Co-feeding dry and live feed in first-feeding gilthead seabream: Effects on functional development of the digestive system, larvae and postlarvae performance
Abstract
In the intensive gilthead seabream larviculture practice, dry feeds are introduced not earlier than on 15 days post hatching (dph). The aim of the present study was to compare such a common protocol (DF15, Dry Feed introduced on 15 dph) with one in which dry feed is co-fed with live feed from the onset of the exogenous feeding (DF4, Dry Feed introduced on 4 dph). The evaluation focused on larvae performance and functional development of the digestive system up to weaning and first grading (Trial 1; conducted in the hatchery; larvae monitored from 5 to 60 dph; first grading on 62 dph), as well as on postlarvae performance (Trial 2; conducted in the laboratory; the graded larvae of each treatment were used; postlarvae growth monitored from 66 to 126 dph). Obtained results showed that introducing dry feed in first-feeding gilthead seabream larvae (DF4) did not affect larvae growth, survival and functional development of the digestive system. Indeed, growth enhancement and lower incidence of total phenotypical deformities are indicated on first grading. Moreover, postlarvae performance was improved. The present study indicates a viable feeding protocol for gilthead seabream larviculture and emphasizes the importance of larval rearing for subsequent production stages.
1 INTRODUCTION
Gilthead seabream Sparus aurata is an altricial species, that is, in larval stages, the onset of exogenous feeding happens before the complete digestive system maturation and the beginning of gastric digestion. Newly hatched larvae are too small and require even smaller feed particles. Since the 1970s, the use of live feeds like rotifers and Artemia nauplii/metanauplii was a critical breakthrough for altricial species successful larviculture and is still applied in commercial hatcheries in first-feeding larvae (Dhont et al., 2013). As the anatomical and functional development of the digestive system is achieved, live feed is gradually replaced by inert diets until weaning (Cahu & Zambonino-Infante, 2001; Rosenlund et al., 1997).
The use of dry instead of live feeds for altricial species larviculture has long been an ambitious expectation for reasons of stability in nutritional value, price and supply. Despite the technological advances in larval feed manufacture (i.e., small-sized particles meeting the nutritional needs, having high acceptability, palatability, water stability and low nutrient leaching) (Hardy & Barrows, 2002), efforts so far to replace live with dry feeds have not been successful. For example, larvae of Senegalese sole Solea senegalensis (Cañavate & Fernández-Díaz, 1999), gilthead seabream S. aurata (Fernández-Díaz & Yúfera, 1997), African catfish Clarias gariepinus (Chepkirui-Boit et al., 2011), black catfish Rhamdia quelen (Salhi & Bessonart, 2013), Nishikigoi Cyprinus carpio (Fosse et al., 2018) and red seabream Pagrus major (Khoa et al., 2020) fed exclusively with dry feeds from mouth opening showed very low growth and survival. These negative effects are considered to be related to the inefficiency of the majority of the newly hatched larvae to detect, ingest and digest the feed particles.
In the hatchery practice of marine altricial species, larvae are weaned to artificial diets after a period of co-feeding with live feed, usually after Artemia introduction. The time of weaning is species dependent, but the general idea is that the co-feeding period starts when larvae have attained a certain size and a developmental stage close to the onset of gastric digestion. It has been shown that the longer the co-feeding period, the better the larval performance at weaning (Baskerville-Bridges & Kling, 2000; Fosse et al., 2018; Hart & Purser, 1996). Co-feeding period of longer duration before weaning is thought to better acclimate larvae to feed particles, thus promoting their ingestion and to control the dependence of larvae on live feed (Baskerville-Bridges & Kling, 2000; Hart & Purser, 1996; Koven et al., 2001; Rosenlund et al., 1997). Moreover, co-feeding of Artemia and dry feed has been suggested to improve the efficiency of dry feeds due to specific nutrient factors found in live Artemia (Koven et al., 2001).
Only a limited number of research papers investigate the effect of co-feeding dry and live feed from as early as on mouth opening (loach Misgurnus anguillicaudatus: Wang et al., 2008; Wang et al., 2009; Senegalese sole: Cañavate & Fernández-Díaz, 1999; Engrola, Figueira, et al., 2009; Engrola, Mai et al., 2009; Engrola, Dinis et al., 2010; Mai et al., 2009; African catfish: Chepkirui-Boit et al., 2011; gilthead seabream: Fernández-Díaz & Yúfera, 1997, Pousão-Ferreira et al., 2003). Results obtained are not uniform probably due to species specific differences and variations in experimental laboratory conditions and/or feeding protocols of live and dry feeds. However, available data clearly suggest that introducing dry feed at the earliest life stages can affect, positively or negatively, both larvae and postlarvae performance (e.g., growth, survival, protein and lipid metabolism).
In commercial hatcheries, the widely adapted feeding practice for gilthead seabream larvae production includes the introduction of dry feed not earlier than on 15 days post hatching (dph) and a co-feeding period with live feed until weaning (58–60 dph). The aim of the present study was (a) to evaluate, under actual production conditions, the effects of co-feeding dry and live feed from mouth opening up to weaning on larvae performance (i.e., growth, survival and phenotypical deformities) and functional development of the digestive system and (b) to monitor postlarvae growth performance during pregrowing under common laboratory conditions.
2 MATERIALS AND METHODS
2.1 Ethical note
The study was carried out in accordance with the EU Directive 2010/63/EU, national laws (PD 160/91) for animal experiments and the Bioethics Committee of the Department of Animal Science (Agricultural University of Athens). None of the fish died or suffered from injuries during the experiments.
2.2 Experimental design
Two trials were conducted. Trial 1 (Hatchery rearing, Hr) was held in a commercial Greek marine fish hatchery (Selonda, Psachna, Evia) under actual production conditions. It is here that the experimental treatments were assigned and larvae performance was monitored from 5 to 60 dph. At the end of Trial 1 (Hr), weaned larvae were graded for the first time to two weight classes (viz., Big and Small; grader 1.5 mm). A sample of the graded fish groups of each experimental treatment was transferred to a laboratory recirculating seawater system to monitor postlarvae performance during pregrowing (Trial 2, Laboratory rearing, Lr). Trial 2 was decided to be performed in the laboratory, because, under production conditions, it is not feasible to keep together and separately a specific hatchery batch due to frequent grading and group mixing.
2.2.1 Trial 1 (Hatchery rearing, Hr)
Four tanks of 18 m3 were stocked with eggs from the same group of broodstock at a mean stocking density of 73 hatched larvae per litre. In two of the tanks, a commercial dry feed (Larviva Prostart 100, Biomar, 80–125 µm) was introduced on 4 dph (DF4), whereas in the other two tanks, the dry feed was introduced on 15 dph (DF15). In all experimental tanks, the larvae were fed with rotifers from 4 dph up to 24 dph and with Artemia (initially nauplii followed by metanauplii) from 13 dph up to 58 dph. Prey density offered was similar between treatments and according to hatchery protocol, that is, live prey density was not reduced in the DF4 treatment. From 59 dph, larvae were fed dry feed only. Larvae handling and live feed feeding followed the hatchery protocol. Dry feed feeding and timing of changing feed particle size followed the manufacturer's protocol (i.e., feeding every hour; gradually increasing daily quantity). Water quality was monitored twice daily (8:00 and 12:00 h) and was maintained as follows (mean ± standard error of the mean [SEM]): temperature, 18 ± 0.6°C; salinity, 35 ± 0.0 g L−1; dissolved oxygen, 7.6 ± 0.10 mg L−1 (100 ± 1.4% saturation); pH, 7.67 ± 0.014; total ammonia nitrogen, NH4++NH3–N, 0.441 ± 0.0181 mg L−1; ammonia nitrogen, NH3–N, 0.003 ± 0.0001 mg L−1; and nitrites nitrogen, NO2–N, 0.021 ± 0.0008 mg L−1.
Daily, samples of larvae were observed under microscope to confirm food consumption. Beginning from 5 dph and up to 60 dph, duplicated samples of larvae were sampled from each tank every 5 days. Larvae were sampled when fully fed, that is, around noon. Larvae used for length measurement and observation of phenotypical deformities were preserved in buffered formalin (approximately 65–75 larvae per treatment per sampling). Larvae used for digestive enzymes analyses (approximately a total of 200 larvae gradually reduced to 60 larvae from 5 to 60 dph) were gently netted, washed in fresh water, immediately frozen to dry ice and transferred to −80°C until analysed.
2.2.2 First grading and transfer to Lr
Each experimental tank was graded on 62 dph to two size classes (grader 1.5 mm). Hereafter, the two size classes of the first grading are named as Big and Small. Big and Small fish were group weighted and counted. The Big and Small fish of each duplicated tank were pooled, and for each treatment, the respective Big and Small fish groups were formed (DF4-Big, DF4-Small, DF15-Big and DF15-Small). On 64 dph, a subgroup of each graded fish group was transferred to the recirculating seawater system of the Laboratory of Applied Hydrobiology (Agricultural University of Athens). No significant mortality was observed during transport.
2.2.3 Trial 2 (Laboratory rearing, Lr)
Upon arrival to the laboratory, fish were gradually (approximately for 5 h) acclimated to the water quality (temperature, salinity and pH) of the recirculating seawater system. Fish were allowed for 2 days acclimation to laboratory tanks during which they were fed to satiation (Biomar Larviva ProStart 300).
On 66 dph, 976 fish from each size class and Hr treatment (DF4-Big, DF4-Small, DF15-Big and DF15-Small) were group weighed (at least 25–30 groups for each tank) and randomly distributed in duplicated tanks (488 fish per tank). Postlarvae growth performance was monitored for 8 weeks (up to 126 dph). The laboratory seawater recirculating system has a total water volume capacity of 11 m3 (renewal 3% make-up water). It is provided with mechanical (polyester filter pad) and biological filters (submerged gravel biofilter), UV sterilization, compressed air supply, water cooling apparatus and photoperiod control (12L:12D, L: 8:00–20:00 h, 220 lx at water surface). Tanks used were glass, rectangular (41 × 49 × 44 cm, water volume 88.4 L), with all sides, apart from the front and top ones, externally covered with light blue styrofoam. The water flow rate in each tank was 1.8 L min−1. All tanks were thoroughly cleaned once a week. Twice daily (11:30 and 17:30 h) uneaten feed was removed. Water physicochemical characteristics were monitored twice daily (before feeding and noon), and mean values were maintained as follows (mean ± SEM): temperature, 24.6 ± 0.02°C; dissolved oxygen, 6.2 ± 0.05 mg L−1 (90 ± 0.1% saturation); pH, 7.62 ± 0.004; salinity, 34 ± 0.0 g L−1; total ammonia nitrogen, NH4++NH3–N, 0.795 ± 0.0260 mg L−1; un-ionized ammonia–nitrogen, NH3–N, 0.010 ± 0.0003 mg L−1; and nitrite–nitrogen, NO2–N, 0.392 ± 0.0198 mg L−1.
Fish were fed to satiation, by hand, six times daily (9:00, 11:00, 13:00, 15:00, 17:00 and 19:00 h) the same commercial diets adjusting feed particle size as fish were grown. Every 2 weeks, fish were group weighted (in groups of 20–25 fish) and counted. During the last 4 weeks of the rearing period, feed offered was recorded to estimate feed consumption and efficiency.
At the end of the experimental period, all fish were individually weighted. Total and standard length was measured to 20% of each fish group. Any kind of phenotypical deformity was also recorded.
2.3 Analyses and calculations
2.3.1 Trial 1 (Hatchery rearing, Hr)
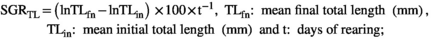
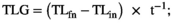
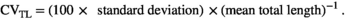
Also, total length (on 60 dph) frequency distribution for each experimental treatment was tabulated.
Phenotypical deformities of 60 dph preserved larvae were also recorded. Deformities identified were jaw and spinal cord malformations. Specific and total deformities were expressed as percentage of the total number of observed fish. Hatchery administration confirmed that number of larvae without swim bladder was equally low (<3%) to all experimental treatments; these data are not quantified or analysed further.
For digestive enzymes analyses, whole larvae were homogenized in five volumes v/w of cold 50 mM Tris–HCl buffer with CaCl2, pH 8.0, followed by centrifugation (3,805 g; 45 min, 4°C). The supernatant was divided in different aliquots so that for each analysis, only fresh, previously unthawed samples were used. All procedures were performed in ice, and samples were kept to −80°C until analysed. Trypsin and chymotrypsin activity was measured using N-a-benzoyl-Arg-p-nitroanilide (L-BAPNA) and succinyl-Ala-Ala-Pro-Phe p-nitroanilide (Suc-AAPF-pNA) as the substrate, respectively (Lainé et al., 1993). Pepsin was measured according to Anson (1938) using haemoglobin as the substrate. Amylase was measured according to Somogyi (1960) using potato starch as the substrate. Lipase was measured according to Choi et al. (2003) using 2,3-dimercapto-1-propanol tributyrate (DMPTB) as the substrate. Enzymatic activities were expressed as specific activities (enzymatic units per mg protein). Protein was determined by the Bradford method (Bradford, 1976).
2.3.2 First grading
- Number of survived larvae;
- Survival (%) = (final number of survived larvae) × (initial number of stocked larvae)−1 × 100;
- Density of survived larvae (larvae L−1);
- Number of Big and Small larvae;
- Big larvae (%) = (number of Big larvae) × (number of survived larvae)−1 × 100;
- Small larvae (%) = (number of Small larvae) × (number of survived larvae)−1 × 100;
- Mean weight of Big and Small larvae (group weighted);
- Weighted body weight = [Big larvae (%) × (mean weight of Big larvae)] + [Small larvae (%) × (mean weight of Small larvae)].
2.3.3 Trial 2 (Laboratory rearing, Lr)
- SGR = (lnWfn − lnWin) × 100 × t−1, Wfn: mean final body weight (g), Win: mean initial body weight (g) and t: days of rearing;
- Thermal growth coefficient, TGC =1000 × [(Wfn1/3 − Win1/3)] × dd−1, dd: degree days;
- Weight gain (g day−1), WG = (Wfn − Win) × t−1;
- FCR (from 30 to 60 days of rearing) = (food consumed, g) × (weight gain, g)−1;
- Feeding rate (% body weight, from 30 to 60 days of rearing), FR = (food consumed) × (mean biomass increase)−1 × t−1 × 100;
- Coefficient of weight variation, CV = 100 × (standard deviation) × (mean body weight)−1;
- Survival (%) = (final number of fish) × (initial number of fish)−1 × 100;
- Final density (kg m−3).
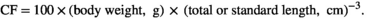
Also, final weight frequency distribution for each experimental treatment was tabulated.
Phenotypical deformities identified were mainly gill operculum, jaw and spinal cord malformations. Specific and total deformities were expressed as percentage of the total number of observed fish. Fish with more than one deformity were denoted as “other.”
2.4 Statistics
Data were analysed by one-way analysis of variance (one-way ANOVA). The experimental tank was the experimental unit. When data did not satisfy the assumptions of ANOVA (i.e., normality and homogeneity of variance), non-parametric analyses was carried out (Mood's Median Test). Where p values were significant (p < .05), multiple comparisons were carried out using the Duncan test. All values presented in the text and tables are means ± SEM. In the case of Laboratory rearing (Lr), the above-mentioned statistical analysis was performed for Big and Small fish separately. For SGR and TGC, initial weight was used as a covariate to account for differences in initial weight. Kolmogorov–Smirnov Test was used to compare larval length or juveniles weight distributions.
3 RESULTS
3.1 Trial 1 (Hatchery rearing, Hr)
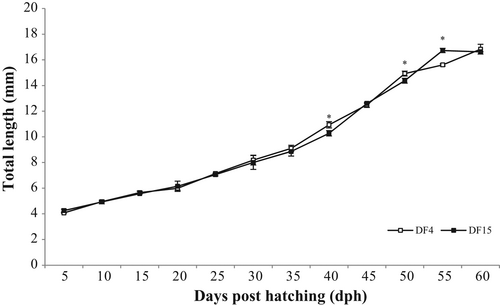
Larvae total length did not show marked differences during development (Figure 1). On 60 dph, larvae total length between DF4 and DF15 was similar. Also, specific growth rate and length gain from 5 to 60 dph did not differ between treatments (Table 1). Total length variation was significantly lower when dry feed was introduced on 4 dph (DF4, Table 1). However, comparison of length distributions did not reveal significant differences (K–S statistic = 0.724031, DN =0.137179, p > .05; data not presented).
| DF4 | DF15 | Significance level (p) | |
|---|---|---|---|
| Growth performance | |||
| Total length (mm; 60 dph) | 16.8 ± 0.38 | 16.6 ± 0.01 | ns |
| CVTL (%; 60 dph) | 9.2 ± 1.80 a | 13.5 ± 1.22 b | * |
| SGRTL (5–60 dph) | 2.58 ± 0.031 | 2.51 ± 0.058 | ns |
| TLG (mm day−1; 5–60 dph) | 0.231 ± 0.0065 | 0.229 ± 0.0036 | ns |
| Phenotypical deformities (60 dph) | |||
| Not-normal (%) | 3.8 ± 3.85 a | 16.0 ± 2.94 b | * |
| Jaw def. (%) | 1.9 ± 1.93 | 2.7 ± 2.71 | ns |
| Spinal cord def. (%) | 1.9 ± 1.93 a | 13.3 ± 0.24 b | * |
| Grading data (62 dph) | |||
| Survival (%) | 31.3 ± 5.63 | 32.8 ± 2.14 | ns |
| Final density (larvae L−1) | 22.4 ± 1.78 | 24.5 ± 2.48 | ns |
| % Big fish | 45.7 ± 7.75 a | 58.7±2.52 b | * |
| % Small fish | 54.3 ± 7.75 b | 41.3 ± 2.52 a | * |
| Weight (g) of Big fish | 0.086 ± 0.0010 b | 0.065 ± 0.0020 a | ** |
| Weight (g) of Small fish | 0.044 ± 0.0015 b | 0.039 ± 0.0000 a | * |
| Weighted body weight (g) | 0.063 ± 0.0035 | 0.054 ± 0.0020 | ns |
Note
- Values are means of duplicated groups ± SEM. Means with different letters in the same row are significantly different.
- Abbreviations: CVTL, coefficient of total length variation; def., deformities; ns, not significant; SGRTL, specific growth rate based on total length; TLG, total length gain.
- * p < .05.
- ** p < .01.
Introducing dry feed on 4 dph (DF4) resulted in significantly lower number of deformed larvae (Table 1). This lower value was related to lower spinal cord malformations. Jaw deformities observed were similar between treatments (Table 1).
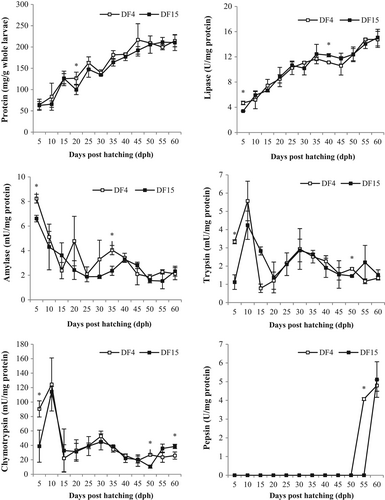
During development, total body protein was progressively increased in a similar way in both DF4 and DF15 larvae (Figure 2). Although in DF4, larvae body protein was generally higher than in DF15, the difference was significant only on 20 dph. Lipase specific activity was also progressively increased from 5 to 60 dph in both treatments. On 5 dph, lipase activity was significantly higher in DF4 than in DF15 larvae, whereas on 40 dph, the opposite was observed (Figure 2). Amylase specific activity was high on 5 dph and then progressively decreased up to 60 dph (Figure 2). DF4 larvae presented significantly higher amylase levels on 5 and 35 dph compared with DF15 larvae. The pattern observed for trypsin and chymotrypsin specific activities during development was generally similar between treatments, that is, lower levels on 5 dph were followed by a sharp increase on 10 dph and then levels were decreased to lower values with a much lower peak around 30 dph (Figure 2). In the case of trypsin, DF4 larvae had significantly higher levels on 5 dph compared with DF15 larvae. In DF4 larvae, the trypsin peak on 10 dph was more intense than in DF15 although the difference was not significant, and the decrease of tryptic activity was observed 5 days earlier (i.e., on 15 dph instead of 20 dph for the DF15 larvae). Chymotrypsin specific activity was also higher in DF4 larvae on 5 dph, but no other differences were observed up to 50 dph, when DF4 larvae showed higher levels than DF15 larvae. On 60 dph, the opposite was observed (Figure 2). Pepsin specific activity was firstly detected on 55 dph for DF4 larvae and on 60 dph for DF15 larvae. On 60 dph, pepsin was found at equal levels in both treatments (Figure 2).
3.2 First grading
Survival and final density did not significantly differ between the two treatments (Table 1). When dry feed was introduced on 4 dph (DF4), significantly lower and higher percentages of Big and Small larvae were obtained compared with the DF15 treatment. However, body weight in both Big and Small larvae was significantly higher when dry feed was introduced on 4 dph (Table 1). The calculated weighted body weight was similar between treatments (Table 1).
3.3 Trial 2 (Laboratory rearing, Lr)
3.3.1 The big fish
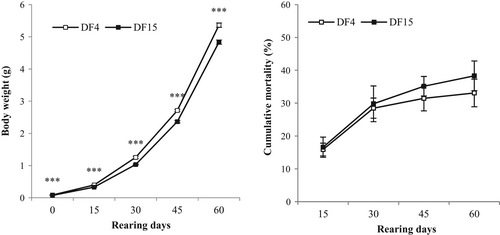
The first weighing of weaned and transferred larvae in the laboratory (on 66 dph) confirmed the data obtained from the hatchery at first grading (on 64 dph), that is, DF4 fish were significantly larger than DF15 fish (Figure 3 and Table 2). This difference was maintained throughout the 8 weeks of experimental Lr (Figure 3 and Table 2). Similar results were obtained for CFTL and CFSL, WG, TGC and final density (Table 2). No significant differences were observed for SGR, feed conversion ratio (FCR), FR and survival (Table 2 and Figure 3). CV did not differ between DF4 and DF15 treatments (Table 2). However, weight distributions were significantly different, and when dry feed was introduced on 4 dph (DF4), lower percentage of small fish were produced (K–S statistic = 2.73814, DN = 0.154711, p < .001; data not presented).
| DF4 | DF15 | Significance level (p) | |
|---|---|---|---|
| Performance | |||
| Initial body weight (g) | 0.087 ± 0.0012 b | 0.074 ± 0.0012 a | *** |
| Final body weight (g) | 5.36 ± 0.070 b | 4.84 ± 0.068 a | *** |
| Total length (cm) | 7.05 ± 0.054 b | 6.85 ± 0.067 a | * |
| Standard length (cm) | 5.93 ± 0.045 b | 5.71 ± 0.057 a | ** |
| CFTL | 1.46 ± 0.009 b | 1.39 ± 0.008 a | *** |
| CFSL | 2.46 ± 0.015 b | 2.41 ± 0.018 a | * |
| CV (%) | 33.5 ± 0.97 | 34.2 ± 1.36 | ns |
| WG (g day−1) | 0.094 ± 0.0014 b | 0.085 ± 0.0040 a | * |
| SGRa | 7.62 ± 0.105 | 7.22 ± 0.105 | ns |
| TGCa | 0.98 ± 0.002 b | 0.88 ± 0.002 a | * |
| FCRb | 1.21 ± 0.005 | 1.23 ± 0.029 | ns |
| FRb (% body weight) | 5.2 ± 0.05 | 5.4 ± 0.21 | ns |
| Survival (%) | 66.9 ± 4.20 | 61.7 ± 4.51 | ns |
| Final density (kg m−3) | 19.8 ± 0.96 b | 16.5 ± 0.45 a | * |
| Phenotypical deformities | |||
| Not normal (%) | 11.8 ± 0.64 a | 15.1 ± 1.44 b | * |
| Gill operculum def. (%) | 7.5 ± 0.02 b | 5.8 ± 0.26 a | * |
| Jaw def. (%) | 3.3 ± 0.71 a | 5.6 ± 1.24 b | * |
| Spinal cord def. (%) | 0.5 ± 0.18 a | 2.8 ± 0.30 b | * |
| Otherc | 0.5 ± 0.13 a | 0.9 ± 0.23 b | * |
Note
- As larvae, they were co-fed live and dry feed from 4 dph (DF4) or 15 dph (DF15). Values are means of duplicated groups ± SEM. Means with different letters in the same row are significantly different.
- Abbreviations: CFSL, condition factor based on standard length; CFTL, condition factor based on total length; CV, coefficient of weight variation; FCR, feed conversion ratio; FR, feeding rate; ns, not significant; SGR, specific growth rate; TGC, thermal growth coefficient (1386 degree days);WG, weight gain.
- a Initial weight was used as a covariate.
- b Thirty to sixty days of rearing.
- c “Other” refers to fish with more than one of the observed deformities.
- * p < .05.
- ** p < .01.
- *** p < .001.
Introducing dry feed on 4 dph (DF4) resulted in significantly lower total number of deformed fish after 8 weeks of Lr compared with DF15 treatment (Table 2). This lower value was related to lower jaw and spinal cord deformities. Instead, gill operculum malformations were higher in DF4 fish (Table 2).
3.3.2 The small fish
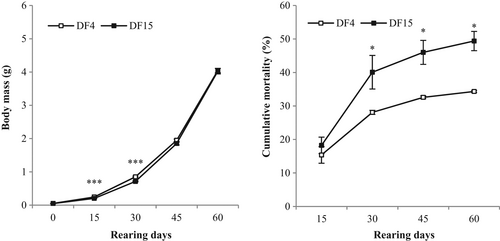
The first weighing of weaned and transferred larvae in the laboratory (on 66 dph) showed no significant differences in Small fish weight between treatments (Figure 4 and Table 3). Although after 15 and 30 days of Lr, fish of the DF4 treatment had significantly higher body weight than the DF15 fish, the difference was no longer obvious after 45 and 60 days of Lr (Figure 4 and Table 3). For DF4, lower condition factor (CFSL) and FR were observed (Table 3). The other growth performance parameters examined did not differ between DF4 and DF15 treatments. Survival was significantly higher for DF4 than for DF15 fish (Table 3 and Figure 4). Neither CV nor weight distributions were significantly different between DF4 and DF15 treatments (Table 3; K–S statistic = 0.753037, DN =0.0450839, p >.05; data not presented).
| DF4 | DF15 | Significance level (p) | |
|---|---|---|---|
| Performance | |||
| Initial body weight (g) | 0.051 ± 0.0010 | 0.049 ± 0.0011 | ns |
| Final body weight (g) | 4.05 ± 0.067 | 4.02 ± 0.081 | ns |
| Total length (cm) | 6.53 ± 0.068 | 6.54 ± 0.074 | ns |
| Standard length (cm) | 5.47 ± 0.058 | 5.47 ± 0.064 | ns |
| CFTL | 1.42 ± 0.007 | 1.44 ± 0.010 | ns |
| CFSL | 2.41 ± 0.013 a | 2.47 ± 0.017 b | * |
| CV (%) | 41.7 ± 1.42 | 44.6 ± 1.66 | ns |
| WG (g day−1) | 0.071 ± 0.0043 | 0.071 ± 0.0022 | ns |
| SGRa | 7.81 ± 0.059 | 7.87 ± 0.059 | ns |
| TGCa | 0.88 ± 0.011 | 0.89 ± 0.011 | ns |
| FCRb | 1.18 ± 0.005 | 1.17 ± 0.003 | ns |
| FRb (% body weight) | 5.3 ± 0.03 a | 5.5 ± 0.05 b | * |
| Survival (%) | 65.7 ± 0.31 b | 50.6 ± 2.87 a | * |
| Final density (kg m−3) | 14.7 ± 0.95 b | 11.2 ± 0.99 a | * |
| Phenotypical deformities (126 dph) | |||
| Not normal (%) | 22.3 ± 1.61 a | 29.1 ± 1.19 b | * |
| Gill operculum def. (%) | 12.9 ± 1.35 b | 10.3 ± 1.24 a | * |
| Jaw def. (%) | 6.2 ± 0.60 a | 12.7 ± 3.15 b | * |
| Spinal cord def. (%) | 1.3 ± 0.63 | 2.7 ± 1.87 | ns |
| Otherc | 1.9 ± 0.31 | 3.4 ± 1.23 | ns |
Note
- As larvae, they were co-fed live and dry feed from 4 dph (DF4) or 15 dph (DF15). Values are means of duplicated groups ± SEM. Means with different letters in the same row are significantly different.
- Abbreviations: CFSL, condition factor based on standard length; CFTL, condition factor based on total length; CV, coefficient of weight variation; FCR, feed conversion ratio; FR, feeding rate; ns: not significant; SGR, specific growth rate; TGC, thermal growth coefficient (1386 degree days);WG, weight gain.
- a Initial weight was used as a covariate.
- b Thirty to sixty days of rearing.
- c “Other” refers to fish with more than one of the observed deformities.
- * p < .05.
Introducing dry feed on 4 dph (DF4) resulted in significantly lower total number of deformed fish after 8 weeks of Lr (Table 3). This lower value was related to lower jaw deformities. Instead, gill operculum malformations were higher in DF4 fish, and no significant differences were observed for spinal cord deformities (Table 3).
4 DISCUSSION
Present results show that introducing dry feed as early as on mouth opening, together with live feed, can be a viable feeding protocol for gilthead seabream larviculture. Larvae development is not compromised while certain benefits can be obtained for larvae and postlarvae performance.
Up to 60 dph, larvae growth, in terms of length (i.e., total length, SGRTL and TLG), was similar between experimental treatments and in agreement, or even better, with previously reported growth data for gilthead seabream larvae reared at comparable ambient temperature and salinity under laboratory (e.g. Fernández et al., 2008; Gisbert et al., 2012; Mata-Sotres et al., 2018; Perera & Yúfera, 2017; Seiliez et al., 2006) or production conditions (Brown, 2003). The absence of differences between treatments on 60 dph suggests that the introduction of dry feed on first-feeding gilthead seabream larvae, together with live feed, does not have a detrimental effect on growth. On the other hand, data of first grading, on 62 dph, indicated that there was a growth advantage for larvae fed dry feed from mouth opening (DF4) since both the Big and the Small fish resulting from the grading were larger than the fish of the later introduction of dry feed (DF15). The fact that this growth difference was not obvious in larvae length measurements up to 60 dph is unlikely to be related to the 2 days difference and is probably misleading because length data refer to ungraded larvae. Actually, the calculated weighted mean of body weight was similar between treatments and in accordance with length data. Similar to present results, that is, no growth effects up to the end of the developmental stages when dry feed is co-fed with live feed from mouth opening and compared with “live feed alone,” have also been reported for ungraded gilthead seabream (Fernández-Díaz & Yúfera, 1997) and Senegalese sole (Cañavate & Fernández-Díaz, 1999). It is important to note that in the latter studies, similar amounts of live feed were offered either in the co-feeding or in the “live feed alone” treatments, as was the case in the present study. Instead, in most other related studies, live feed was reduced in the co-feeding treatment, and no growth effect (Senegalese sole, Cañavate & Fernández-Díaz, 1999; loach, Wang et al., 2008; Wang et al., 2009) or lower growth performance (gilthead seabream Pousão-Ferreira et al., 2003; Senegalese sole, Engrola, Figueira, et al., 2009; Engrola, Dinis et al., 2010; Mai et al., 2009) have both been reported.
The introduction of dry feed from the onset of exogenous feeding in gilthead seabream larvae (DF4) resulted in a lower percentage of Big fish (grading data on 62 dph) than the DF15 treatment. Taking under consideration the lower length variation on 60 dph and the absence of differences in larvae total survival, the lower percentage of Big fish may be related to a differential size-related larvae mortality during the larviculture. However, the hypothesis could not be verified because in actual production conditions, it is very difficult to obtain an accurate estimation of larvae mortality during the first life stages and before the first grading, due to tank volume, number and size of larvae. Furthermore, to our knowledge, no related data have been reported in the literature. On the other hand, under laboratory conditions, it has been shown that mortality during development may differ among treatments imposed despite similar total survival rates (Senegalese sole, Cañavate & Fernández-Díaz, 1999; gilthead seabream, Castanho et al., 2017; Nishikigoi Cyprinus carpio, Fosse et al., 2018).
Phenotypical deformities detected on 60 dph were mainly jaw and spinal cord malformations. In the present study, observation was restricted to obviously visible deformities, and no staining, microtomal and, generally, histological techniques were applied. However, because these techniques can offer more accurate deformities characterization (Koumoundouros et al., 1997; Morel et al., 2010; Thuong et al., 2017; Verhaegen et al., 2007), the possibility that results obtained may differ from those that would be obtained through such methods cannot be excluded. Nevertheless, co-feeding live and dry feed from the onset of exogenous feeding (DF4) resulted in a lower incidence of total deformities (mainly spinal cord deformities) as compared with DF15. Taking into account that other parameters which may have affected the incidence of deformities were kept as similar as possible between treatments (e.g., broodstock, water quality, rearing conditions and larvae handling) (Boglione et al., 2001; Divanach et al., 1996; Koumoundouros et al., 1997), present results suggest that the dry feed has a deformities-reducing potential when introduced at early life stages of gilthead seabream. This effect could be related to the specific feed composition which may have provided larvae with the necessary nutrients to form a healthy skeletal system at the sensitive early stages of osteogenesis (Faustino & Power, 1998; Riera-Heredia et al., 2018).
The digestive enzymes pattern observed during the early life stages is in agreement with the pattern reported for gilthead seabream or other altricial gastric fish species (e.g. Gisbert et al., 2009; Hamza et al., 2015; Suzer et al., 2008; Yúfera et al., 2011) and indicates a normal functional development of the digestive system in both treatments. Briefly, even during the endogenous feeding, the pancreatic tissue is functional secreting digestive enzymes (i.e., amylase, trypsin, chymotrypsin and lipase) for effective feed digestion. As the digestive tract matures, the relative importance of the pancreatic tissue in feed digestion is reduced. In particular, as the ingestion of exogenous feed increases (especially during first feeding), trypsin and chymotrypsin rise sharply. The subsequent reduction is related to the development of the intestine which supports protein digestion with intestinal proteolytic enzymes, whose activity increases. Trypsin and chymotrypsin activity may be observed to rise again during later developmental stages, usually following changes in live feeds (i.e., introduction of Artemia) or the introduction of dry feeds. Finally, the detection of pepsin signals the development of the stomach. The presence of gastric protein digestion signifies a reduction in the relative importance of the other proteolytic enzymes (i.e., pancreatic and intestinal). On the other hand, amylase activity is high on mouth opening and soon drops to lower levels, indicating the ability of larvae for carbohydrates digestion during the first developmental stages and the following adaptation of carnivorous feeding habits. The case of lipase is different; in most cases, a slow but steady increase is observed during development, indicative of the ingestion of feeds (live and/or dry) rich in lipids (for review, see Cahu & Zambonino-Infante, 2001, and Yúfera et al., 2011).
The above-mentioned pattern of digestive enzymes activity was generally present in all experimental groups. Some specific points could be pointed out. First, the higher levels of lipase, amylase, trypsin and chymotrypsin on 5 dph in DF4 larvae compared with DF15 larvae are probably indicative of the ingestion and digestion of dry feed beyond rotifers, since in DF15, no dry feed had been yet introduced. Besides, it has been previously shown that first-feeding gilthead seabream larvae are capable of ingesting (Fernández-Díaz et al., 1994) and digesting (Fernández-Díaz & Yúfera, 1995) dry feed particles of appropriate size and preparation technology. The subsequent peak of proteolytic enzymes, on 10 dph, suggests feed intake from an increasing number of larvae in both treatments, because not all larvae start feeding at once (Andrade et al., 2011; Parra & Yúfera, 2000). The higher (although not significant) trypsin activity of DF4 larvae on 10 dph and the earlier reduction on 15 dph compared with DF15 larvae may indicate an earlier maturation of the intestine and the secretion of brush border-related proteolytic enzymes (Guo et al., 2016; Suzer et al., 2007, 2008). Second, the following mild increase of trypsin and chymotrypsin up to 30 dph suggests the ingestion of Artemia (firstly introduced on 13 dph) and, in the case of DF15 larvae, the ingestion of dry feed also. Third, in both treatments, gastric digestion was functional on 55–60 dph in accordance with previously reported results (Elbal et al., 2004; Yúfera et al., 2011). The 5 days difference between treatments may suggest an earlier stomach maturation for the DF4 larvae. However, both trypsin and pepsin secretion timing differences between treatments (i.e., DF4 on 15 dph vs. DF15 on 20 dph for trypsin and DF4 on 55 dph vs. DF15 on 60 dph for pepsin) may be attributed simply to the “inaccuracy” of the 5-day interval of samplings, and further elucidation before concluding on an earlier maturation of the digestive system is needed. On the other hand, in Senegalese sole larvae co-fed live and dry feed from mouth opening and compared with a “live feed alone” feeding regime, no differences were found for trypsin and amylase specific activities on 2, 5, 9 and 20 dph, and an earlier maturation of the digestive capacity was suggested based on the higher alkaline phosphatase specific activity detected on 20 dph (Engrola, Figueira, et al., 2009).
Overall results obtained up to 60 dph are probably related not only to the introduction of dry feed from mouth opening but also to the concomitant maintenance of live prey density at a full ration level in both treatments. It is true not only that the larvae have a preference for live against dry feed (Fernández-Díaz et al., 1994) but also that the longer the exclusive presence of live prey, the stronger the larvae addiction and the harder their acclimation to dry feed (Baskerville-Bridges & Kling, 2000; Cañavate & Fernández-Díaz, 1999; Koven et al., 2001). In the present study, the introduction of dry feed from mouth opening without restricting access to plenty of live feed has probably allowed the larvae to be smoothly acclimated to the dry feed without the threat of starvation. Although, in early co-feeding studies, the reduction in live feed prey density is totally reasonable for cost-saving reasons, results obtained so far have not been very promising because in most cases, lower larvae performance has been reported (gilthead seabream Pousão-Ferreira et al., 2003; Senegalese sole, Engrola, Figueira, et al., 2009; Engrola, Dinis et al., 2010; Mai et al., 2009).
Performance of graded postlarvae after weaning was found to be improved when dry feed was introduced to first-feeding larvae (DF4). Compared with a more common feeding protocol (DF15), benefits were observed for both the Big (better growth, more favourable size distribution and fewer deformities) and the Small (better survival and fewer deformities) fish resulting from the first grading. Actually, the re-evaluation of deformities at this stage, that is, when they are obviously distinguishable, confirmed deformities results obtained on 60 dph larvae. Importantly, the other performance parameters were similar between treatments and not compromised by the early introduction of dry feed. In agreement to present results, co-feeding regimes from mouth opening have been reported to enhance growth and survival of Senegalese sole (Cañavate & Fernández-Díaz, 1999; Engrola, Figueira, et al., 2009) and loach (Wang et al., 2009) after weaning. Present results also corroborate the scientific findings that indicate that changes in environment or nutrition during the critical developmental stages may influence the subsequent postlarvae and juvenile performance (Koedijk et al., 2010; López-Albors et al., 2003; Perera & Yúfera, 2016; Séité et al., 2019; Vagner et al., 2007).
In conclusion, introducing dry feed in gilthead seabream larvae from mouth opening did not have detrimental effects on larvae growth, survival and functional development of the digestive system. Indeed, growth enhancement and lower incidence of total phenotypical deformities are suggested. Dry feed was ingested and digested, even if it was offered before stomach maturation. The fact that live feed was maintained at full ration may have helped the larvae to better acclimate to the dry feed. Moreover, postlarvae performance was improved emphasizing the importance of larval rearing for subsequent production stages. Obtained results establish a basis for further investigation on more cost-efficient feeding protocols for gilthead seabream larvae (e.g., by rationally reducing live feed) without compromising larvae performance. Despite the experimental difficulties, it would also be of great interest to evaluate the possible long-term effects by following fish performance up to commercial size.
ACKNOWLEDGEMENTS
This research was financially supported by BioMar Hellenic SA. We cannot thank enough all the staff of the Selonda hatchery for their fundamental contribution to the successful experimental larval rearing. We are most grateful to G. Konstantinou for his valuable technical laboratory assistance.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request.




