Study supplementation of astaxanthin in high-fat diet on growth performance, antioxidant ability, anti-inflammation, non-specific immunity and intestinal structure of juvenile Trachinotus ovatus
Abstract
A 6-week feeding experiment was conducted to evaluate whether dietary supplementation of astaxanthin could mitigate negative effects of high-fat diet on growth performance, antioxidant capacity, anti-inflammation, non-specific immunity and intestinal structure of Trachinotus ovatus. Three isonitrogenous diets were formulated supplementing with or without high-fat or astaxanthin (lipid positive (LP): 130 g/kg lipid, lipid negative (LN): 210 g/kg lipid, lipid negative astaxanthin (LNA): 210 g/kg lipid with 0.5 g/kg astaxanthin). Results showed that the growth performance (weight gain (WG) and specific growth rate (SGR)) of T. ovatus in the LN group were significantly lower than that of growth performance in the LP group (p < .05), while growth performance data in the LNA group were significantly higher than those data in the LN group (p < .05) and without statistical difference with the LP group (p > .05). There were significantly lower levels of antioxidant contents (malondialdehyde (MDA) and Glutathione (GSH)) and transcriptional level of antioxidant-related gene (Nrf2, keap1, Mn-SOD, GSH-PX, HO-1 and CAT) in the LNA group when compared to the LN group (p < .05), while no statistical differences were detected between the LNA and LP groups (p > .05). NF-κB signal pathway key gene (p65) and non-specific immunity-related genes (IRF3, IRF7 and MyD88) of T. ovatus were significantly lower in the LNA and LP groups when compared to the LN group (p < .05). Fewer hepatic lipid droplets and more goblet cells of mid-gut were found in T. ovatus from the LNA group than those from the LN group. These results indicate that a diet supplementation of 0.5 g/kg astaxanthin mitigates detrimental effects of T. ovatus growth performance caused by high-fat diet by decreasing hepatic lipid accumulation and improving antioxidant, anti-inflammation, non-specific immunity and intestine barrier capacities.
1 INTRODUCTION
When farming fishes, the largest proportion of cost is spent on the diet, meaning that diet is generally regarded as the most expensive item in aquaculture. A balanced mixture of proteins, lipids and carbohydrates is a common choice in fish diets to provide energy because of the relatively low price. Regarding carnivorous fishes, they prefer lipid as an energy source rather than carbohydrates due to an inadequate utilization capacity of carbohydrates (Council, 2011). Previous reports demonstrated that an optimal lipid level could spare proteins and enhance the growth performance of fishes (Chatzifotis et al., 2010; Cho & Kaushik, 1990; Kaushik & Medale, 1994). On the contrary, excessive lipids in the diet may cause several disadvantages, including severe lipid accumulation in hepatic and abdominal tissues, causing metabolic disturbances (Rueda-Jasso et al., 2004), inflammation (Xie et al., 2020), oxidative stress (Guo et al., 2019) and stress to the intestine (Trenzado et al., 2018), eventually inhibiting the growth performance of fishes.
Astaxanthin, found in some yeast, algae and marine aquatic animals (Ambati et al., 2014), has been intensively investigated in recent years. Dietary supplementation of astaxanthin provides several benefits to marine animals, including the promotion of growth performance (Cheng et al., 2018), pigmentation (Song et al., 2017), and enhancements of their antioxidative and anti-inflammatory capacities under stress (Yang et al., 2013). Moreover, astaxanthin regulates lipid metabolism in terrestrial animals (Gao et al., 2020; Wang, Liu, et al., 2019; Wang, Ishikawa, et al., 2019). However, there are limited data on the role of astaxanthin on lipid metabolism in aquatic animals.
Trachinotus ovatus belongs to the Carangidae family and it is a vital economic sea fish raised in southern China (Liu et al., 2019; Yin et al., 2015). This species has many advantages, such as high nutritional value and rapid growth, thus being widely consumed (Tan et al., 2018). However, to decrease the budget, high-fat diet has been widely given to T. ovatus, causing high mortality rates and economic losses. Therefore, in this study, astaxanthin was supplemented in the high-fat diet of fishes to study its effect on growth performance, antioxidant capacity, anti-inflammatory activity, non-specific immunity and intestine structure of T. ovatus. These results may offer a reference to high-fat diet formulas of T. ovatus.
2 MATERIALS AND METHODS
2.1 Experimental diet
Three dietary interventions were performed and are shown in Table 1: lipid positive (LP): 130 g/kg lipid group, lipid negative (LN): 210 g/kg lipid group and lipid negative astaxanthin (LNA): 210 g/kg lipid with 0.5 g/kg astaxanthin (Carophyll Pink, 10% astaxanthin, DSM) group.
| Ingredients | LP | LN | LNA |
|---|---|---|---|
| Fish meal | 32 | 32 | 32 |
| Soybean meal | 22 | 22 | 22 |
| Microcrystalline cellulose | 7.05 | 0.05 | 0 |
| Wheat four | 10.75 | 10.75 | 10.75 |
| Krill meal | 3 | 3 | 3 |
| Peanut meal | 10 | 10 | 10 |
| Fish oil | 8 | 15 | 15 |
| Soy lecithin | 2 | 2 | 2 |
| Ca(H2PO4)2 | 2 | 2 | 2 |
| Previtamina | 1 | 1 | 1 |
| Premineralb | 1 | 1 | 1 |
| Choline | 0.5 | 0.5 | 0.5 |
| DL-Met | 0.3 | 0.3 | 0.3 |
| Lys-HCL (99%) | 0.4 | 0.4 | 0.4 |
| Astaxanthinc | 0 | 0 | 0.05 |
| Sum | 100 | 100 | 100 |
| Nutrient levelsd | |||
| Moisture | 9.22 | 9.48 | 9.15 |
| Crude protein | 40.13 | 40.26 | 39.94 |
| Crude lipid | 13.58 | 20.42 | 20.51 |
- a Vitamin premix provides the following per kg of diet: VB1 25 mg, VB2 45 mg, pyridoxine HCl 20 mg, VB12 0.1 mg, VK3 10 mg, inositol 800 mg, pantothenic acid 60 mg, niacin acid 200 mg, folic acid 20 mg, biotin 1.20 mg, retinal acetate 32 mg, cholecalciferol 5 mg, α-tocopherolα 120 mg, ascorbic acid 2000 mg, choline chloride 2,500 mg, ethoxyquin 150 mg, wheat middling 14.012 g.
- b Mineral premix provides the following per kg of diet: NaF 2 mg, KI 0.8 mg, CoCl2·6H2O 50 mg, CuSO4·5H2O 10 mg, FeSO4·H2O 80 mg, ZnSO4·H2O 50 mg, MnSO4·H2O 60 mg, MgSO4·7H2O 1,200 mg, Ca(H2PO4)2·H2O 3,000 mg, NaCl 100 mg, zeolite 15.447 g.
- c Carophyll pink from DSM Nutritional Products France, 10% astaxanthin.
- d Measured values.
Dietary lipid levels were determined according to the published manuscript of Fang et al. (2021). Diets were prepared and processed according to the methods of Zhao et al. (2020). Briefly, all dry ingredients were finely ground through a 60-mesh screen, thoroughly mixed and homogenized in a Hobart-type mixer (A-200T Mixer Bench Model unit, Resell Food Equipment Ltd.). Soybean lecithin was added to fish oil and mixed until homogenous. Then, the oil mix and distilled water were added to the mixture. The 2.5-mm-diameter puffed pellets were produced by using a puffing apparatus (Institute of Chemical Engineering, South China University of Technology). The pellets were then dried in a forced-air oven at 40°C and then stored at −20°C until used.
2.2 Fish breeding and management
Experimental fishes were obtained and cultured at the Chinese Academy of Fishery Science (Lingshui, Hainan). Before the dietary intervention, juvenile T. ovatus were cultured in sea cages with the LP group diet for acclimatization for 2 weeks, and then fishes were starved for 1 day. Afterwards, 240 fishes (average weight 7.26 ± 0.11 g) were stochastically distributed to 12 sea cages (1 × 1 × 2 m). Fishes were fed twice 1 day (9:00 and 16:00) slowly until they stopped eating to prevent waste (Liu et al., 2018). Fishes were fed their respective diets for 6 weeks. The weight of feeding diet, number and weight of dead fishes were recorded for every cage every day. Water temperature was maintained between 28 and 31°C. Water quality was maintained as follows: PH, 8.1–8.2; NH4, <0.01 mg/L; dissolved oxygen, >8.33 mg/L; salinity, 28–31 g/L.
2.3 Sample collecting
After the dietary intervention, fishes were starved for 1 day and the total weight and number of fishes in each cage were recorded. Seven fishes were selected stochastically from each cage and anaesthetized (MS-222, Sigma) for sampling. Four fishes were put in ice for whole-body inspection, while three fishes were euthanized and the hepatic tissue was separated and quickly frozen into liquid nitrogen quickly to determine enzyme levels and gene expression. Meanwhile, the intestinal and hepatic tissues were separated and fixed in 4% paraformaldehyde for morphology assessment.
All of the procedures were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH). All research protocols were approved by Experimental Animal Ethics Committee of Sun-Yat San University.
2.4 Detection of fish body composition
Crude protein, crude lipid and moisture of the fish body and diet were detected by the AOAC method (Horwitz, 2010). Briefly, crude protein content was detected using the Kjeldahl method (1030-Auto-analyzer, Tecator), crude lipid was determined by the Soxhlet extractor method (Soxtec System HT6, Tecator) and moisture was analysed by drying in an oven at 105°C to acquire dry weight.
2.5 Detection of hepatic antioxidant enzymes
Hepatic samples were homogenized and centrifuged based on the methods of Zhao et al. (Zhao et al., 2020). Supernatants were collected to measure hepatic antioxidant index, including superoxide dismutase (SOD) (A001-1), total antioxidant capacity (T-AOC) (A015-2-1), malondialdehyde (MDA) (A003-1) and Glutathione (GSH) (A006-2). The analysis of enzyme activities was performed based on manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute).
2.6 Histological evaluation of hepatic and intestinal tissues
The staining method to evaluate liver and intestine tissues was performed according to our previous report(Zhao et al., 2020). Briefly, tissue sections (mid-gut and liver, 0.5 μm thick) were stained using haematoxylin and eosin. Afterwards, the intestinal and hepatic morphologies were inspected with an optical microscope (Olympus CKX41 microscope). The length of intestine villi, thickness of muscle and number of goblet cells were assessed as described by Zhao et al. (2020).
2.7 RNA extraction and gene expression analysis
Total RNA was extracted from the liver using Trizol® reagent (Invitrogen) based on the manufacturer's instructions. A 1% agarose gel electrophoresis and spectrophotometer (NanoDrop 2000, Thermo Fisher) were used to assess RNA quality and concentration, respectively. Afterwards, cDNA was synthesized using a PrimeScript TM RT reagent kit (Takara), based on the manufacturer's instructions. More specifically, we mixed the following compounds in a centrifuge tube: oligo dT primers, PrimeScriptTM RT enzyme Mix I, 5 *PrimeScriptTM buffer, Random 6 mers and RNase-free dH2O. Reverse transcription was performed at 37°C for 15 min followed by inactivation at 85°C for 5 s. Primers used in our study are shown in Table 2.
| Gene name | Primer sequence (5′−3′) |
|---|---|
| β-actin F | TACGAGCTGCCTGACGGACA |
| β-actin R | GGCTGTGATCTCCTTCTGCA |
| Nrf2 F | TTGCCTGGACACAACTGCTGTTAC |
| Nrf2 R | TCTGTGACGGTGGCAGTGGAC |
| GSH-PX F | GCTGAGAGGCTGGTGCAAGTG |
| GSH-PX R | TTCAAGCGTTACAGCAGGAGGTTC |
| CAT F | GGATGGACAGCCTTCAAGTTCTCG |
| CAT R | TGGACCGTTACAACAGTGCAGATG |
| Keap1 F | CAGATAGACAGCGTGGTGAAGGC |
| Keap1 R | GACAGTGAGACAGGTTGAAGAACTCC |
| HO-1 F | AGAAGATTCAGACAGCAGCAGAACAG |
| HO-1 R | TCATACAGCGAGCACAGGAGGAG |
| Mn-SOD F | CCTCATCCCCCTGCTTGGTA |
| Mn-SOD R | CCAGGGAGGGATGAGAGGTG |
| P65 F | CGTGAGGTCAGCGAGCCAATG |
| P65 R | ATGTGCCGTCTATCTTGTGGAATGG |
| IL-1β F | CGGACTCGAACGTGGTCACATTC |
| IL-1β R | AATATGGAAGGCAACCGTGCTCAG |
| IκB F | GCTGGTCCATTGCCTCCTGAAC |
| IκB R | GTGCCGTCTTCTCGTACAACTGG |
| IKK F | CCTGGAGAACTGCTGTGGAATGAG |
| IKK R | ATGGAGGTAGGTCAGAGCCGAAG |
| MyD88 F | AATACCTTGACAGCGATGCCTG |
| MyD88 R | GTGCAAGGCCTGGTGTAATCA |
| IRF3 F | GCTGGGATCCGCTTAGTCTACA |
| IRF3 R | GCCCAGCTTGTCCAGGATG |
| IRF7 F | CGAATACACCAACCGCATCCT |
| IRF7 R | AGCTTTCTTGGGTCTGGGTCA |
2.8 Statistical analysis
Data are shown as means ± standard error (SE). Data were analysed by SPSS 22.0 (SPSS) and differences between groups were determined by the one-way analysis of variance (ANOVA) and Duncan's test. p < .05 was considered statistically significant.
3 RESULTS
3.1 Growth performance of T. ovatus
The growth performance of T. ovatus fed with or without high-fat diet and astaxanthin are shown in Table 3. Weight gain (WG) and specific growth rate (SGR) of T. ovatus in the LN group was significantly lower than those of T. ovatus in the LP group, while data from the LNA group was significantly higher than those from the LN group (p < .05). In parallel, we observed no statistical difference in growth performance between LP and LNA groups (p > .05). After 6 weeks of dietary intervention, the survival rate (SR) of all groups was higher than 90% and no statistical differences were detected (p > .05). Different dietary interventions did not alter the feed conversion rate (FCR) of T. ovatus (p > .05).
| LP | LN | LNA | |
|---|---|---|---|
| IBW (g) | 7.24 ± 0.07 | 7.29 ± 0.08 | 7.23 ± 0.08 |
| WGR (%) | 123.54 ± 10.7b | 81.81 ± 9.2a | 139.71 ± 11.33b |
| SGR (%) | 1.91 ± 0.11b | 1.42 ± 0.12a | 2.08 ± 0.11b |
| SR (%) | 96 ± 2.92 | 91 ± 1.87 | 96.25 ± 2.39 |
| FCR | 1.47 ± 0.03 | 1.44 ± 0.02 | 1.45 ± 0.02 |
Note
- Values are mean ± SE (n = 4). Means in the same row with different superscripts are significantly different (p < .05).
- a IBW (g per fish): initial mean body weight.
- b Weight gain rate (WGR, %) = 100 * (final body weight−initial body weight)/initial body weight.
- c Specific growth rate (SGR, % day-1): 100 × (Ln final fish weight−Ln initial fish weight)/the experimental duration in days.
- d Survival rate (SR) (%) = 100 * (final number of fish)/(initial number of fish).
- e Feed conversion ratio (FCR) = dry diet fed/wet weight gain.
3.2 Whole-body composition
The whole-body composition of T. ovatus fed with or without high-fat diet and astaxanthin are shown in Table 4. The lipid content of T. ovatus fed a high-fat diet with astaxanthin (LNA group) was significantly lower than that of the LN group (p < .05), while it was higher than that of the LP group (p < .05). Regarding crude protein, data in the LN group was significantly lower than that of the LP group (p < .05), and no statistical differences were observed between the LNA group and LN groups (p > .05). No statistical difference was found in the moisture contents of T. ovatus between groups (p > .05).
| LP | LN | LNA | |
|---|---|---|---|
| Moisture (%) | 72.59 ± 0.56 | 70.88 ± 1.07 | 70.41 ± 0.3 |
| Lipid (%, DWB) | 25.51 ± 0.29a | 35.43 ± 0.69b | 32.32 ± 0.57c |
| Protein (%, DWB) | 56.76 ± 0.78b | 51.97 ± 1.00a | 54.59 ± 0.04ab |
Note
- Values are mean ± SE (n = 4). Means in the same row with different superscripts are significantly different (p < .05).
- Abbreviation: DWB, Dry weight basis.
3.3 Antioxidant capacity
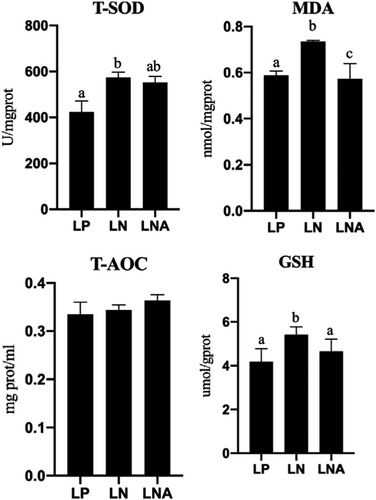
Results of dietary astaxanthin on antioxidant capacity of T. ovatus are depicted in Figure 1. The hepatic T-SOD of T. ovatus in the LN group was significantly higher than that of T. ovatus in the LP group (p < .05), while the LNA group showed lower level of hepatic T-SOD when compared to the LN group (p > .05). GSH in the LNA group was significantly lower than that in the LN group (p < .05), but without statistical difference with the LP group (p > .05). The lowest MDA levels were obtained in the LNA group among all treatments (p < .05). No statistical difference was found for T-AOC among groups (p > .05).
3.4 Expression of immunity-related genes
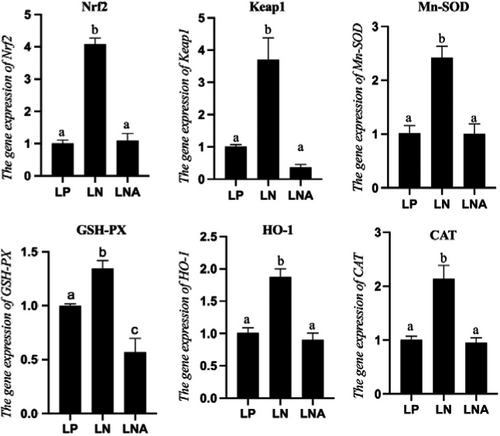
RNA expression levels of antioxidant-related genes were influenced by dietary interventions (Figure 2). Genes of the Nrf2-Keap1 signalling pathway, such as Nrf2, Keap1, manganese superoxide dismutase (Mn-SOD), glutathione peroxidase (GSH-Px), haeme oxygenase-1 (HO-1) and catalase (CAT), were significantly higher in the LN group when compared to the LP group (p < .05). Meanwhile, the above genes were lower in the LNA group when compared to the LN group (p < .05) while there were no statistical differences with the LP group (p > .05) except for GSH-PX.
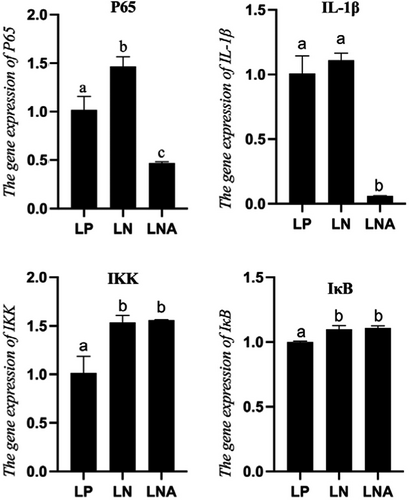
The RNA expression of genes related to the NF-κB signalling pathway is shown in Figure 3. The RNA expressions of transcription factor p65 (p65) and Interleukin 1 beta (IL-1β) of T. ovatus fed a high-fat diet were higher than those of the LP group, while diet supplementation with astaxanthin (LNA group) led to significantly lower levels than those of the LP and LN groups (p < .05). Regarding RNA expression of IκB kinase (IKK) and Inhibitor of κB (IκB), no statistical difference was observed between the LN and LNA groups (p > .05).
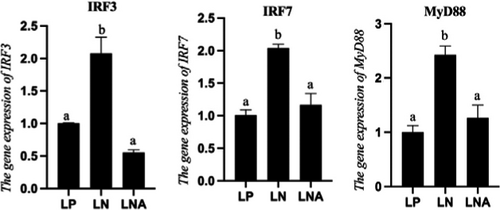
RNA expressions of non-specific immunologic genes were also influenced by dietary interventions (p < 0.05) (Figure 4). RNA expressions of IRF3, IRF7 and MyD88 of T. ovatus from the LN group were significantly higher than those of T. ovatus from the LP group (p < .05). Meanwhile, fishes in the LNA group had significantly lower levels of non-specific immunology related genes than those of the LN group (p < .05) whereas there was no statistical difference with the LP group (p > .05).
3.5 Hepatic and intestinal morphology
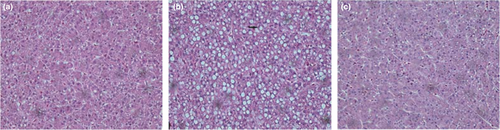
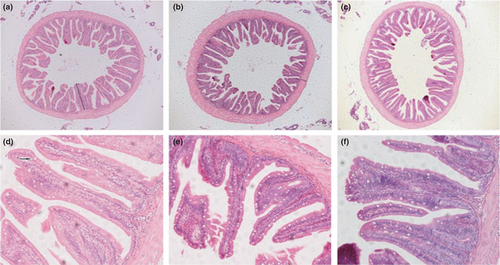
The hepatic morphology of T. ovatus from different experimental groups is shown in Figure 5. It was evident that T. ovatus fed a high-fat diet had more hepatic vacuole lipid droplets than those of the LP and LNA groups. In parallel, intestinal morphology and related data are depicted in Figure 6 and Table 5. A significant increase of goblet cells was observed in the LNA group when compared to the two other groups (p < .05). No statistical difference in villi length was found between the two high-fat diet treatment groups (p > .05). The muscular thickness of T. ovatus fed with high-fat diet (LN group) was significantly higher than that of T. ovatus in the LP and LNA groups (p < .05).
| LP | LN | LNA | |
|---|---|---|---|
| Villi length (um) | 365.19 ± 6.47a | 278.58 ± 8.9b | 296.75 ± 11.59b |
| Muscle thickness (um) | 81.07 ± 3.99a | 116.6 ± 9.84b | 73.85 ± 6.57a |
| Goblet cell | 23.5 ± 0.87a | 23.25 ± 0.63a | 34.5 ± 1.04b |
Note
- Values are mean ± SE. Means in the same row with different superscripts are significantly different (p < .05).
4 DISCUSSION
Lipids, which are a dietary energy resource, play a substantial role in fish growth. High utilization of dietary lipids is generally associated with protein-saving (Council, 2011; López et al., 2009). However, Excessive dietary fat might cause adverse effects to fish (Yin et al., 2021). Our present study showed that the growth performance (WG and SGR) of T. ovatus exposed to high-fat diet without astaxanthin was significantly lower than that of the LP group after 6 weeks of dietary intervention, indicating that excessive intake of lipids in the diet impairs the growth performance of T. ovatus. Several reports have shown impaired growth performance of fishes fed a high-fat diet, such as Ctenopharyngodon idellus (Li et al., 2016), Micropterus salmoides (Yin et al., 2021) and Micropterus salmoides (Zhou et al., 2020). There are two main reasons that high-fat diet causes lower growth performance. First, high-fat diet provides exceeding energy to fish, resulting in the reduction of food intake and lower absorption of other nutrients (Ding et al., 2020). On the other hand, excessive lipid intake may cause fat deposition in hepatic tissue, causing metabolic disturbances and reduction of liver health (Wang et al., 2005; Zhou et al., 2020). Our study demonstrated that T. ovatus in the LNA group had a similar growth performance when compared to the LP group, suggesting that dietary astaxanthin supplementation can prevent the detrimental impact caused by high-fat diet treatment on the growth performance of T. ovatus. These results may be a consequence of the enhanced lipid utilization and anti-stress properties of astaxanthin (Cheng et al., 2018; Cheng & Wu, 2019; Gao et al., 2020; Wang, Liu, et al., 2019; Wang, Ishikawa, et al., 2019). However, previous studies have reported that dietary astaxanthin supplementation did not affect the growth performance of fishes, such as Pagrus pagrus (Nogueira et al., 2021), Pagrus auratus (Doolan et al., 2008), Paralichthys olivaceus (Pham et al., 2014) and Sparus aurata (Wassef et al., 2010). These different results may be due to environmental conditions, different fish species and the dose of astaxanthin used.
Our study demonstrated that fishes fed 210 g/kg lipid had a higher lipid and lower protein concentration in their body when compared to fishes fed 130 g/kg lipid. These results are in accordance with data in Sebastes schlegeli (Lee et al., 2002), T. ovatus (Fang et al., 2021) and Scortum barcoo (Song et al., 2009). In our present study, an increased trend of protein and significant reduction of whole-body lipids were found for the LNA group when compared to the LN group, meaning that astaxanthin plays a substantial role in decreasing whole-body lipid contents and promoting protein deposition. Regarding liver morphology, we observed more vacuoles, which reflect lipid droplets (Li et al., 2019), livers of fishes fed a high-fat diet when compared to fishes from the LP group, while the LNA group had less vacuoles. These results showed that 0.5 g/kg astaxanthin could decrease hepatic lipid droplet accumulation under a high-fat diet intervention.
Previous literature has reported that high-fat diet intake might cause lipid accumulation and peroxidation, leading to the generation of reactive oxygen species (ROS) and inflammatory response (Milagro et al., 2006; Vial et al., 2011). T-SOD is a vital enzyme in the antioxidant system, while GSH is a non-enzymatic antioxidant (Zhai et al., 2018). Both of them play a significant role in scavenging ROS and alleviate cells from oxidative stress (Lesser, 2006; Misra & Niyogi, 2009; Nan et al., 2004). On the other hand, the accumulation of MDA, a lipid peroxidation product, may cause cell toxicity (Tsikas, 2017). In our present study, higher hepatic T-SOD and GSH were found for T. ovatus fed a 210 g/kg diet lipid compared to those of fishes fed a 130 g/kg diet lipid, indicating that T. ovatus respond to adverse effects of high-fat dietary by improving their antioxidant enzymatic activity. However, the increase of antioxidant enzyme capacity could not scavenge excessive ROS, resulting in increased MDA levels upon 210 g/kg lipid diet intake. Previous studies have reported that carotenoids could act as an antioxidant, thus scavenging ROS (Chisté et al., 2014; Rodrigues et al., 2012). Here we showed lower levels of T-SOD, GSH and MDA in the LNA group when compared to those of the LN group, indicating that T. ovatus were not exposed to oxidative stress caused by high-fat diet treatment because astaxanthin could scavenge ROS. As a consequence, fishes did not need to produce T-SOD and GSH. These results suggested that astaxanthin can protect T. ovatus from oxidative stress caused by high-fat diet.
The Nrf2-ARE pathway is key to prevent oxidative stress in various tissues by activating Nrf2 and upregulating the transcription level of antioxidant-related gene, such as Mn-SOD, GSH-PX, HO-1 and CAT (Qin et al., 2017; Shaw & Chattopadhyay, 2020). When cells do not exhibit oxidative stress, Nrf2 is coupled with Keap1, a negative regulator of Nrf2, in the cytoplasm (Tong et al., 2006). However, when cells suffer from various stresses, Nrf2 dissociates from Keap1 and enters into the nucleus (Osburn & Kensler, 2008). After entering the nucleus, Nrf2 binds to small Maf proteins as a dimer, resulting in the interaction of Nrf2 with antioxidant response element (ARE) and upregulating the transcription of antioxidant-related genes (Motohashi et al., 2004). Our present study showed that RNA expressions of antioxidative-related genes (Mn-SOD, GSH-PX, HO-1 and CAT) were significantly higher in the LN group than those of the LP and LNA groups. These results indicated that T. ovatus responded to oxidative stress by upregulating the Nrf2-ARE pathway, while astaxanthin protected fishes from the oxidative stress caused by high-fat diet.
Inflammatory responses of organisms can be stimulated by various factors, including cytokines and prostaglandins, resulting in several diseases (Chen et al., 2018). IL-1β, a predominant pro-inflammatory cytokine, plays a significant role in inflammatory responses (Kistowska et al., 2014). Our present findings show that the mRNA expression of IL-1β was significantly lower in the LNA and LP groups than in the LN group, indicating that astaxanthin down-regulated the RNA expression of IL-1β caused by high-fat diet. NF-κB signalling pathway is considered an essential mechanism associated with inflammatory diseases and regulate the transcription of cytokines, such as IL-1β (Libermann & Baltimore, 1990). Under normal physiological conditions, the p65/p50 heterodimer is restricted to the cytoplasm by an interaction with IκB. However, if cells are exposed to environmental stresses, the IKK signalosome activates and phosphorylates and then degrades IκB, resulting in the dissociation of the p65/p50 complex and translocate into the nucleus, which induce the expression of inflammatory-related gene (Gao et al., 2013; Giridharan & Srinivasan, 2018). Suppressing NF-κB pathway-related genes can decrease pro-inflammatory cytokines, resulting in a milder inflammatory response (Kim et al., 2013). In our present study, the RNA expression of p65 was significantly higher in the LN group when compared to that of the LP group, while supplementation of 0.5 g/kg astaxanthin could decrease the expression of genes related to the NF-κB signalling pathway. Altogether, this indicates that astaxanthin decreases inflammatory responses in fishes exposed to a high-fat diet.
Innate immunity is generally regarded as an initial self-protective response against invading microbes based on pattern recognition receptors (Meylan et al., 2006). The toll-like receptor (TLR) family, a vital pattern recognition receptor, regulates downstream defensive signals by two pathways, including the MYD88-dependent and the MyD88-independent TRIF-dependent pathways (Kim et al., 2018; Medzhitov, 2001). First, MyD88 interacts with corresponding IL-1R-associated kinases (Krishnan et al., 2007; Wesche et al., 1997). In addition, the TRIF adapter stimulates transcription factors and signalling pathways, such as IRF3 and IRF7 (Takeda & Akira, 2005). These two pathways cause the production of type I IFNs and inflammatory cytokines (Takeda & Akira, 2005). In our present study, high-fat diet intervention group presented higher mRNA expressions of MyD88, IRF3 and IRF7 than those of the LP group. In contrast, fishes fed a high-fat diet supplemented with astaxanthin maintained these markers at a low level, indicating that supplementation of 0.5 g/kg astaxanthin in high-fat diet was essential to enhance the non-specific immune ability of fishes exposed to high-fat diet by activating the MYD88-dependent and MyD88-independent/TRIF-dependent pathways.
A healthy intestine morphology reflects better capacity of nutrient absorption and immune barrier (Dawood, 2021). Higher villi and thicker muscle indicate better intestinal digestion and absorption of fishes (Chen & Wang, 2013). In our present study, no statistical difference was found for the villi length of the mid-gut between high-fat diet supplemented with or without astaxanthin, indicating that astaxanthin was unable to improve the intestinal structure of T. ovatus fed a high-fat diet. Interestingly, the muscle thickness of the intestines in the LN group was higher than that of the LP group. This suggests that the intestines have developed a thicker muscle to digest and absorb the high-fat diet (Chen et al., 2020). However, thinner muscles were seen in the LNA group when compared to those of the LN group, which may be associated with the improved hepatic capacity caused by astaxanthin. This resulted in lower lipid absorption through the intestines. Therefore, the muscle thickness of fishes fed a high-fat diet does not need to improve, although more research should investigate this in the future.
Goblet cells, the secretory cell distributed between epithelial cells, can enhance the barrier capacity by synthesizing and secreting mucin glycoproteins to cover the intestinal epithelium and prevent intestinal pathogens (Deplancke & Gaskins, 2001). In our present study, T. ovatus fed a high-fat diet with astaxanthin had the largest number of intestine goblet cells among all groups, indicating that dietary astaxanthin could enhance the barrier capacity of intestines caused by high-fat diet ingestion.
5 CONCLUSION
Overall, diet supplementation with 0.5 g/kg astaxanthin mitigates detrimental effects on growth performance of T. ovatus caused by high-fat diet ingestion by decreasing lipid accumulation in the hepatic tissue and improving the antioxidant, anti-inflammatory, non-specific immunity and intestine barrier abilities.
ACKNOWLEDGEMENTS
This research was supported by Project of National Natural Science Foundation of China (31872580), and Project of Science and Technology of Guangzhou City (201803020006), Project of Science and Technology of Guangdong Province (2019B110209005), and Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (2019KJ143).
CONFLICT OF INTEREST
No competing interests.
AUTHOR CONTRIBUTIONS
Thanks to my supervisors and colleagues who assisted my experiment. H-H F, Y-J L and L-X T designed the study. W Z carried out the feeding experiment. Z-L L analysed enzyme activities. H-H F, J-J X and J N analysed data. H-H F wrote this paper.
Open Research
DATA AVAILABILITY STATEMENT
The experiment data in this study are available from the corresponding author based on reasonable request.




