Effects of tributyrin on growth performance, immune response and intestinal barrier function of juvenile grass carp (Ctenopharyngodon idellus) fed diets with high cottonseed and rapeseed meal
Yi Hu and Yong Shi contributed to this work equally.
Abstract
Tributyrin (TB) has applications as a growth and intestinal health promoter for animals. A 10-week feeding trial was conducted to evaluate the application of TB to relieve the negative effects induced by high cottonseed and rapeseed meal (CRM) in juvenile grass carp (Ctenopharyngodon idellus; initial weight 9.59 ± 0.01 g). Grass carp were fed a normal fish meal (FM) diet (positive control [PC], 50 g/kg FM, 340 g/kg CRM), high CRM diet (T0, 20 g/kg FM, 540 g/kg CRM) or a T0 diet supplemented with 500 (T500), 1000 (T1000) and 1500 (T1500) mg/kg TB. The results showed that (1) grass carp fed diet T0 displayed the poorest growth (p < .05), whilst no significant differences in growth performance were observed in those fed diets PC and T1000. (2) Complement 3, glutathione peroxidase and superoxide dismutase levels in the T0 group were significantly lower (p < .05) than those in the PC group. The immune indices of fish fed the 1000 or 1500 mg/kg TB diet were significantly higher (p < .05) than those of fish fed the T0 diet, whilst there was no significant difference with PC group. (3) Compared with that of the T0 group, pro-inflammatory cytokine (il-6, il-8, nf-κb and tlr-4) expression in the 1000 mg/kg tributyrate group was significantly downregulated, whilst the expression of anti-inflammatory cytokines (il-10 and thf-β) and intestinal tight junction proteins (zo-1, zo-2, claudin-b, claudin-c and occludin) was significantly upregulated (p < .05). In conclusion, high CRM diets induced negative effects on grass carp, and dietary TB supplementation promoted growth, improved antioxidant capacity and enhanced intestinal barrier function of grass carps. And the optimum supplemental level of TB was 1000 mg/kg.
1 INTRODUCTION
Aquaculture production continues to grow steadily (FAO, 2020). With the rapid development of intensive aquaculture, fish meal (FM) and soybean meal are high-quality protein sources in aquatic feed (Shi et al., 2019). The substitution of FM and soybean meal in feeds with sufficient and inexpensive plant protein sources in feeds has received much attention in research (Lim & Lee, 2009; Ye et al., 2005). Cottonseed meal and rapeseed meal are widely used in aquatic feeds because of their high protein content, low price, abundant resources, and convenient processing (Hu et al., 2014). However, high cottonseed meal diets have been found to significantly decrease the growth performance and damage the intestinal tissue of grass carp (Ctenopharyngodon idellus; Liu et al., 2019), and reduce the immunity and disease resistance of Ussuri catfish (Pseudobagrus ussuriensis; Bu et al., 2017). Previous studies in our laboratory have shown that high cottonseed meal feed can reduce the growth and impair liver function of black carp (Mylopharyngodon piceus; Hu et al., 2015). In addition, a high proportion of rapeseed meal diets has been shown to induce negative effects on the growth performance and immunity of grass carp (Tan et al., 2013; Yao et al., 2019) and red sea bream (Pagrus major; Dossou et al., 2019).
High cottonseed and rapeseed meal (CRM) diets have many anti-nutritional factors, such as gossypol, tannin, sinapine and glucosinolate (Barros et al., 2002; Francis et al., 2001). These anti-nutritional factors can reduce growth and immunity. These anti-nutritional factors may cause oxidative damage and destruction in the intestines of aquatic animals. Studies have shown that dietary gossypol inhibits the expression of the TOR signalling pathway in the intestines of grass carp, subsequently affecting intestinal digestion and absorption and destroying intestinal structure (Wang et al., 2018). In addition, von Danwitz and Schulz (2020) demonstrated that excessive levels of glucosinolates in feed can lower the immunity and impair the intestinal health of turbot (Psetta maxima L.), which in turn leads to growth impairment. The health of aquatic animals should be considered when using inexpensive vegetable protein sources to replace FM or soybean meal. Therefore, exploring effective dietary strategies is imperative to alleviate the negative effects of high CRM on aquatic animals.
Butyric acid is a short-chain fatty acid present in the intestinal tract and plays an important role in regulating the microecological balance of the gastrointestinal tract, maintaining the normal state of intestinal mucosal epithelial cells, and promoting intestinal digestion and absorption (Aalamifar et al., 2020; Chow et al., 2017). Tributyrin (TB), a precursor of butyric acid, can pass through the stomach without changing the biological characteristics of butyric acid and cannot be digested by digestive enzymes (Chen & Breitman, 1994; Su et al., 2004). Studies have reported that the addition of TB into high soybean meal diets can improve the growth performance, immunity and intestinal health of common carp (Cyprinus carpio L.; Xie et al., 2021), yellow drum (Nibea albiflora; Zhu et al., 2020), blunt snout bream (Megalobrama amblycephala; Liang et al., 2021) and black sea bream (Acanthopagrus schlegelii; Volatiana et al., 2020). Moreover, TB can alleviate the growth performance decline and lipid deposition caused by high levels of soybean oil diets in large yellow croaker (Larimichthys crocea; Xu et al., 2021).
Grass carp is the most productive freshwater species in China, and its production was estimated to be close to 5,704,000 tons in 2018 (FAO, 2020). Currently, grass carp commercial feed formulation uses soybean meal and FM as high-quality protein sources. Therefore, in this study, high proportions of FM and soybean meal were replaced by CRM, and TB was added to high CRM diets to study the effects of TB on growth, immune response and intestinal barrier function of grass carp. This study gets insight into the high utilization level of low-quality protein sources such as CRM in aquatic feed and provides support for the application of TB in aquatic animals.
2 MATERIALS AND METHODS
2.1 Preparation of experimental diet
Peruvian steamed FM, soybean meal, cottonseed meal and rapeseed meal were used as the main protein sources, and soybean oil was used as the lipid source. The basal diet was formulated (50 g/kg FM, 200 g/kg soybean meal, 170 g/kg cottonseed meal and 170 g/kg rapeseed meal) as the positive control (PC; Table 1). The high CRM diets (20 g/kg FM, 80 g/kg soybean meal, 270 g/kg cottonseed meal and 270 g/kg rapeseed meal) were supplemented with 0 (T0), 500 (T500), 1000 (T1000) and 1500 (T1500) mg/kg TB. The TB source was provided by Guangdong Yiduoli Biotechnology Co., Ltd. The purity of TB was ≥98%.
| Items | PCa | T0a | T500a | T1000a | T1500a |
|---|---|---|---|---|---|
| Ingredients | |||||
| Fish mealb | 50.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Soybean mealb | 200.0 | 80.0 | 80.0 | 80.0 | 80.0 |
| Cottonseed mealb | 170.0 | 270.0 | 270.0 | 270.0 | 270.0 |
| Rapeseed mealb | 170.0 | 270.0 | 270.0 | 270.0 | 270.0 |
| Rice branb | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Wheat flourb | 242.7 | 192.7 | 192.2 | 191.7 | 191.2 |
| Choline chlorideb | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Fish oilb | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Soybean oilb | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Monocalcium Phosphateb | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Premixc | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Mold inhibitorb | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Tributyrind | 0 | 0 | 0.5 | 1.0 | 1.5 |
| Nutrient compositione | |||||
| Crude protein | 305.0 | 304.4 | 304.9 | 305.4 | 305.1 |
| Crude lipid | 52.0 | 51.7 | 52.1 | 51.5 | 51.5 |
| Crude ash | 71.6 | 71.2 | 71.5 | 71.5 | 71.3 |
| Gross energy (KJ/g) | 17.36 | 17.18 | 17.21 | 17.19 | 17.23 |
| Tributyrin (g/kg) | 0 | 0 | 0.47 | 0.93 | 1.47 |
- a PC: the positive control with 50 g/kg fish meal and 340 g/kg cottonseed and rapeseed meal; T0: the high cottonseed and rapeseed meal diet with 20 g/kg fish meal and 540 g/kg cottonseed and rapeseed meal; T500, T1000 and T1500 represent supplemented with 500, 1000 and 1500 mg/kg TB to the T0 diet, respectively.
- b Hunan Aohua Agriculture and Animal Husbandry Technology Co. Ltd.
- c Provided by MGOTer Bio-Tech Co. Ltd, Mineral Premix composition (per kg) as follows: vitamin A, 120,000 IU; vitamin D3, 40,000 IU; vitamin E, 480 mg; vitamin B1, 200 mg; vitamin B2, 280 mg; vitamin B8, 240 mg; vitamin K3, 200 mg; vitamin B12, 0.6 mg; calcium pantothenate, 720 mg; nicotinic acid, 1000 mg; folic acid, 60 mg; biotin, 1.2 mg; VC phosphatase, 6850 mg; acid, 3200 mg; iron, 4800 mg; magnesium, 4000 mg; zinc, 2000 mg; manganese, 800 mg; copper, 160 mg; cobalt, 12 mg; selenium, 4 mg; and iodine, 40 mg.
- d Guangdong Yiduoli Biotechnology Co. Ltd.
- e Crude protein, crude lipid, crude ash, gross energy and tributyrin contents were measured values.
The ingredients were finely ground, sieved (0.25 mm), mixed and supplemented with fish oil and soybean oil. A 10% volume of water of the weight of the ingredients was added. Then the ingredients were mixed with a feed mixer (Huacan Machinery Equipment Co., LTD) for 10 min. After mixing, the 1.5-mm diameter pellets were extruded using a single-screw cooking extruder (Zhengchang Cereal and Feed Machinery Co., LTD), and the processing conditions were as follows: 80 rpm speed screw and 100℃ temperature. And then the pellets were dried naturally in the shade. The experimental diets were then stored at −20℃ until use.
2.2 Experimental animals and feeding experiment
The experiment was carried out in the Reservoir using outdoor cage culture. Grass carp were obtained from the Hunan Fisheries Science Institute, China, and were housed for 10 days in a 5 m × 4 m × 3 m cage to acclimatize. During the acclimation, fish were fed with a commercial diet (crude protein 30%; crude lipid 5%; Changde, China). After the acclimation, grass carp fingerlings (average weight 9.59 ± 0.01 g) were randomly distributed into 15 floating cages (polyethylene material, 2 m × 2 m × 2 m, outdoor), and each diets had three cages, with each cage consisting 50 fish. The cages were fixed with a spacing of 2 m between each cage. The fish were fasted for 24 h prior to the experimental period.
For the next 10 weeks (from 9 July 2018 to 16 September 2018), the feeding rate was 3%–5% of body weight. Grass carp fingerlings were hand fed three times daily at 08:00, 12:00 and 17:00, and the fish could eat all diets within 20 min after each feeding. The water depth was approximately 20 m. The physicochemical parameters of the water, such as the temperature (27–30℃; measured using a thermometer), pH (6.5–7.5), dissolved oxygen (≥6.5 mg/L) and ammonia and nitrate (≤0.1 mg/L; measured using an LH-M900 portable colorimeter), were kept stable during the experimental period. The fish were cultured under a natural photoperiod.
2.3 Sample collection
All procedures involving live animals were performed in accordance with the Hunan Agriculture University regulations concerning the protection of experimental animals. At the end of the feeding trial, all fish were starved 24 h prior to the sample collection and weighed to determine growth performance. Prior to sampling, the fish were narcotized with 100 mg/L MS-222 (tricaine methanesulfonate, Sigma-Aldrich Co. LLC.; Shi et al., 2019). Three fish from each cage were selected for body index analysis. Three fish from each cage were used for collection of blood samples using 2 ml syringes (caudal vein). The blood was stored at 4°C for 24 h, and then the serum was isolated by centrifuged at 3000 g for 10 min (4°C) and kept at −80°C for detection of serum index. The midgut and hindgut were immersed in 4% paraformaldehyde. The midgut samples were removed in 2.0-ml enzyme-free centrifuge tube and then stored at −80°C.
2.4 Growth and morphology parameters
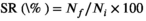
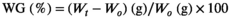
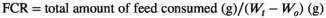
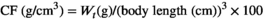
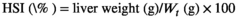
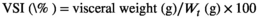
2.5 Composition analysis
Crude ash content of diets and whole fish was determined in a combustion oven at 550℃ for 6 h (P300, Nabertherm). The crude protein was determined by Kjeldahl digestion method (nitrogen ×6.25; InKjel 1225 M, WD 30, Behr). Soxhlet method was used to determine crude lipid content of diets (Soxtherm, C. Gerhardt GmbH & Co. KG; AOAC, 1995).
2.6 Analysis of serum immune indices
The levels of catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), total antioxidant capacity (T-AOC), glutathione peroxidase (GPx) and alkaline phosphatase (AKP) in serum were spectrophotometrically measured at 405, 450, 532, 405, 412, and 520 nm, respectively(Shi et al., 2020). At 340 nm wavelength, complement 3 (C3), complement 4 (C4) and immunoglobulin M (IgM) contents were determined by immunoturbidimetry (Zhejiang Yilikang Biotechnology Co., Ltd.). The used kits were as follows: CAT (A007-1-1), SOD (A001-3-2), MDA (A003-1-2), T-AOC (A015-2-1), GPx (A005-1-2), AKP (A059-2-2), C3 (Y10025), C4 (Y10026) and IgM (Y10024).
2.7 Intestinal histological assays
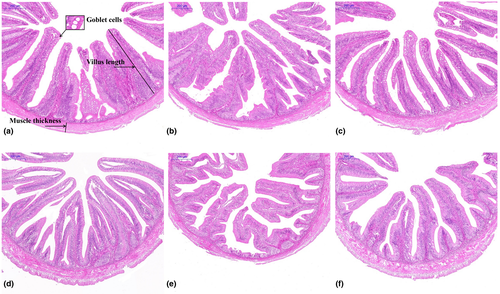
For intestinal histological analysis, samples of fish from each cage of the PC, T0 and T1000 groups were chosen based on growth data. Paraffin sections were prepared from the midgut and hindgut of each fish. The samples were immediately fixed in paraformaldehyde solution, and slides were prepared by subjecting the samples to washing, dehydration using different grades of alcohol, clearing with xylene and embedding in paraffin wax. The wax blocks were sectioned to a thickness of 5 μm and stained with hematoxylin and eosin (H&E). The height of the six highest intestinal villi was measured, and the average value was calculated. Goblet cells were counted on the previous selected highest villi, and then the average number of goblet cell per villus was determined (Liu et al., 2020).
2.8 Intestine RNA extraction, cDNA synthesis and qRT-PCR analysis
Total RNA was extracted from the muscle using TRIzol reagent (Invitrogen), following the manufacturer's protocol. The RNA samples were analysed using 1.5% agarose electrophoresis and quantified at 260 nm using a NanoDrop ND-2000 UV-Visible Spectrophotometer with OD260/OD280 values of 1.8–2.0. First-strand cDNA was synthesized using SuperScript III RNase H-reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Real-time polymerase chain reaction (PCR) analysis of the mRNA was performed using a Bio-Rad CFX96 System with SYBR Premix Ex TaqII (Takara). Each PCR assay (total volume of 25 μl) was composed of SYBR Premix Ex TaqII (2×, 12.5 μl), forward primer (1 μl), reverse primer (1 μl), cDNA (2 μl) and sterile double-distilled water (8.5 μl). The primer sequences were designed based on the published grass carp sequences or by RNA-seq in our laboratory (Table 2). The β-actin gene was used as the internal control. Each assay was performed in triplicates. Gene expression was calculated using the 2−ΔΔCT method (Livak & Schmittgen, 2001).
| Gene | Forward primer sequence (5′→3′) | Reverse primer sequence (5′→3′) | PCR efficiency (%) | Accession number |
|---|---|---|---|---|
| IL-1β | CCCTCACCTGGTCTTGGA | TACTTGGCACCTGGCACA | 98 | JQ692172.1 |
| IL-6 | CAGCAGAATGGGGGAGTTATC | CTCGCAGAGTCTTGACATCCTT | 97 | JN663841 |
| IL-8 | ATGAGTCTTAGAGGTCTGGGT | ACAGTGAGGGCTAGGAGGG | 98 | JN663841 |
| IL-10 | AATCCCTTTGATTTTGCC | GTGCCTTATCCTACAGTATGTG | 96 | HQ388294 |
| TGF-1β | TTGGGACTTGTGCTCTAT | AGTTCTGCTGGGATGTTT | 97 | EU099588 |
| NF-κB p65 | GTAGAGGGTGTAGGAAGAAG | GAGTCGGGTAGATGCTGTTGT | 100 | KJ526214 |
| IκBα | TCTTGCCATTATTCACGAGG | TGTTACCACAGTCATCCACCA | 99 | KJ125069 |
| TLR4 | TTCCACCTATTCATCTTTGC | ACTTTACGGCTGCCCATT | 99 | EU699768.1 |
| TLR7 | GAGCATACAGTTGAGTAAACGCAC | TCTCCAAGAATATCAGGACGATAA | 97 | FJ610253.1 |
| ZO-1 | CGGTGTCTTCGTAGTCGG | CAGTTGGTTTGGGTTTCAG | 100 | KJ000055 |
| ZO-2 | TACAGCGGGACTCTAAAATGG | TCACACGGTCGTTCTCAAAG | 100 | KM112095 |
| Claudin-12 | CCCTGAAGTGCCCACAA | GCGTATGTCACGGGAGAA | 98 | KF998571 |
| Claudin-b | GAGGGAATCTGGATGAGC | ATGGCAATGATGGTGAGA | 98 | KF193860 |
| Claudin-c | GAGGGAATCTGGATGAGC | CTGTTATGAAAGCGGCAC | 97 | KF193859 |
| Occludin | TGAGCAGCGGTTCAGAGT | TATTAGCAATGGGAGGGA | 97 | KF193855 |
| MLCK | GAAGGTCAGGGCATCTCA | GGGTCGGGCTTATCTACT | 98 | KM279719 |
| β-actin | GATGATGAAATTGCCGCACTG | ACCGACCATGACGCCCTGATGT | 100 | M25013 |
2.9 Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences statistical software package (version 24.0; SPSS Inc.), and the data are presented as the mean ± standard error. All data were checked for normality and homogeneity of variance using the Shapiro-Wilk and Levene's tests, respectively. Significant differences between means were determined using a one-way analysis of variance, and Duncan's multiple range test was used to determine the significance level of p < .05.
3 RESULTS
3.1 Growth and morphology parameters
There were no significant differences in the SR, CF, and HSI of grass carp among the different treatment groups. Compared with that of the PC group, the FCR of the T0 group was significantly increased (p < .05), and the supplementation of 1000 mg/kg tributyrate significantly reduced the FCR compared with that of the T0 group (p < .05). The WG in the T0 group was significantly lower than that in the PC group (p < .05). There was no significant difference in the WG between the T1000 and PC groups (Table 3).
| Index | PC | T0 | T500 | T1000 | T1500 | p Value |
|---|---|---|---|---|---|---|
| Initial weight (g) | 9.59 ± 0.01 | 9.62 ± 0.04 | 9.56 ± 0.01 | 9.59 ± 0.02 | 9.60 ± 0.01 | .436 |
| Final weight (g) | 73.63 ± 0.20b | 67.10 ± 1.05a | 67.40 ± 3.14a | 70.06 ± 1.33ab | 67.92 ± 1.00a | .022 |
| WGR (%) | 667.15 ± 2.40b | 597.41 ± 8.05a | 605.17 ± 33.79a | 630.26 ± 13.63ab | 606.94 ± 10.32a | .017 |
| FCR (%) | 1.14 ± 0.02a | 1.28 ± 0.05b | 1.22 ± 0.04ab | 1.13 ± 0.01a | 1.26 ± 0.03b | .020 |
| SR (%) | 98.00 ± 1.15 | 97.33 ± 1.76 | 97.33 ± 0.67 | 98.67 ± 0.67 | 98.00 ± 0.00 | .089 |
| CF (g/cm3) | 2.02 ± 0.02 | 1.99 ± 0.03 | 2.02 ± 0.06 | 1.89 ± 0.09 | 2.02 ± 0.03 | .783 |
| HSI (%) | 2.33 ± 0.16 | 2.52 ± 0.09 | 2.37 ± 0.07 | 2.48 ± 0.16 | 2.42 ± 0.06 | .543 |
| VSI (%) | 18.46 ± 0.26c | 17.86 ± 0.46bc | 16.87 ± 0.64ab | 17.95 ± 0.37bc | 16.47 ± 0.48a | .023 |
Note
- Data indicate the mean values of three replicate cages per treatment (three fish per replicate cage). Mean values with different superscripts in a row are significantly different (one-way ANOVA, p < .05). PC: the positive control with 50 g/kg fish meal and 340 g/kg cottonseed and rapeseed meal; T0: the high cottonseed and rapeseed meal diet with 20 g/kg fish meal and 540 g/kg cottonseed and rapeseed meal; T500, T1000 and T1500 represent supplemented with 500, 1000 and 1500 mg/kg TB to the T0 diet, respectively. Weight gain rate (WGR, %) = (final body weight − initial body weight)/initial body weight × 100; feed conversion rate (FCR) = total amount of the feed consumed/(final body weight − initial body weight); survival rate (SR, %) = final number of fish/initial number of fish × 100; condition factor (CF, g/cm3) = 100 × whole body weight/(body length)3; hepatosomatic index (HSI, %) = liver weight/whole body weight × 100; viserosomatic index (VSI, %) = visceral weight/whole body weight × 100.
3.2 Chemical proximate composition of whole body
As shown in Table 4, there were no significant differences in moisture, crude protein, crude lipid and crude ash contents among the treatment groups (p > .05).
| Index | PC | T0 | T500 | T1000 | T1500 | p Value |
|---|---|---|---|---|---|---|
| Moisture | 722.3 ± 5.4 | 720.6 ± 1.1 | 724.7 ± 5.6 | 728.1 ± 3.0 | 708.5 ± 10.2 | .250 |
| Crude lipid | 90.5 ± 2.1 | 90.8 ± 2.7 | 89.1 ± 3.0 | 87.8 ± 0.3 | 97.7 ± 5.1 | .250 |
| Crude Protein | 138.4 ± 1.7 | 140.1 ± 1.5 | 140.6 ± 0.4 | 135.5 ± 2.2 | 141.3 ± 1.3 | .139 |
| Crude ash | 25.0 ± 0.6 | 25.0 ± 0.4 | 24.5 ± 1.1 | 24.3 ± 0.5 | 25.3 ± 0.5 | .805 |
Note
- Data indicate the mean values of three replicate cages per treatment (three fish per replicate cage). Mean values with different superscripts in a row are significantly different (one-way ANOVA, p < .05). PC: the positive control with 50 g/kg fish meal and 340 g/kg cottonseed and rapeseed meal; T0: the high cottonseed and rapeseed meal diet with 20 g/kg fish meal and 540 g/kg cottonseed and rapeseed meal; T500, T1000 and T1500 represent supplemented with 500, 1000 and 1500 mg/kg TB to the T0 diet, respectively.
3.3 Serum immune indices
The levels of CAT and AKP were not significantly different among the treatment groups (Table 5). The levels of SOD, GPx and C3 in the T0 group were significantly lower than those in the PC group (p < .05). Compared with that of the T0 group, the levels of SOD, T-AOC and IgM were significantly higher in the T1000 group, and the levels of SOD and C3 were significantly higher in the T1500 group (p < .05). The MDA content in the T1000 and T1500 groups was significantly lower than that in the PC group (p < .05). Moreover, there were no significant differences in the levels of SOD and C4 between the T1000 and PC groups. The levels of T-AOC and IgM in the T1000 group were significantly higher than those in the PC group (p < .05).
| Index | PC | T0 | T500 | T1000 | T1500 | p Value |
|---|---|---|---|---|---|---|
| CAT (U/ml) | 1.60 ± 0.35 | 1.25 ± 0.05 | 1.53 ± 0.25 | 1.88 ± 0.47 | 1.68 ± 0.03 | .640 |
| SOD (U/ml) | 63.37 ± 0.75b | 59.34 ± 1.61a | 62.01 ± 0.81ab | 62.73 ± 0.21b | 62.98 ± 0.63b | .041 |
| MDA (nmol/ml) | 8.15 ± 0.04bc | 8.45 ± 0.60c | 7.87 ± 0.41abc | 6.82 ± 0.41a | 7.11 ± 0.14ab | .028 |
| T-AOC (mmol/ml) | 0.08 ± 0.01b | 0.07 ± 0.00ab | 0.08 ± 0.00ab | 0.11 ± 0.01c | 0.07 ± 0.00a | .000 |
| GPx (µmol/L) | 129.01 ± 2.16b | 108.06 ± 5.63a | 111.00 ± 3.77a | 117.10 ± 2.73a | 110.00 ± 1.72a | .001 |
| C3 (g/L) | 0.61 ± 0.04b | 0.43 ± 0.03a | 0.48 ± 0.02a | 0.48 ± 0.04a | 0.66 ± 0.04b | .000 |
| C4 (g/L) | 0.11 ± 0.00c | 0.10 ± 0.01abc | 0.09 ± 0.01ab | 0.11 ± 0.01bc | 0.09 ± 0.01a | .029 |
| IgM (g/L) | 0.34 ± 0.02a | 0.34 ± 0.03a | 0.47 ± 0.03b | 0.45 ± 0.05b | 0.40 ± 0.01ab | .009 |
| AKP (King's unit/100 ml) | 6.43 ± 0.16 | 5.97 ± 0.08 | 6.12 ± 0.14 | 6.25 ± 0.14 | 5.97 ± 0.08 | .059 |
Note
- Data indicate the mean values of three replicate cages per treatment (three fish per replicate cage). Mean values with different superscripts in a row are significantly different (one-way ANOVA, p < .05). PC: the positive control with 50 g/kg fish meal and 340 g/kg cottonseed and rapeseed meal; T0: the high cottonseed and rapeseed meal diet with 20 g/kg fish meal and 540 g/kg cottonseed and rapeseed meal; T500, T1000 and T1500 represent supplemented with 500, 1000 and 1500 mg/kg TB to the T0 diet, respectively. CAT, catalase; SOD, superoxide dismutase; MDA, malondialdehyde; T-AOC, total antioxidant capacity; GPx, glutathione peroxidase; C3, complement 3; C4, complement 4; IgM, immunoglobulin M; AKP, alkaline phosphatase.
3.4 Intestinal histological structure
In the midgut of the grass carp, compared to that of the T0 group, the villus height in the T1000 group was higher; however, the villus height of those in the T1000 group was not significantly different from that of the PC group. In the hindgut of the grass carp, the muscle thickness in the T0 group was significantly lower than that in the PC and T1000 groups (p < .05). The number of goblet cells in the T1000 group tended to be higher than those in the T0 group (Table 6).
| Index | PC | T0 | T1000 | p Value |
|---|---|---|---|---|
| Midgut | ||||
| Villus height (μm) | 746.86 ± 22.56 | 655.76 ± 16.61 | 712.49 ± 23.96 | .060 |
| Muscle thickness (μm) | 31.14 ± 1.47a | 54.71 ± 6.25b | 52.25 ± 2.11b | .010 |
| Goblet cells (A/root) | 61.20 ± 4.33 | 53.00 ± 3.39 | 62.20 ± 2.37 | .162 |
| Hindgut | ||||
| Villus height (μm) | 658.89 ± 27.93 | 616.67 ± 19.27 | 643.91 ± 0.72 | .366 |
| Muscle thickness (μm) | 55.37 ± 1.69b | 29.49 ± 1.21a | 66.44 ± 3.64c | .000 |
| Goblet cells (A/root) | 82.20 ± 4.15 | 74.20 ± 1.83 | 79.80 ± 1.50 | .152 |
Note
- Data indicate the mean values of three replicate cages per treatment (three fish per replicate cage). Mean values with different superscripts in a row are significantly different (one-way ANOVA, p < .05). PC: the positive control with 50 g/kg fish meal and 340 g/kg cottonseed and rapeseed meal; T0: the high cottonseed and rapeseed meal diet with 20 g/kg fish meal and 540 g/kg cottonseed and rapeseed meal; T1000: supplemented with 1000 mg/kg TB to the T0 diet.
In the midgut and hindgut of the grass carp, the intestinal villus in the T0 group were not arranged neatly compared to those of the PC group. The intestinal histological structure in the T1000 group was intact, and the shape of the villus clusters was regular compared with that in the T0 group (Figure 1).
3.5 Gene expression related to the intestinal immune barrier
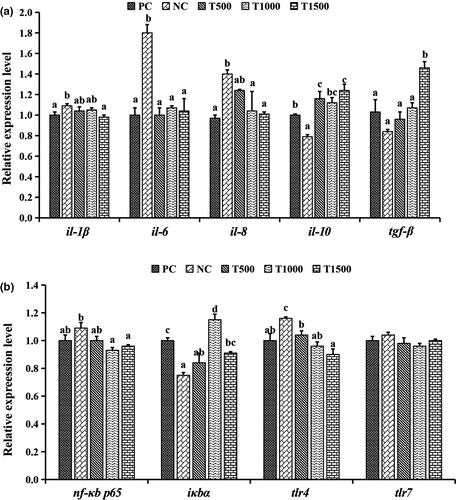
There was no significant difference in the tlr-7 gene expression among the treatment groups. The mRNA expression levels of il-1β, il-6, il-8 and tlr-4 in the intestines of the grass carp in the T0 group were significantly upregulated (p < .05), and the mRNA expression levels of il-10, tgf-β and iκbα were significantly downregulated (p < .05) compared with that of the PC group. Compared with that of the T0 group, TB supplementation significantly upregulated (p < .05) the mRNA expression level of intestinal IL-10 and significantly downregulated (p < .05) the mRNA expression levels of il-6 and tlr-4. Compared with the T0 group, the mRNA expression levels of il-8, nf-κb p65 and tlr-4 in the T1000 and T1500 groups were significantly downregulated (p < .05), and the mRNA expression level of iκbα was significantly upregulated (p < .05). In addition, there were no significant differences in the mRNA expression levels of il-1β, il-6, il-8, nf-κb p65 and tlr-4 between fish fed the PC- and TB-supplemented diets (Figure 2).
3.6 Gene expression related to the intestinal physical barrier
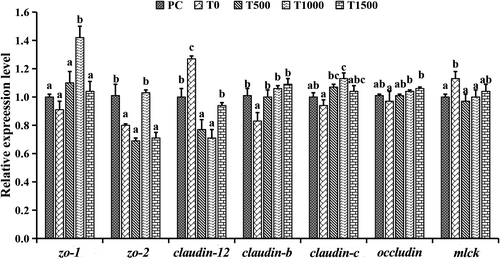
The mRNA expression levels of zo-2 and Claudin-b in the T0 group were significantly lower than those in the PC group (p < .05). The mRNA expression levels of claudin-12 and mlck in the T0 group were significantly higher than those in the PC group (p < .05). The mRNA expression levels of zo-1, zo-2, claudin-b, claudin-c and occludin in the T1000 group were significantly upregulated compared to those in the T0 group after dietary supplementation of TB (p < .05). The mRNA expression levels of claudin-12 and mlck in the T500 and T1000 groups were significantly lower than those in the T0 group (p < .05). There were no significant differences in the mRNA expression levels of claudin-b, occludin and mlck between fish fed the PC- and TB-supplemented diets. The mRNA expression levels of zo-1 and claudin-c in the T1000 group were significantly higher than those in the PC group (p < .05; Figure 3).
4 DISCUSSION
Currently, the substitution of CRM for FM and soybean meal has been reported in grass carp (Liu et al., 2019; Yao et al., 2019), Yellow River carp (Cyprinus carpio var; Wang et al., 2020) and other aquatic animals. In the present study, CRM instead of FM and soybean meal significantly lowered the WG of grass carp. Yuan et al. (2014) also showed that the specific growth rate of grass carp was significantly reduced when the content of CRM in the diet was 530 g/kg of the diet. The main reason is that CRM contains many anti-nutrition factors (Prabu et al., 2017). For example, the binding of free gossypol and lysine in cottonseed meal leads to a decrease in lysine activity, which subsequently decreases growth performance (Wilson et al., 1981). Further, under the action of myrosinase, thioglucosinolates in rapeseed hydrolyse to isothiocyanate, azolidin, nitrile and other toxic substances (Clay et al., 2009).
The present study showed that the addition of TB to high CRM diets tended to improve the growth performance of grass carp so that the addition of 1000 mg/kg TB presented the same growth as compared with the PC group. These results are consistent with results obtained from common carp (Xie et al., 2021) and shrimp (Liu et al., 2021). This result may be due to the fact that TB can improve the intestinal health of fish. Studies have shown that the intestinal digestive capacity by the regulation of growth and development is closely related to intestinal villus length in common carp (Xie et al., 2021). Villus length and muscular thickness are important indicators of the efficiency of intestinal digestion and absorption (Pirarat et al., 2011). In this study, we found that villus length and goblet cell numbers of the midgut and hindgut of grass carp fed the high CRM diet were reduced compared with the PC group, whilst the addition of 1000 mg/kg TB to the diet increased villus length and the number of goblet cells. Studies have shown that TB is broken down into butyric acid in the intestine (Conley et al., 1998); however, the previous research indicated that dietary sodium butyrate supplementation to low FM feed improved the intestinal structure of rice field eels (Monopterus albus) and promoted the digestion and absorption of nutrients (Zhang et al., 2020). Moreover, butyric acid is easily absorbed by colonic mucosal epithelial cells before being oxidized into ketone bodies, which participate in the synthesis of adenosine triphosphate (ATP) and provide energy for the growth and development of intestinal cells (Macfarlane & Macfarlane, 2011). However, the addition of 1500 mg/kg TB in this study appeared to induce adverse effects on the growth performance of grass carp. High dietary TB levels (400 and 800 mg/kg) have also been reported to reduce the growth performance of black sea bream (Volatiana et al., 2020). Because TB has a bitter odour (Donovan et al., 2016), adding high concentrations of TB to aquaculture diets may affect the feed intake of grass carp.
Aquatic animal health status is closely related to antioxidant capacity (Liang et al., 2018). Free radicals and reactive oxygen species are continuously generated under normal or stressful conditions, and to avoid or repair the damage that these compounds may cause in tissues, organisms possess protection systems, such as antioxidant defense enzymes (i.e., SOD, GSH, CAT, MDA and T-AOC; Liu et al., 2018). High CRM has been shown to have negative effects on the antioxidant capacity of red sea bream (Dossou et al., 2019). The results of the present study are consistent with these results. The levels of CAT, SOD, GPx and T-AOC in the serum of grass carp were decreased. In addition, the results of the present study indicated that the addition of 500–1500 mg/kg TB to the high CRM diet significantly improved the antioxidant enzyme activity in the serum, with an optimal concentration of 1000 mg/kg. Similar studies have confirmed that TB can significantly increase the serum content of SOD, GPx and CAT and significantly reduce the content of MDA under oxidative stress in blunt snout bream (Liang et al., 2021). A possible mechanism is that TB can increase the content of ATP (Li, Hou, et al., 2015; Li, Feng, et al., 2015), which plays an important role in activating PI3K/Akt signalling (Chen et al., 2014), further activating the Nrf2 signalling pathway and subsequently promoting the transcription of antioxidant enzymes and improving its gene expression, which improves the activity of antioxidant enzymes (Wu et al., 2018).
The development of animal immunity depends on a series of immune substances, such as complement factor, immunoglobulin and antimicrobial peptides (Ni et al., 2016). The immune system of fish is regulated by nutrients, which can exhibit immunomodulatory functions (Pulsford et al., 1995). This study showed that the content of high CRM in the diet significantly decreased the immunity of grass carp. Studies have shown that dietary TB supplementation can improve immune response (Li, Hou, et al., 2015; Li, Feng, et al., 2015). Complements 3 and 4 are the main components of the complement system (Mori et al., 1989), which immunoglobulin M is the most important immunoglobulin in fish and is the first antibody to be produced when the body is stimulated by antigens (Pilström & Bengtén, 1996). AKP is a phosphohydrolase that is considered an important non-specific indicator in crustaceans and an evaluation index that reflects the health status of aquatic animals (Han et al., 2015). In this study, the addition of TB to a high CRM diet increased the levels of AKP, C3, C4 and IgM in the serum, which indicated that TB can improve immune function of grass carp. Similarly, various previous studies have reported that TB supplementation in feed can also increase the production of immunoglobulins (Liang et al., 2021) and the AKP activity (Liu et al., 2021). A possible reason is that TB can improve immune function by controlling the inflammatory response and regulating the expression of anti-inflammatory factors (Leonel et al., 2013).
Our experiment contributed to the knowledge of the expression of inflammation-related genes in the intestines of grass carp. Intestinal inflammation is a biological response involving multiple types of immune and non-immune cells, usually triggered by exogenous substances and products of tissue damage, accompanied by the production of pro-inflammatory cytokines and the recruitment and activation of immune cells (Karin & Clevers, 2016). Intestinal immunity is related to inflammation, and cytokines play an important role in regulating intestinal inflammation in fish. Studies have shown that the upregulation of pro-inflammatory factors, such as il-1β, il-6 and il-8, aggravates intestinal inflammation (Papadakis & Targan, 2000). In order to prevent endothelial cell damage caused by inflammation, the body inhibits the production of inflammatory factors by secreting the anti-inflammatory factors il-10 and tgf-β (Chen & Manning, 1996). The results of the present study indicated that the high CRM diets significantly upregulated the expression levels of il-1β, il-6 and il-8 and downregulated the expression levels of il-10 and tgf-β. The addition of TB significantly downregulated the expression levels of il-1β, il-6 and il-8 and upregulated the expression levels of il-10 and tgf-β in the intestine. Previous studies have reported that TB supplementation can increase the expression levels of anti-inflammatory cytokines, including il-10, in the intestine of juvenile yellow drum (Zhu et al., 2020) and tgf-β in the intestine of blunt snout bream (Liang et al., 2021) and can reduce the mRNA levels of the pro-inflammatory cytokine il-1β in broiler chickens (Hansen et al., 2021). These results are consistent with our findings and indicate that TB supplementation can improve immunocompetence by regulating the production of inflammatory cytokines. The addition of 1500 mg/kg TB in this study appeared to induce adverse effects on the intestinal immune barrier. This may be related to the bitter taste of TB that deterred meal consumption; however, this requires further study.
NF-κB is a key transcriptional regulator of immune regulation and plays a key role in the regulation of cytokine activation (Zhu et al., 2013). TB attenuates the inflammatory response by inhibiting NF-κB activation (Miyoshi et al., 2011). The results from the present study showed that the high CRM diet upregulated the expression level of nf-κb p65, and the addition of appropriate quantities of TB downregulated its expression in the intestine. This expression trend was consistent with the expression levels of il-1β, il-6 and il-8 in the intestine. Therefore, the results indicate that TB may affect the expression of pro-inflammatory and anti-inflammatory factors in the intestines of grass carp by regulating the transcription of nf-κb. Generally, nf-κb binds to i-κb in its inactive form in cells. When stimulated by various activating factors, i-κb is degraded by ubiquitination, and nf-κb is complexed with nf-κb/iκb (Kumar et al., 2012). The substance dissociates and translocates into the nucleus and binds to the target gene to regulate gene transcription. Studies on grass carp have shown that the downregulation of iκbα expression promotes the nuclear translocation of nf-κb (Chen et al., 2018). The results of this experiment indicated that the high CRM downregulated the mRNA expression level of iκbα, and the addition of appropriate TB upregulated its expression level of iκbα in the intestine. In addition, nf-κb gene expression is regulated by upstream Toll-like receptor (TLR) signalling molecules (Cario et al., 2007). TLRs are a vital source recognition receptor in vertebrates, and tlr4 and tlr7 play a key important role in maintaining intestinal immune barriers in animals (Cario & Podolsky, 2000). It has been reported that the upregulation of TLR expression in grass carp leads to the activation of the nf-κb signalling pathway (Guo et al., 2018). The results of this experiment also indicated that the high CRM upregulated the expression levels of tlr4 and tlr7, and the addition of appropriate TB downregulated these expression levels in the intestine. Therefore, we hypothesize that TB may regulate the intestinal immune barrier of grass carp by regulating the tlr/nf-κb signalling pathway.
The fish intestinal tract is the first physical barrier to prevent pathogen invasion, which is crucial to ensure the intestinal health of fish (Cario et al., 2007). Tight junction proteins between fish cells are divided into two major categories: cytoplasmic proteins (such as ZOs) and transmembrane proteins (such as occludin and claudins; Luo et al., 2014). Studies in aquatic animals have shown that upregulation of zo-1, zo-2, occludin, claudin-b and claudin-c gene expression and downregulation of claudin-12 gene expression can enhance intercellular structural integrity (Li, Hou, et al., 2015; Li, Feng, et al., 2015). This study showed that a high CRM diet downregulated the expression levels of claudin-b, claudin-c, occludin, zo-1 and zo-2 in the intestines of grass carp and upregulated the expression levels of claudin-12 in the intestine, indicating that high CRM may increase the intestinal barrier structural damage caused by the cell gap by regulating the expression of tight junction protein genes. Cunningham and Turner (2012) reported that the expression of tight junctions in the intestinal epithelium is regulated by the signalling molecule myosin light chain kinase (mlck). Studies in mice have shown that the upregulation of mlck expression in the intestine results in the downregulation of occludin, claudin-1 and zo-1 gene expression, leading to intestinal damage (Nahidi et al., 2013). In addition, dietary zinc (Zn) deficiency upregulates mlck gene expression to downregulate zo-1, zo-2, occludin, claudin-b and claudin-c mRNA levels, impairing the physical barrier function in the intestine of grass carp (Song et al., 2017). This study showed that a high CRM diet significantly upregulated mlck gene expression. Compared with that of T0 group, the addition of TB in the diet significantly downregulated mlck gene expression in the intestines of grass carp, and the mRNA levels of claudin-b, claudin-c, zo-1, zo-2 and occludin were negatively correlated with mlck, whilst Claudin-12 mRNA levels were positively correlated with mlck. Similar studies have shown that TB supplementation in a high soybean meal diet significantly downregulated mlck gene expression in the intestines of juvenile yellow drum (Tan et al., 2020). These results suggest that adding TB to high CRM diets can enhance intestinal physical barrier function and protect intestinal health by downregulating mlck gene expression in the intestine.
5 CONCLUSIONS
The high CRM diet of grass carp reduced growth performance, induced immune damage and impaired intestinal barrier function. The addition of an appropriate level of TB to these diets improved the growth performance of juvenile grass carp. Furthermore, this study found that TB supplementation can protect intestinal immunity and physical barrier function, which may be related to TLR/NF-κB and MLCK signalling pathway. The suggested optimal TB level was 1000 mg/kg in a high CRM diet to maximize the growth rate of juvenile grass carp. Thus, we will study the regulatory effect of tributyric acid on TLR/NF-κB and MLCK signalling pathways and its antiviral ability in the future.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (32172985), the National Key R&D Program of China (2019YFD0900200), the State Key Laboratory of Developmental Biology of Freshwater Fish (2019KF003), the Hunan Provincial Natural Science Foundation (2020JJ4044) and the Hunan Provincial Key Laboratory of Nutrition and Quality Control of Aquatic Animals (2018TP1027). The authors thank the personnel of these teams for their kind assistance.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




