopancreas, as well as T-AOC, Mn-SOD, T-SOD and MDA in haemolymph, were analysed by using commercial kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's instructions by Multiskan spectrum (Thermo, USA).
2.8 Gene expression
Gene expression was determined by reverse-transcriptase quantitative polymerase chain reaction (qPCR). RNA extraction, reverse transcription and real-time qPCR were carried according to methods described in detail previously (Luo et al., 2020). Briefly, total RNA of hepatopancreas were extracted by using TRIzol Reagent (Vazyme, China) according to manufacturer's instructions. Quality and quantity were assessed by 1.2% agarose gel electrophoresis and spectrophotometer NanoDrop 2000 (Thermo Fisher Scientific, USA), respectively. RNA samples were reverse transcribed to cDNA using the PrimeScript® RT reagent kit (Vazyme, China) following the manufacturer's protocol. The specific primers for the candidate genes were designed by Primer Premier 5.0 software (Table 2). Amplification efficiency was performed using the equation: E = 10(–1/Slope) −1 (Jothikumar et al., 2006), and the amplification efficiencies of all genes were approximately equal and ranged from 99% to 106%. PCR amplification was performed using a quantitative thermal cycler (Lightcycler 96, Roche, Switzerland), with each reaction containing 0.4 μl of each primer, 10 μl of 2× ChamQ SYBR qPCR Green Master Mix (Vazyme), 0.8 μl of one-eighth diluted cDNA and 8.4 μl DEPC-water. The qPCR procedure contained an initial activation step at 95°C for 2 min, followed by 45 cycles of 95°C for 10 s and 58°C for 10 s, and 72°C for 20 s. Relative expression levels of the target genes were computed by way of 2–ΔΔCt (Livak & Schmittgen, 2001). The beta-actin gene was used as the house-keeping gene, and the 9.8 mg/kg treatment group was used as the control/reference group.
| Genes | Nucleotide sequence (5′–3′) | Size (bp) | GenBank NO/references |
|---|---|---|---|
| cMn sod | F: GATCCATCACACCAAGCACC | 193 | FJ031018.1 |
| R: GTTCTCCTCCAGCTTCAGGT | |||
| cat | F: GCTCTGACCCTGACTATGCT | 180 | FJ152102.2 |
| R: CGACCAACAGGAATTAGCGG | |||
| prx | F: TGACCAGCAACATGAGCAAC | 215 | FJ174664.1 |
| R: CAATCTTGCGGAACTCCTCG | |||
| gpx | F: TACCGTTCAGCAAGTACCGT | 171 | KY216076.1 |
| R: GTGGTGTTTTCCTGGTGACC | |||
| β-actin | F: GAAGTAGCCGCCCTGGTTGTG | Pan et al. (2010) | |
| R: GGGTCAGAATACCTCGCTTGCTC |
- Abbreviations: cat, catalase; cMn sod, cytosolic manganese superoxide dismutase;gpx, glutathione peroxidase; prx, peroxiredoxin.
2.9 Calculations and statistical analysis
- Absolute weight gain (AWG, g) = final body weight − initial body weight;
- PWG (%) =100 × (final body weight − initial body weight) / initial body weight;
- Specific growth ratio (SGR, %/day) =100 × (Ln (final body weight) – Ln (initial body weight)) / t;
- Survival (%) =100 × (final number of crabs) / (initial number of crabs);
- FE = weight gain (g, wet weight) / feed consumed (g, dry weight);
- MR =2 × Moulting times / (final number of crabs + initial number of crabs);
- Manganese retention rate (MRR, %) =100 × (Wt × Mt – Wi × Mi) / (Wd × Md).
Wt is the final tissue weight (g), Wi is the initial tissue weight (g), Mt is the final Mn concentration (mg/kg), Mi is the initial Mn concentration (mg/kg), Wd is the weight of fed diet (g) and Md is the Mn concentration of the diet (mg/kg). The calculation of these parameters was based on three experimental replicates per diet.
Results were presented as means ± standard error of the mean (SEM) of three replicates (n = 3). Data were checked for normality and homogeneity of variances and were normalized when appropriate. The relative gene expression results (qPCR analyses) were expressed as mean normalized ratios corresponding to the ratio between the copy numbers of the target genes and the copy numbers of the reference gene, beta-actin. The homogeneity of variances between means values were compared by one-way analysis of variance (ANOVA) followed by Tukey's multiple-range test (SPSS Statistics 20, Chicago, USA).
3 RESULTS
3.1 Growth performance and feed utilization
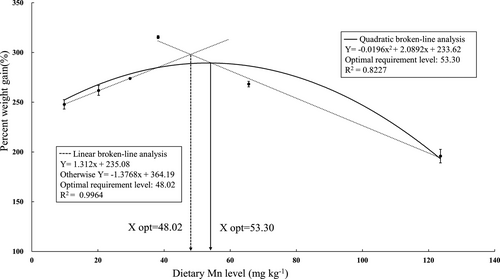
The effects of different dietary Mn levels on growth performance, feed utilization, survival and MR in juvenile swimming crab are showed in Table 3. Mean final weight (MFW), AWG, PWG and SGR significantly increased with dietary Mn levels increasing from 9.8 to 38.2 mg/kg and then significantly decreased with dietary Mn levels increasing from 38.2 to 123.6 mg/kg; the highest MFW, AWG, PWG and SGR were observed in crabs fed with the diet containing 38.2 mg/kg Mn (p < .05). Survival ranged from 63.3% to 86.7%; crabs fed with the diet containing 38.2 mg/kg Mn had significantly higher survival than those fed with the 123.6 mg/kg Mn diet. However, no significant differences in FE and MR were found among all treatments (p > .05). Based on two slope broken-line and quadratic regression analysis of PWG against dietary Mn levels, the Mn requirements for maximum PWG was estimated to be 48.02 and 53.30 mg/kg for juvenile swimming crab (Figure 1).
| Parameters | Dietary Mn levels (mg/kg) | |||||
|---|---|---|---|---|---|---|
| 9.80 | 20.20 | 29.70 | 38.20 | 65.60 | 123.60 | |
| Mean initial weight (g) | 9.34 ± 0.36 | 9.44 ± 0.32 | 9.13 ± 0.37 | 8.51 ± 0.30 | 8.62 ± 0.33 | 8.94 ± 0.35 |
| Mean final weight (g) | 31.14 ± 2.03ab | 34.49 ± 1.44b | 34.29 ± 0.80b | 38.35 ± 1.80b | 35.14 ± 0.73b | 24.58 ± 2.06a |
| Weight gain (g) | 21.56 ± 1.89ab | 24.96 ± 0.98bc | 25.13 ± 0.55bc | 29.15 ± 1.38c | 25.61 ± 0.60bc | 17.05 ± 1.18a |
| PWG (%) | 247.79 ± 4.69b | 261.88 ± 4.95bc | 273.89 ± 0.63c | 315.41 ± 1.46d | 268.27 ± 2.79c | 195.83 ± 6.77a |
| SGR (%/day) | 2.17 ± 0.04b | 2.29 ± 0.02bc | 2.34 ± 0.01c | 2.54 ± 0.004d | 2.31 ± 0.01c | 1.83 ± 0.03a |
| Survival (%) | 73.33 ± 3.33ab | 76.67 ± 3.33ab | 73.33 ± 3.33ab | 86.67 ± 3.33b | 76.67 ± 6.67ab | 63.33 ± 3.33a |
| FE | 0.66 ± 0.07 | 0.62 ± 0.03 | 0.66 ± 0.02 | 0.68 ± 0.08 | 0.69 ± 0.01 | 0.59 ± 0.03 |
| MR (%) | 1.59 ± 0.03 | 1.89 ± 0.16 | 1.92 ± 0.11 | 1.78 ± 0.22 | 1.75 ± 0.08 | 1.74 ± 0.14 |
Note
- Data are presented as means ± SEM (n = 3). Values in the same row with different superscript letters are significantly different (p < .05) as determined by analysis of variance and Tukey's test.
- Abbreviations: FE, feed efficiency; MR, moulting rate; PWG, percent weight gain; SGR, specific growth ratio.
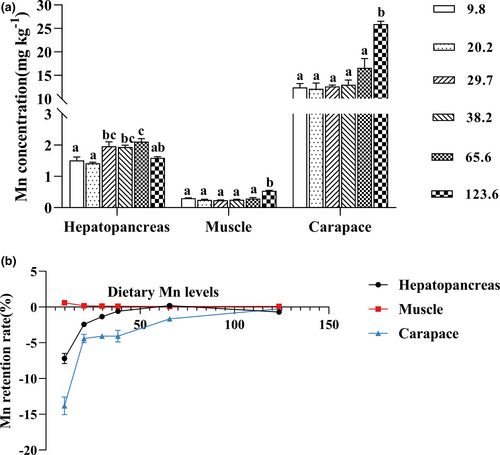
3.2 Proximate compositions and Mn concentration in tissues
Crabs fed with the diet containing 123.6 mg/kg Mn showed higher Mn concentrations in muscle and carapace than the other treatments (p < .05); however there were no significant differences of Mn concentrations in muscle and carapace among those dietary Mn levels ranging from 9.8 to 65.6 mg/kg. However, Mn concentration in hepatopancreas markedly increased with dietary Mn levels increasing from 9.8 to 65.6 mg/kg, and the highest concentration of Mn was found in crabs fed with the diet containing 65.6 mg/kg Mn (p < .05) (Figure 2a). The MRR in hepatopancreas and carapace significantly increased with increase of dietary Mn levels; however, MRR in muscle significantly decreased with dietary Mn contents increasing from 9.8 to 123.6 mg/kg (Figure 2b).
Moisture, protein and lipid contents in muscle and hepatopancreas were not significantly affected by dietary Mn levels (p > .05) (Table 4).
| Parameters (g/kg) | Dietary Mn levels (mg/kg) | |||||
|---|---|---|---|---|---|---|
| 9.80 | 20.20 | 29.70 | 38.20 | 65.60 | 123.60 | |
| Muscle | ||||||
| Moisture | 804.3 ± 3.7 | 810.9 ± 6.5 | 792.4 ± 8.5 | 799.4 ± 4.3 | 810.6 ± 6.6 | 808.6 ± 3.1 |
| Protein | 166.8 ± 1.0 | 160.4 ± 7.1 | 174.1 ± 6.0 | 168.5 ± 3.1 | 160.3 ± 4.6 | 157.5 ± 2.4 |
| Lipid | 22.8 ± 1.3 | 22.8 ± 1.5 | 24.3 ± 0.9 | 23.3 ± 1.2 | 23.4 ± 0.3 | 20.4 ± 0.5 |
| Hepatopancreas | ||||||
| Moisture | 711.3 ± 6.5 | 707.9 ± 3.9 | 690.2 ± 7.3 | 697.2 ± 9.0 | 700.6 ± 2.3 | 719.1 ± 11.0 |
| Protein | 116.7 ± 2.5 | 125.9 ± 5.6 | 117.1 ± 2.7 | 119.1 ± 2.9 | 119.5 ± 3.5 | 116.4 ± 4.0 |
| Lipid | 149.9 ± 12.2 | 170.7 ± 11.3 | 185.4 ± 12.5 | 170.7 ± 6.8 | 189.1 ± 8.8 | 171.0 ± 10.6 |
Note
- Data are presented as means ± SEM (n = 3). Values in the same row with different superscript letters are significantly different (p < .05) as determined by analysis of variance.
3.3 Haemolymph biochemical analysis
Haemolymph biochemical parameters of crabs fed with diets containing different Mn levels are presented in Table 5. GGT and HDL in haemolymph were not significantly influenced by dietary Mn levels (p > .05). GLU, LDL and CHO contents in haemolymph significantly increased with dietary Mn levels increasing from 9.8 to 29.7 mg/kg but then significantly decreased with dietary Mn levels increasing from 38.2 to 123.6 mg/kg (p < .05).
| Treatments | Dietary Mn levels (mg/kg) | |||||
|---|---|---|---|---|---|---|
| 9.80 | 20.20 | 29.70 | 38.20 | 65.60 | 123.60 | |
| GGT (U/L) | 8.23 ± 0.52 | 7.09 ± 0.60 | 7.66 ± 0.35 | 7.86 ± 0.09 | 7.10 ± 0.12 | 8.61 ± 0.18 |
| GLU (mmol/L) | 0.74 ± 0.01ab | 0.71 ± 0.07ab | 0.93 ± 0.07b | 0.89 ± 0.04b | 0.58 ± 0.06a | 0.55 ± 0.03a |
| CHO (mmol/L ) | 0.18 ± 0.02ab | 0.18 ± 0.02ab | 0.29 ± 0.02c | 0.25 ± 0.02bc | 0.26 ± 0.02bc | 0.16 ± 0.01a |
| HDL (mmol/L ) | 0.82 ± 0.01 | 0.82 ± 0.01 | 0.83 ± 0.01 | 0.85 ± 0.01 | 0.84 ± 0.01 | 0.84 ± 0.01 |
| LDL (mmol/L ) | 0.87 ± 0.01a | 0.85 ± 0.02a | 0.92 ± 0.01b | 0.90 ± 0.01ab | 0.90 ± 0.01ab | 0.87 ± 0.01a |
Note
- Data are presented as means ± SEM (n = 3). Values in the same row with different superscript letters are significantly different (p < .05) as determined by analysis of variance and Tukey's test.
- Abbreviations: CHO, cholesterol; GGT, gamma-glutamyl transpeptidase; GLU, glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride.
3.4 Antioxidant enzyme activities in haemolymph and hepatopancreas
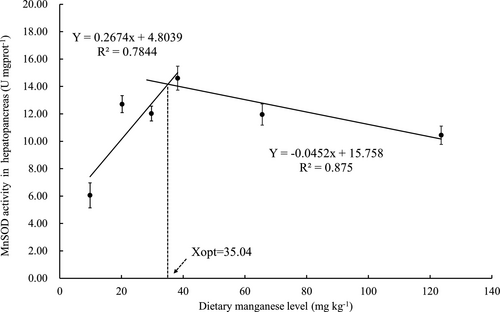
The activities of antioxidant enzymes in hepatopancreas and haemolymph of crabs fed with the experimental diets are exhibited in Figure 3a,b. The highest activity of CAT in hepatopancreas was observed in crabs fed with diet containing 65.6 mg/kg Mn. Crabs fed with the diets containing 29.7 and 38.2 mg/kg Mn exhibited significantly higher T-AOC activities than those fed with the other diets (p < .05). Crabs fed with the diet containing 123.6 mg/kg Mn were significantly lower T-SOD activity compared with those fed with the other diets (p < .05). Activity of Mn-SOD increased as dietary Mn levels increasing from 9.8 to 38.2 mg/kg mg/kg and then decreased as dietary Mn levels increasing from 38.2 to 123.6 mg/kg. MDA concentration in hepatopancreas was not significantly affected by dietary Mn levels. Based on two slope broken-line analysis of Mn-SOD activitiy in hepatopancreas against dietary Mn levels, the Mn requirements for maximum Mn-SOD was estimated to be 35.04 mg/kg for juvenile swimming crab (Figure 4).
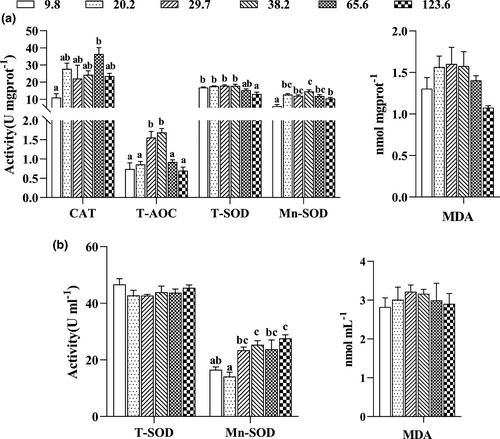
Crabs fed with the diets containing 9.8 and 20.2 mg/kg Mn exhibited significantly lower Mn-SOD activity in haemolymph than those fed with the other diets (p < .05). However, activity of T-SOD and content of MDA in haemolymph were not significantly influenced by dietary Mn levels (p > .05).
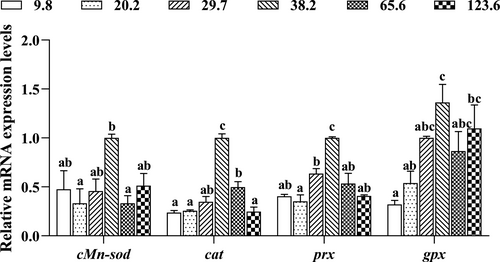
3.5 Expression of genes involved into antioxidant in hepatopancreas
The expression levels of genes related to antioxidant system in hepatopancreas are shown in Figure 5. The highest expression levels of cMn-sod, cat, prx and gpx were found in crabs fed with the diet containing 38.2 mg/kg Mn (p < .05). The mRNA expression levels of those genes in hepatopancreas were significantly upregulated as dietary Mn levels increased from 9.8 to 38.2 mg/kg and then downregulated as dietary Mn levels increased from 38.2 to 123.6 mg/kg.
4 DISCUSSION
The results of present study indicated that crabs fed with the Mn deficient diet exhibited poor growth, reduced Mn concentration in tissues and activities of Mn-SOD, CAT and T-AOC in hepatopancreas. Mn deficiency signs have been also observed in several crustacean species, such as giant freshwater prawn, oriental river prawn and Chinese mitten crab (Asaikkutti et al., 2016; Ding et al., 2019; Zhao et al., 2017). Similar studies in fish were also reported that dietary Mn deficiency lead to poor growth, reduced whole body and vertebrae Mn concentration, increased lipid content in whole body and decreased hepatic Mn-SOD activity, such as gibel carp (Carassius auratus gibelio), grouper (Epinephelus coioides), yellow catfish (Pelteobagrus fulvidraco), cobia (Rachycentron canadum) and stinging catfish (Heteropneustes fossilis) (Liu et al., 2018; Pan et al., 2008; Tan et al., 2012; Ye et al., 2009; Zafar & Khan, 2019). Moreover, in the present study, crabs fed with the diet containing 123.6 mg/kg had the lowest MFW, weight gain, PWG and SGR among all treatments, but even more important, crabs fed with the diet containing 123.6 mg/kg Mn had lower MFW, WG, PWG and SGR than those fed with the deficient Mn diet (9.8 mg/kg). The results indicated that excessive Mn levels were more toxic than those caused by deficient Mn diet. Based on the above results, it may be concluded that dietary Mn is essential and adequate inclusion is necessary for optimum growth, and retarded growth in swimming crab may be attributed to deficient or excessive Mn levels.
In the present study, PWG and SGR significantly increased with dietary Mn levels increasing from 9.8 to 38.2 mg/kg, and crabs fed with the diet containing 123.6 mg/kg Mn had lower PWG and SGR compared with those fed with the other diets. Based on two slope broken-line and quadratic regression analysis of PWG against dietary Mn levels, the optimum dietary Mn requirements for maximum PWG was estimated to be 48.02 and 53.30 mg/kg for juvenile swimming crab, respectively. This requirement value was higher than those studies reported in fish which ranged from 2.4 to 24.93 mg/kg (Domínguez et al., 2020; Liu et al., 2013; NRC, 2011; Prabhu et al., 2016; Tan et al., 2012; Tang et al., 2016; Zafar & Khan, 2019; Zhang et al., 2016). In comparison, some studies on optimal Mn requirement in crustacean were similar to the results of present study. The Mn requirements for juvenile Pacific white shrimp and Chinese mitten crab were 32.26 and 30.34 mg/kg, respectively (Cai et al., 2016; Zhao et al., 2017). Previous results suggested that crustaceans have a higher requirement for Mn than fish. Meanwhile, Mn can be added in diets with different forms such as inorganic salts and amino acids chelated salts; the absorption and utilization of different forms of Mn for animals are different (Katya et al., 2016). For example, in the form of MnSO4⸱H2O, Mn requirement was estimated to be 10.5, 46.3 or 12.9 mg/kg for turbot (Scophthalmus maximus) based on the SGR, vertebra Mn concentration or liver Mn-SOD activity; however, the Mn requirement was evaluated to be 7.6, 43.0 or 22.5 mg/kg for turbot fed amino acid chelated Mn, respectively (Ma et al., 2014). Moreover, the optimal dietary Mn requirement was estimated to be 32.26 mg/kg manganese sulphate (MnSO4) and 23.90 mg/kg manganese methionine (Mn-Met) for Pacific white shrimp. These results indicated that different Mn sources affected the Mn requirement of Pacific white shrimp (Cai et al., 2016). Therefore, the factors that may affect the minimal dietary levels of mineral and trace element to fish or crustaceans can be one or a combination of the following: biological factors such as species, life stage, sex, trophic level, feeding habits and the nutritional status; dietary factors such as diet composition, availability and nutrient interactions; and environmental factors such as water mineral (or trace element) concentration, salinity and temperature of the rearing system (Prabhu et al., 2016).
In the present study, data obtained for PWG and Mn-SOD activity in hepatopancreas against dietary Mn levels were subjected to quadratic and/or two slope broken-line regression analysis which reflected a high value of the coefficient of determination (R2). Based on the above analysis, the optimal dietary Mn requirement were estimated to range between 35.04 and 53.30 mg/kg. Previous studies have also reported the dietary optimal Mn requirement for other crustacean ranged from 16 and 80 mg/kg (NRC, 2011); the results of present study are similar to those of the previous study for crustaceans. The main reason for this wide variation in requirements reported for different species may be the differences in crustacean species, stage of life, experimental design, diets, dietary interactions, form of supplemented Mn, rearing water Mn concentration, response variables and statistical models (Zafar & Khan, 2019).
In the present study, crabs fed with the diet containing 123.6 mg/kg Mn had higher Mn concentration in muscle and carapace than those fed with the other diets; the results indicated that there were differences of Mn accumulation in the tissues, and the order of Mn accumulation from high to low was carapace, hepatopancreas and muscle. At the same time, the trend of accumulation in different tissues is not the same; there is tissue specificity; the Mn content in carapace and muscle was positively correlated with the dietary Mn levels. Similar results were also found in Pacific white shrimp (Cai et al., 2016) and Chinese mitten crab (Zhao et al., 2017). However, Mn concentration in hepatopancreatic increased when crabs were fed with the diets ranging from 9.8 to 65.6 mg/kg Mn and then decreased with the increasing of dietary Mn levels. Similar studies showed that Mn levels in the whole body and vertebrae increased at first and then plateaued once the dietary Mn optimal requirement is achieved (Liu et al., 2013; Maage et al., 2000). Moreover, the Mn retention rate in hepatopancreas and carapace significantly increased with increase of dietary Mn levels; however, MRR in muscle significantly decreased with dietary Mn contents increasing from 9.8 to 123.6 mg/kg. Previous studies indicated that the maintenance of stable tissue Mn level through tight homeostatic control, intestine absorption and endogenous excretion, aquatic animal can enhance Mn absorption and reduce Mn excretion to compensate dietary Mn deficiency (Malecki et al., 1996; Zafar & Khan, 2019).
The superoxide dismutase (SOD) is one of the principal defence systems against oxygen free radicals (Fang et al., 2002). SOD removes O2− by greatly accelerating its conversion to H2O2 (Aruoma, 1998). Animal cells contain two SOD forms, which are Cu/Zn-SOD and the Mn-SOD forms. Meanwhile, copper, zinc and manganese are essential metals for the Cu/Zn-SOD and Mn-SOD activities (Aruoma, 1998; Fattman et al., 2003). As a consequence, dietary deficiencies of these trace elements will decrease the activities of Cu/Zn-SOD and Mn-SOD in tissues and cause peroxidative damage and mitochondrial dysfunction (Fang et al., 2002). CATs in peroxisomes convert H2O2 into water and O2 and helps to dispose of H2O2 generated by the action of the oxidase enzymes that are located in these organelles (Valko et al., 2007). T-AOC, which represents the total level of antioxidation, directly reflected the antioxidant capacity of crustacean (Hong et al., 2018). MDA is produced during oxidative damage-induced lipid peroxidation (Raharjo & Sofos, 1993). In the present study, dietary optimal Mn levels significantly could enhance the activities of antioxidant defence system such as Mn-SOD, CAT and T-AOC in hepatopancreas. In addition, the results of present study also indicated that crabs fed with Mn deficient diet had the lowest Mn-SOD activity in haemolymph; the results demonstrated that dietary Mn deficiency could induce oxidative stress. However, MDA content in hepatopancreas and haemolymph had no significant difference among all treatments. Moreover, the lowest activity of T-SOD in hepatopancreas was found in crabs fed with diet containing 123.6 mg/kg Mn. Some studies had demonstrated that dietary excessive Mn level will suppress Mn-SOD activity in fishes (Lin et al., 2008; Liu et al., 2013; Tan et al., 2012) and crustaceans (Cai et al., 2016; Ding et al., 2019; Zhao et al., 2017). Mn is indispensable metal for the activity of Mn-SOD, and therefore, dietary deficient or excessive Mn markedly decreased tissue Mn-SOD activity and resulted in peroxidative damage and mitochondrial dysfunction (Aruoma, 1998). Previous study also showed that dietary Mn deficiency resulted in the depression of antioxidant enzyme activity to impair the antioxidant ability for grass crap (Tang et al., 2016). Similar results were observed in stinging catfish which reported that lowest T-SOD, Mn-SOD and CAT activities were occurred at stinging catfish fed with Mn-deficiency diet (Zafar & Khan, 2019). Acute exposure of goldfish to Mn induced a significant increase of activities in SOD, CAT in serum, suggesting that Mn2+ exposure caused a generalized oxidative stress which leads to activation of protective mechanisms necessary for scavenging of produced O2− radicals in erythrocytes (Aliko et al., 2018). Moreover, the phenomenon of excess dietary Mn that leads to decreased T-SOD activity was also found in Pacific white shrimp, which may demonstrate that excess Mn can also cause oxidative stress (Cai et al., 2016). Therefore, the results of present study indicated that crabs fed with the Mn deficient or excessive diets confronted with oxidative stress, which consisted with suppressed antioxidant responses.
The gene cytosolic Mn-SOD (cMnsod) is only found in crustaceans (Li et al., 2010), and the function of cytoplasmic Cu Zn-SOD protein was substituted by the cytoplasmic Mn-SOD in crustaceans (Brouwer et al., 2003). Peroxiredoxin (prx), a ubiquitous antioxidant enzyme, possesses a basic catalytic mechanism (Wood et al., 2003). Glutathione peroxidase (gpx), a selenium-containing peroxidase, involved in cell antioxidant (Arthur, 2001). In the present study, crabs fed with diet containing 38.2 mg/kg Mn exhibited the higher expression levels of cMnsod, cat, prx and gpx in hepatopancreas compared with those fed with the other diets. These results were consistent with the trend of Mn-SOD, CAT and T-AOC activities in hepatopancreas, which indicated a positive correlation between antioxidative gene expression and enzymatic activities. The expression of cMnsod in the hepatopancreas increased with increase of dietary Mn levels and then plateaued in Pacific white shrimp (Cai et al., 2016) and Eriocheir sinensis (Zhao et al., 2016); similar results was also observed in present study which indicated the dietary optimal Mn levels could activate expression of cMnsod. Moreover, it has been reported that expression of cMnsod significantly decreased with increase of dietary Mn levels over 30 mg/kg Mn in gilthead sea bream (Domínguez et al., 2020), and similar results were found in the present study; these results may suggest a reduction in oxidation resistance with excessive Mn in diet. Other genes such as cat, prx and gpx showed the same trend with cMnsod, which were upregulated in crabs fed with diets containing 20.2 to 38.2 mg/kg Mn then downregulated with the further increase of dietary Mn levels. Thus, it is concluded that the expression of antioxidant genes in swimming crab could be improved by adding proper amount of Mn in diets.
5 CONCLUSION
In conclusion, it can be concluded that dietary Mn levels significantly influenced growth performance, Mn bioaccumulation in tissue and expression of genes involved in antioxidant response of swimming crab. The results also clearly demonstrated that swimming crab have a dietary Mn requirement for growth and proper physiological function, dietary deficient or excessive Mn levels resulted in reduced growth and induced oxidative stress. Moreover, crabs fed with optimal Mn diets could improve the expression levels and activities of antioxidant enzymes, therefore, enhance the antioxidant defence of swimming crab.
ACKNOWLEDGEMENTS
This study was supported by National Natural Science Foundation of China (32072987), Nature Science Foundation of Zhejiang Province (LY21C190006), China Agriculture Research System-48 (CARS-48), National Key R&D Program of China (2018YFD0900400), Industrial chain Collaborative Innovation Project of the Demonstration Work on Innovative Development of The Marine Economy of the State Oceanic Administration (NBHY-2017-S2), Key Research Program of Zhejiang Province of China (2018C02037) and Zhejiang Aquaculture Nutrition & Feed Technology Service Team (ZJANFTST2017-2). This study was also sponsored by the K. C. Wong Magna Fund in Ningbo University. The authors graciously thank Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences (NIMTE, CAS) for use of the ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometer, PE2100DV, Perkin Elmer, USA).
CONFLICT OF INTEREST
The authors have declared that no competing interests exist.
AUTHOR CONTRIBUTION
Q. C. Z., M. J. and X. Y. H. conceived and designed the research; X. Y. H. and B. S. conducted the research; X. Y. H. and M. J. performed the statistical analysis; X. Y. H. and B. S. contributed reagents/materials/analysis tools; X. Y. H., X. X. W., Y. Y., J. X. L., X. C., M. M. Z. and L. F. J. made the diets; X. Y. H., B. S., X. X. W., Y. Y., J. X. L., P. S., X. C. and M. M. Z. collected the samples; and X. Y. H., B. S., M. J., Y. Y., P. S. and Q. C. Z. wrote the paper. All authors contributed to manuscript revision, read and approved the submitted version.




