Supplementing tributyrin to cottonseed protein concentrate-based diets can improve growth performance, lipid metabolism and distal intestinal immunity in hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂)
Abstract
An 8-week trial was performed to evaluate tributyrin effects on growth, disease resistance, lipid metabolizing, distal intestinal inflammation-, tight junctions- and apoptosis-related gene expressions caused by high-level cottonseed protein concentrate (CPC) substitution of fishmeal. Fish were fed one fishmeal (50% fishmeal) and six CPC diets (27.5% fishmeal and 25.2% CPC). Seven isonitrogenous (48.0%) and isolipidic (9.0%) were formulated to contain 0.0 (FM), 0.0 (S00), 0.2 (S02), 0.4 (S04), 0.8 (S08), 1.6 (S16) and 3.2 (S32) g/kg tributyrin. Fish (initial body weight: 7.3 ± 0.0 g) were randomly assigned to 21 300-L fiberglass buckets. Results indicated that optimal tributyrin improved weight gain (WG), specific growth rate (SGR), feed utilization, reduced serum triglyceride and total cholesterol levels. Based on Vibrio alginolyticus challenge test, supplementation with 0.4–3.2 mg/kg tributyrin significantly improved percent survival. Optimal tributyrin enhanced distal intestinal development by increasing fold height/intestinal diameter ratio and goblet cell number, and upregulated jam1, claudin3 and ZO-1; and upregulated TGF-β1, hepcidin, downregulated TNF-α, IL-1β, caspase-2 and caspase-8 mRNA levels. Generally, optimal tributyrin could improve growth performance, disease resistance, hepatic lipid metabolism capacity, distal intestinal immunity and tight junctions. Based on SGR, dietary optimal tributyrin supplementation in hybrid grouper was estimated to be 790.0 mg/kg.
1 INTRODUCTION
Aquaculture contributes high-quality protein at a relatively reasonable cost, providing nearly 16% of the world's human-acquired protein (Naseem et al., 2021). Protein is also one of the main nutrients for aquatic animals, and the quality and quantity of protein directly determine the cost of feed. Fishmeal has been used as the main protein source for aquafeeds for its high palatability and balance of amino acids (Han et al., 2018). Due to the impact of El Niño and other climatic events, global fishmeal production has decreased by 26.5% from 2000 to 2018 and the price of fishmeal has increased from 452 USD/T in 2000 to 1596.54 USD/T in 2018 (Jannathulla et al., 2019), which has severely constrained the development of the aquaculture industry. To alleviate the contradiction between the fast growing aquaculture industry and the scarce fishmeal resources, all the scholars have suggested to find new fishmeal protein substitutes (Agboola et al., 2021). Cottonseed protein concentrate (CPC) is a protein product obtained from cottonseed through low-temperature extraction, dephenolization and detoxification process, which reduces the Maillard reaction and reduces the content of gossypol from 1000 ppm to 0.79 ppm (tested by SGS, China). And it has a greater advantage compared with the traditional process of high-temperature pressing. Previous researches in our lab found that CPC can be used relatively well on both juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) (Yin et al., 2018), juvenile golden pompano (Trachinotus ovatus) (Shen et al., 2020) and silver sillago (Sillago sihama Forsskál) (Liu et al., 2020). In hybrid grouper, CPC can replace 34.37% of fishmeal protein without significant negative effects. However, further increase in CPC addition level could inhibit growth performance and intestinal development, which is closely related to cytokines (Yin et al., 2018). Tributyrin is a lipid substance produced from three molecules of butyric acid and one molecule of glycerol catalysed by sulfuric acid and is a precursor substance of butyric acid (Li et al., 2020). Compared with the traditional butyrate, tributyrin is characterized by slow release at a fixed point in the distal intestine after passing through the stomach, performs the corresponding physiological functions, and will not react with acidic gastric juice to produce harmful substances (Jin, 2018; Wu, 2019). Tributyrin can improve the antioxidant capacity of the intestinal tract of crucian carp (Carassius auratus gibelio) and activate the mTOR pathway through insulin-like growth factor I to improve the utilization of feed protein in fish (Jiang, 2019). It can also improve the expression and activity of PepT1 through the transcription factor CDX2, Sp1, facilitate the efficient utilization of feed protein by grass carp (Ctenopharyngodon idellus) (Liu, 2018) and enhance digestive enzyme activity, liver antioxidant activity and intestinal tissue development of hybrid grouper (Jin, 2018). However, there are few reports on the role of tributyrin in improving the tolerance of fish to plant protein.
In this study, we investigated the effects of tributyrin on the growth, hepatic lipid metabolism capacity, serum biochemical parameters and intestinal health of hybrid grouper using CPC after replacing 45.0% of fishmeal protein in the control group and supplemented with different levels of tributyrin to evaluate its positive effects and the optimal additional level in low fishmeal diets. This will help provide a theoretical basis for the development of low fishmeal feeds for hybrid grouper.
2 MATERIALS AND METHODS
2.1 Experimental diets
CPC was utilized to replace 45% of fishmeal protein in the control diet (50% fishmeal), after which 0.0, 0.2, 0.4, 0.8, 1.6 and 3.2 mg tributyrin per 1 kg diet (purity ≥ 50%) were supplemented to the replacement diets (27.5% fishmeal, 25.2% CPC) to make seven groups of isonitrogenous and isolipidic experimental diets. TASA red fishmeal, CPC, US soybean meal, corn gluten meal and wheat gluten meal were used as the main protein sources; fish oil and soy lecithin were used as the main lipid sources; wheat flour were used as the carbohydrate source. All solid raw materials were crushed and passed through 60 mesh sieve and weighted according to Table 1. First, the V-mixer was used to mix TASA brown fishmeal, CPC, US soybean meal, corn gluten meal, wheat gluten and wheat flour for 5 min at the primary level. At the same time, calcium monophosphate, vitamin, mineral premix, vitamin C, antioxidant, choline chloride, microcrystalline cellulose and tributyrin were also mixed for 5 min using the V-mixer. And then the two mixtures were mixed together simultaneously for 10 min to obtain the raw material mixture. Fish oil and soy lecithin were added to the raw material mixture after thorough mixing and blending through a 40 mesh sieve. After that, various oil and water containing choline chloride were supplemented to the mixture and mixed thoroughly again using a Hobart-type mixer (Food mixer B60, Guangdong Henglian Food Machinery Co., Ltd.) for 10 min. A twin-screw extruder (South China University of Science and Technology General Factory, F-75) was used to process the final mixture into pellets (diameter of 2.5 mm). Air dry the molded feed at room temperature for 48 h and stored in sealed Ziploc bags at −20°C until used. The approximate compositions and the amino acid contents of the seven group diets are shown in Tables 1 and 2, respectively.
| Ingredients | Dietary tributyrin levels (g/kg dry diet) | ||||||
|---|---|---|---|---|---|---|---|
| FM | S00 (0.0) | S02 (0.2) | S04 (0.4) | S08 (0.8) | S16 (1.6) | S32 (3.2) | |
| TASA brown fishmeala | 500.0 | 275.0 | 275.0 | 275.0 | 275.0 | 275.0 | 275.0 |
| CPCb | 0.0 | 251.6 | 251.6 | 251.6 | 251.6 | 251.6 | 251.6 |
| US soybean meal | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Corn gluten meal | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Wheat gluten | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Wheat flour | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 |
| Fish oil | 13.4 | 33.6 | 33.6 | 33.6 | 33.6 | 33.6 | 33.6 |
| Soy lecithin | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Calcium monophosphate | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Vitamin, mineral premixc | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Vitamin C | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Antioxidant | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Choline chloride | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| MCCd | 60.6 | 13.8 | 13.6 | 13.4 | 13.0 | 12.2 | 10.6 |
| Tributyrin | 0.0 | 0.0 | 0.2 | 0.4 | 0.8 | 1.6 | 3.2 |
| Proximate composition (g/kg)e | |||||||
| Crude protein | 479.4 | 480.2 | 480.8 | 480.6 | 479.9 | 481.8 | 481.2 |
| Crude lipid | 89.7 | 91.4 | 92.1 | 91.8 | 92.7 | 93 | 92.4 |
| Moisture | 94.5 | 97 | 96.8 | 94.4 | 95 | 96.1 | 96.3 |
- a TASA brown fishmeal: 68.2% crude protein, 9.0% crude lipid, bought from Zhanjiang HaiBao Feed Co. Ltd.
- b CPC: cottonseed protein concentrate, 610.0% crude protein, 0% crude lipid, bought from Jinlan plant protein Co. Ltd.
- c Vitamin, mineral premix (mg/kg of diet): thiamine, 5.0; riboflavin, 10.0; vitamin A, 5000.0 IU; vitamin E, 40.0; vitamin D3, 1000.0 IU; menadione, 10.0; pyridoxine, 10.0; cyanocobalamin, 0.02; biotin, 0.1; calcium pantothenate, 20.0; folic acid, 1.0; niacin, 40.0; vitamin C, 150.0; iron, 100.0; iodine, 0.8; cupper, 3.0 mg; zinc, 50.0 mg; manganese, 12.0; selenium, 0.3; cobalt, 0.2.
- d MCC: Microcrystalline cellulose.
- e Crude protein, crude lipid and moisture content are measured values.
| Amino acids | Diets | |
|---|---|---|
| FM | S00 (0.0) | |
| Methionine | 13.4 | 10.0 |
| Lysine | 33.7 | 24.9 |
| Threonine | 19.0 | 15.2 |
| Isoleucine | 19.9 | 13.7 |
| Histidine | 15.2 | 14.8 |
| Valine | 22.4 | 19.6 |
| Leucine | 32.8 | 28.8 |
| Arginine | 25.9 | 34.2 |
| Phenylalanine | 20.0 | 20.2 |
| Tyrosine | 12.7 | 8.7 |
| Aspartic acid | 38.4 | 36.4 |
| Serine | 19.1 | 19.7 |
| Glutamate | 73.4 | 86.9 |
| Glycine | 27.8 | 19.4 |
| Alanine | 25.0 | 16.1 |
| Cystine | 6.1 | 9.3 |
| Proline | 31.4 | 29.6 |
2.2 Feeding trial
The juvenile hybrid grouper with an average initial body weight of 7.3 ± 0.0 g used in this experiment were purchased from a local commercial hatchery (Zhanjiang, China). Prior to the formal breeding experiment, they were temporarily housed in concrete ponds under the same breeding conditions for 1 week and fed commercial feed (crude protein ≥52.0% and crude protein ≥8.0%; Guangdong Yuequn Biotechnology Co., Ltd.). Each treatment group consisted of three replicates, which were cultured in three 300-L fiberglass buckets, each consisting of 30 fish and feed twice daily at 8:00 and 17:00 until apparent satiation. The water dissolved oxygen was more than 7 mg/L, and ammonia and nitrate <0.03 mg/L with temperature ranging from 28°C to 30°C.
2.3 Challenge test
The Vibrio alginolyticus used in this experiment was obtained by isolation and purification from hybrid grouper as described by Amoah et al. (2021). Towards the end of the culture experiment, 100 fish fed in the control diet (weight similar to that of the fish in the challenge test, about 50–60 g) were selected and randomly divided into five groups, each injected with 200 uL V. alginolyticus, and subjected to a semi-lethal concentration experiment in 300-L fiberglass bucket without feeding during the period. Finally, the semi-lethal concentration of V. alginolyticus at 168 h was calculated based on the deaths (Furuta et al., 2007; data not shown).
After the 8-week feeding trial, 45 fish (30 for challenge test and 15 for blank test) from each treatment (15 fish per replicate) were randomly chosen for 1-week V. alginolyticus (1.00 × 1010 CFU/ml) challenge test. Bacteria cell pellets were re-suspended, and the optical density measured at 600 nm and found to be 3.505. Each fish was injected with 200 μL of V. alginolyticus solution. Blank test fish were injected with equal volume of sterilized phosphate-buffered saline. After injection, the number of deaths in each bucket was counted every 24 h without feeding for 1 week.
2.4 Sample collection
At the end of the 8-week feeding, all fish were fasted for 24 h before collecting samples. Fish from each bucket were counted and weighted for WGR (weight gain rate), SGR (specific growth rate), FCR (feed coefficient ratio) and SR (survival) analysis. After that, two fish from each bucket were randomly chosen for blood sample with 1-mL sterile syringes, put the blood in 1.5-ml Eppendorf tubes and stored 12 h at 4°C to allow serum to precipitate, and then collected the serum and stored at −80°C for triglyceride (TG), total cholesterol (TC), alanine aminotransferase (GPT) and aspartate aminotransferase (GOT) determination. Livers from two fish of each bucket were also collected, homogenized and centrifuged, and the supernatant was collected and stored at −80°C for lipase (LPS), lipoprotein lipase (LPL), GPT and GOT determination. Liver enzyme activities and serum biochemical parameters were determined by the kits purchased from Nanjing Jiancheng Bioengineering Institute, China (TG: A110-1-1, TC: A111-1-1, GPT: C009-2-2, GOT: C010-2-1, LPS: A054-2-1 and LPL: A067-1-2). Another two livers of each bucket were collected and stored at 4% formaldehyde solution for oil red O staining. The distal intestines were taken from four fish in each bucket near the cloacal pore for one-third of full length. Two of the distal intestines were stored in 4% formaldehyde solution for AB-PAS staining and TUNEL staining; the other two distal intestines were stored at −80°C for reverse transcription polymerase chain reaction (RT-PCR) samples.
2.5 Real-Time quantitative PCR analysis
Trizol (TRI reagent solution, Invitrogen) was used for the extraction of total RNA from distal intestinal tissues, and the obtained RNA was subsequently examined for quality and quantity using 1% agarose gel electrophoresis with electrophoresis instrument and A260: 280 values with NanoDrop 2000 spectrophotometer (Thermo Fisher), respectively. PrimeScript™ RT-PCR Kits (Takara) were used to perform the first-strand cDNA synthesis in RT according to the manufacturer's instructions. SYBR GreenPro Taq HS qPCR Kit II (Accurate Biology) was used to perform real-time quantitative PCR by Applied Biosystems 7500 Real-Time PCR System. The primers used in this study (Table 3) were designed by the intestinal full-length transcriptome sequences of hybrid grouper (Zhang, 2020). β-actin was used as a reference gene, and all the CT values were analysed using the 2−ΔΔCT method according to Livak and Schmittgen (2001).
| Gene name | Sequence (5′-3′) |
|---|---|
| jam1 | F: CACGACAACGATGGCTCACCTC |
| R: GCATTTCTGAAGGCGGCAATCTTG | |
| jam4 | F: CCGACACTACCAAGCCCACAATC |
| R: GAGCAAAACCAGCAGAGCAAAACC | |
| Occludin | F: CTGTCACTGTCTATAAGCTACGCTC |
| R: TCTTAACACTTTGCACATGAAGTGGA | |
| Claudin3 | F: AAGCAAGGTCAACATGGCGGA |
| R: GCGCTGCATGTGAAGTGTGATAG | |
| Claudin12 | F: AGGGATCGCTGTGGCAACG |
| R: CAGCCCGTCATACACGCTG | |
| Claudin15 | F: ACTTCAGGACCAGGTCAAAGTTAGG |
| R: CGATCCAGATTCAGCCAGAGCT | |
| ZO-1 | F: TGGAGCTGCGCTTACCTCAC |
| R: GGTCAATGAGCACAGACACACAGT | |
| TNF-α | F: AACTGTGTGTCCCCACTGCC |
| R: CCACAGATGGCCCAGGTCAT | |
| IL-1β | F: AAGGTGGACGCCAACAGACA |
| R: GTTCACTGCAGGCTCAGGGA | |
| TGF-β1 | F: CTTCTCCTCCTCCTCGCTGC |
| R: GATGTTGCTGAGGGCTTCGC | |
| Hepcidin | F: TGTCAATGACCCACTGAGCCTCG |
| R: TCCACTGCAAACTGCTGGGC | |
| IFN-γ | F: CGATTCGGTCATCAAGAGCAT |
| R: CTCCGTCACGACCGACACCA | |
| IL-8 | F: TGTGGCACTCCTGGTTCTCC |
| R: GGGTTCACCTCCACCTGTCC | |
| IL-6 | F: CAATCCCAGCACCTTCCAC |
| R: CCTGACAGCCAGACTTCCTCT | |
| Caspase-2 | F: TCGGACATGATCTGTGGCTTTGC |
| R: GGAGACGCAGTGTGGTGTTGAG | |
| Caspase-3 | F: TGGATCAACGTCTGTTTCCTTGTACTG |
| R: GTTCATTGCCTTTCCCCGTGTTTTC | |
| Caspase-6 | F: AGAATCACTGAAGCCGCAGAAGC |
| R: ATCATCATTGCCGTGACTCAGGAAG | |
| Caspase-8 | F: TCCTCCTCCCTTCTTGATCCAACTG |
| R: AGCCTTCGCATCCTCCTGAGTC | |
| FOXO-1 | F: GGGAGATACAAGGGAAGCAGTAACAC |
| R: GCGGTGGTCAGCTTGATGTCTC | |
| FasL | F: CCTTCATCAAGAGCTGGCTGACTATC |
| ACCTTCTCCTCCTTGTCTGACTCAC | |
| β-actin | F: TACGAGCTGCCTGACGGACA |
| R: GGCTGTGATCTCCTTCTGC |
2.6 Statistic analysis
The data from this experiment were statistically analysed using Statistical Package for the Social Sciences Statistics (v. 22, SPSS Inc.). And the data were tested for normality and homogeneity of variance. Two analytical methods were used in this experiment. First, we subjected the FM and each S groups (S00 to S32, respectively) to a Dunnett test, and second, orthogonal polynomial contrasts were used to determine if the effect was linear or quadratic. The optimum addition level of tributyrin was obtained according to the maximum Y value of the quadratic broken-line trend through the SAS software (v. 9.4; SAS Institute Inc.; Li et al., 2019). Cumulative survival of challenge test was identified by the Kaplan-Meier plot Log-Rank (Mantel-Cox) test (Liao et al., 2019).
3 RESULTS
3.1 Growth performance
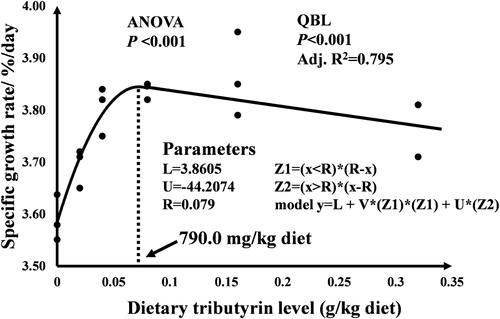
The effects of CPC and dietary tributyrin on the growth performance, feed utilization and morphology indices of hybrid grouper were shown in Table 4. Substitution of 45.0% fishmeal protein in the FM by CPC significantly reduced the FBW, WGR, SGR and feed utilization of hybrid grouper. Meanwhile, FBW, WGR, SGR and FCR all showed a quadratic trend with the increase of dietary tributyrin levels, respectively. Among them, FBW and FCR reached extreme values in the S08, and WGR and SGR reached extreme values in the S16. Feed cost for producing 1 kg of fish reached the lowest value in the S16. There were no significant differences in survival (SR), hepatosomatic indices (HSI) and intestinosomatic indices (ISI) among the seven groups. SGR value was used in the quadratic function model for the estimation of optimum dietary tributyrin levels (Figure 1), and the analysis results showed that optimal dietary tributyrin supplementing level for maximum SGR of hybrid grouper was estimated to be 0.186%.
| Groups | IBWa | FBWb | WGRc | SGRd | FCRe | SR | HSI | ISI |
|---|---|---|---|---|---|---|---|---|
| FM | 7.3 | 1850.3 | 777.2 | 3.9 | 0.8 | 97.0 | 0.03 | 0.01 |
| S00 | 7.3 | 1576.1*** | 646.5*** | 3.6*** | 0.9** | 96.7 | 0.03 | 0.01 |
| S02 | 7.3 | 1687.5** | 690.3** | 3.7** | 0.8 | 97.8 | 0.03 | 0.01 |
| S04 | 7.3 | 1836.0 | 741.0 | 3.8 | 0.8 | 100.0 | 0.03 | 0.01 |
| S08 | 7.3 | 1853.2 | 758.5 | 3.8 | 0.8 | 98.9 | 0.03 | 0.01 |
| S16 | 7.2 | 1844.4 | 771.2 | 3.9 | 0.8 | 97.8 | 0.03 | 0.01 |
| S32 | 7.3 | 1770.4 | 729.7 | 3.8 | 0.8 | 97.8 | 0.02 | 0.01 |
| Pooled SEM | 0.007 | 26.92 | 11.34 | 0.02 | 0.010 | 0.55 | 0.0008 | 0.0002 |
| Linear | ||||||||
| Adj. R2 | −.017 | .063 | .122 | .119 | −.026 | −.061 | .112 | .011 |
| p value | .409 | .163 | .085 | .088 | .465 | .891 | .026 | .544 |
| Quadratic | ||||||||
| Adj. R2 | .302 | .536 | .677 | .664 | .312 | −.096 | .093 | .019 |
| p value | .027 | .001 | <.001 | <.001 | .024 | .780 | .076 | .278 |
Note
- The * in the upper right corner of the number indicates the significance of each S group versus the FM by Dunnett test. * indicates p < .05; ** indicates p < .01; *** indicates p < .001; no * indicates no significance between this group and the FM.
- a IBW (g per fish): initial mean body weight.
- b FBW (g per fish): final mean body weight.
- c WGR (%): weight gain rate, 100 × (FBW−IBW)/IBW.
- d Specific growth rate (SGR, %/day): 100 × (Ln FBW−Ln IBW)/days of breeding.
- e FCR: dry feed/weight gain.
3.2 Challenge test
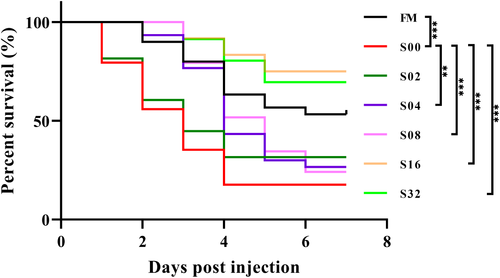
After a 7-day challenge test using V. alginolyticus, CPC substitution for fishmeal significantly reduced the survival of hybrid grouper, and supplementation with 0.16% tributyrin could effectively improve the survival of hybrid grouper (Figure 2).
3.3 Hepatic lipid deposition
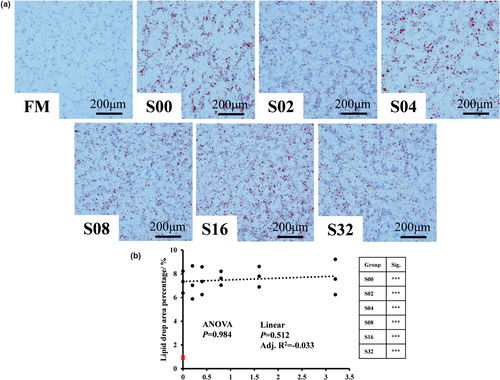
The effects of CPC and dietary tributyrin on the hepatic oil red O staining were shown in Figure 3. CPC significantly exacerbated hepatic lipid deposition of hybrid grouper after replacing fishmeal. Dietary tributyrin could not alleviate hepatic lipid deposition caused by high replacement levels of CPC.
3.4 Hepatic lipid metabolism-related enzyme activities
The effects of CPC and dietary tributyrin on the hepatic enzyme activities LPS, LPL, GPT and GOT associated with lipid metabolism were shown in Table 5. Substitution of fishmeal by CPC significantly reduced the enzyme activities of hepatic LPS, LPL, GPT and GOT in hybrid grouper. Dietary tributyrin did not significantly affect the LPS, LPL, GPT and GOT activities.
| Groups | LPS (U/mg prot) | LPL (U/mg prot) | GPT (U/mg prot) | GOT (U/mg prot) |
|---|---|---|---|---|
| FM | 3.4 | 0.7 | 317.3 | 24.4 |
| S00 | 2.1*** | 0.2*** | 226.5*** | 13.2*** |
| S02 | 2.0*** | 0.2*** | 239.0** | 13.5*** |
| S04 | 2.2*** | 0.2*** | 222.3*** | 12.8*** |
| S08 | 2.1*** | 0.2*** | 232.0*** | 13.1*** |
| S16 | 2.1*** | 0.2*** | 259.1* | 13.7*** |
| S32 | 2.1*** | 0.2*** | 249.0** | 13.9*** |
| Pooled SEM | 0.04 | 0.01 | 5.37 | 0.40 |
| Linear | ||||
| Adj. R2 | −.029 | −.027 | .050 | −.015 |
| p value | .926 | .759 | <.001 | .493 |
| Quadratic | ||||
| Adj. R2 | −.058 | −.040 | .045 | −.046 |
| p value | .964 | .721 | .176 | .790 |
Note
- The * in the upper right corner of the number indicates the significance of each S group versus the FM by Dunnett test. * indicates p < .05; ** indicates p < .01; *** indicates p < .001; no * indicates no significance between this group and the FM.
3.5 Serum biochemical parameters
The effects of CPC and dietary tributyrin on the serum biochemical parameters of hybrid grouper were shown in Table 6. CPC substitution for fishmeal significantly increased serum TG and TC levels and GPT and GOT activities. TG, TC, GPT and GOT all showed a quadratic trend with the increase of dietary tributyrin levels, respectively. All these four parameters showed a decrease in the S00 to S08, and after reaching extreme values in the S08, they showed an increasing trend in the S08 to S32.
| Groups | TG (mmol/L) | TC (mmol/L) | GPT (U/L) | GOT (U/L) |
|---|---|---|---|---|
| FM | 0.6 | 3.3 | 25.6 | 13.2 |
| S00 | 1.0*** | 6.3*** | 71.5*** | 25.3*** |
| S02 | 0.9** | 5.8*** | 59.9*** | 23.7*** |
| S04 | 0.7 | 4.9*** | 49.6*** | 19.0*** |
| S08 | 0.6 | 3.5 | 27.7 | 14.2 |
| S16 | 0.8 | 4.8*** | 33.2* | 17.8** |
| S32 | 0.8* | 5.3*** | 31.1 | 22.7*** |
| Pooled SEM | 0.03 | 0.16 | 2.83 | 0.74 |
| Linear | ||||
| Adj. R2 | −.022 | .003 | .453 | −.025 |
| p value | .616 | .302 | <.001 | .694 |
| Quadratic | ||||
| Adj. R2 | .198 | .460 | .783 | .522 |
| p value | .010 | <.001 | <.001 | <.001 |
Note
- The * in the upper right corner of the number indicates the significance of each S group versus the FM by Dunnett test. * indicates p < .05; ** indicates p < .01; *** indicates p < .001; no * indicates no significance between this group and the FM.
3.6 Distal intestinal histomorphological observation
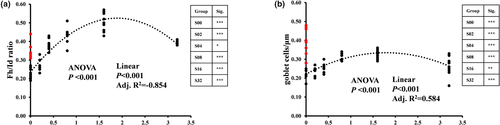
The effects of CPC and dietary tributyrin on the distal intestinal histomorphological indices of hybrid grouper were shown in Figure 4. Fish meal substitution by CPC significantly reduced the Fh/Id ratio (Figure 4a) and the number of goblet cells per unit fold height (Figure 4b). Dietary tributyrin had a significant effect on Fd/Id ratio and the number of goblet cells per unit fold height. Both Fd/Id ratio and the number of goblet cells per unit fold height showed a quadratic trend with the increase of dietary tributyrin levels, and both reached the maximum in the S16.
3.7 Distal intestinal barrier and immune-associated genes expression
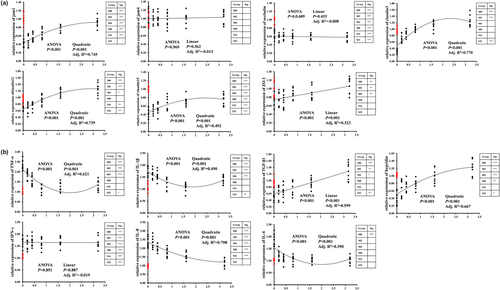
The effects of CPC and dietary tributyrin on the distal intestinal tight junctions and cytokines of hybrid grouper were shown in Figure 5. For tight junctions (Figure 5a), CPC substitution for fishmeal significantly downregulated the relative expressions of jam1, occludin, claudin3, claudin12, claudin15 and ZO-1 in the distal intestine of hybrid grouper but had no significant effect on the relative expression of jam-4. After supplementation with different levels of tributyrin, jam1, claudin3, claudin12 and claudin15 showed a quadratic function model of increasing and then decreasing with dietary tributyrin levels, and ZO-1 showed a linear upregulation trend, while the expressions of jam4 and occludin were not affected by dietary tributyrin levels. For cytokines (Figure 5b), CPC substitution for fishmeal significantly upregulated the relative expressions of TNF-α, IL-1β, IFN-γ, IL-8 and IL-6 in the distal intestine of hybrid grouper and significantly downregulated the relative expressions of TGF-β1 and hepcidin. After supplementation with different levels of tributyrin, TNF-α, IL-1β, hepcidin, IL-8 and IL-6 showed a quadratic function model with dietary tributyrin levels, among them, TNF-α, IL-1β, IL-8 and IL-6 showed a decreasing trend followed by an increasing trend, and hepcidin showed an increasing trend followed by a decreasing trend. And TGF-β1 showed a linear upregulation trend, while the expression of IFN-γ was not affected by dietary tributyrin levels.
3.8 Distal intestinal TUNEL staining and apoptosis-associated genes expression
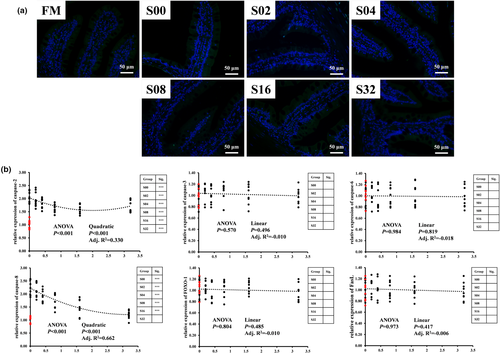
The effects of CPC and dietary tributyrin on the distal intestinal TUNEL staining and apoptosis-associated genes expressions of hybrid grouper were shown in Figure 6. From the TUNEL staining (Figure 6a), no significant apoptosis was found in any of the seven groups, and there was no significant difference in intestinal epithelial cell apoptosis among the seven groups. From the mRNA levels (Figure 6b), the replacement of fishmeal by CPC significantly increased the relative expressions of caspase-2 and caspase-8, meanwhile, caspase-2 and caspase-8 showed a quadratic trend of decreasing and then increasing with the dietary tributyrin levels. However, neither CPC substitution for fishmeal nor tributyrin supplementations had significant effects on the relative expressions of caspase-3, caspase-6, FOXO-1 and FasL.
4 DISCUSSION
In this study, we determined the optimum supplementary level of tributyrin under the condition of using CPC to replace 45% fishmeal protein in the control group. Using SGR as a criterion, a quadratic model was developed, and the results showed that hybrid grouper obtained the maximum SGR at a tributyrin level of 0.186% in feed percentage. In common carp (Cyprinus carpio), supplementation of whole plant protein with 0.2%–0.4% tributyrin was effective in increasing SGR and decreasing FCR of common carp whilst also significantly improving HSI and ISI (Xie et al., 2020). The addition of 0.2% tributyrin to the diet of white shrimp (Litopenaeus vannamei) significantly increased SGR and decreased FCR, whilst having no significant effect on survival (Lee et al., 2020). After replacing 33% of fishmeal in the black seabream (Acanthopagrus schlegelii) diet using soybean meal, supplementation with tributyrin was effective in increasing SGR but had no significant effect on FCR and SR. The 0.224% addition level resulted in maximum growth for black seabream (Volatiana et al., 2020). These showed a similar trend to the results of this experiment. Tributyrin can improve the intestinal trypsin and pepsin activities of Channa argus (Hou et al., 2018) and enhance the immunity of white shrimp (Lee et al., 2020). We speculate that the increased growth of tributyrin might be related to enhanced digestibility and immunity in hybrid grouper. But the optimal dietary tributyrin levels showed some differences, which may be caused by the different types of plant proteins in the feed and the differences in the tolerance and utilization of tributyrin by different aquatic species.
There are many causes of diseases in mariculture fish; apart from nutritional and environmental factors, bacterial infection is also an important cause. Among them, Vibrio is recognized as one of the most harmful diseases in marine fish culture. As a Gram-negative bacterium, V. alginolyticus is an important conditional pathogen and has caused significant economic losses in the mariculture industry (Chen et al., 2010; Lim et al., 2021). To investigate the effect of CPC substitution for fishmeal and tributyrin on the disease resistance of hybrid grouper, we conducted a 1-week challenge test using V. alginolyticus and found that CPC significantly reduced the survival of hybrid grouper, and supplementation with 0.16% tributyrin significantly improved the survival. Studies have shown that organic acids have an inhibitory effect on Vibrio spp. strains, and this effect is closely related to the dose of organic acids (Chuchird et al., 2015). This indicated that the butyric acid in tributyrin, an organic acid, could effectively help hybrid grouper to resist V. alginolyticus. Similar results were also found in white shrimp (Lee et al., 2020). According to the results in Figure 2, at least 0.04% tributyrin should be supplemented to the feed to significantly improve the disease resistance of hybrid grouper. In addition, the slow release of butyric acid from tributyrin in the intestine could enhance the intestinal barrier, whilst it can supply energy to the intestinal epithelial cells and enhance host immunity (Yin et al., 2021). This may also be related to enhancing disease resistance in hybrid grouper.
From the growth performance, we tentatively determined that tributyrin could be utilized as a good additive in hybrid grouper feed. To further investigate in depth how tributyrin positively affects hybrid grouper, we did the following studies. Lipid is deposited in the organs and tissues of fish when there is a disorder of lipid metabolism in fish. The deposition of lipid in the liver would cause disruption of lipid catabolism and metabolism in fish (Cai et al., 2017; Liu et al., 2016). From the oil red O staining, this study showed that CPC significantly exacerbated hepatic lipid deposition in hybrid grouper. This indicated that CPC, similar to other plant proteins such as soybean meal and peanut meal, could cause abnormal hepatic lipid deposition in hybrid grouper after high levels of fishmeal substitution (Yin et al., 2020). Lipoprotein lipase and hepatic lipase are two key enzymes in lipolysis metabolism, collectively known as total lipases. The main function of lipoprotein lipase is to hydrolyse triglycerides carried by very low-density lipoproteins and into small molecules of fatty acids for tissue storage and utilization (Cheng et al., 2021; Suauki et al., 1981). Hepatic lipase can also catalyse the hydrolysis of chylomicrons and promote the hydrolysis of triglycerides in very low density lipoproteins, preventing the development of fatty liver (Zhang et al., 2021). The activity of hepatic transaminases of fish can reflect the degree of hepatic tissue damage, and the lower the activity of hepatic alanine aminotransferase and aspartate aminotransferase, the more serious the degree of hepatic damage (Wang, 2014). In this experiment, CPC substituted for fishmeal significantly reduced the activities of hepatic LPS, LPL, GPT and GOT in hybrid grouper, indicating that excessive intake of CPC would reduce the hepatic ability to catabolize and metabolize lipid and impair hepatic function, leading to abnormal lipid deposition in the liver. Dietary tributyrin did not have a significant effect on hepatic lipid deposition, which may be associated with the low activities exhibited by LPS, LPL, GPT and GOT, indicating that dietary tributyrin could not improve hepatic lipid metabolism and repair the normal structure of hepatic tissue in hybrid grouper. However, interestingly, growth performances were significantly higher despite impaired hepatic function. Dietary 0.25% tributyrin significantly promoted the growth performance of Channa argus and did not have any significant effects on hepatic LPL, LPS, GPT and GOT (Hou et al., 2018), similar to the results of this experiment.
Under normal physiological metabolic conditions, GPT and GOT are relatively constant and low in serum. When stress caused hepatic damage, the permeability of the hepatocyte membrane of fish would increase, and GPT and GOT would cast across the hepatocyte membrane, leading to an increase in the activity of these two enzymes in the blood (Casillas et al., 1983). The increase in serum GPT and GOT activities after CPC substitution of fishmeal further demonstrated that CPC could cause hepatic damage in hybrid grouper. The absorption, transport and storage sites of lipids in feed by fish are generally accomplished in the form of TG-bound proteins forming chylomicron. Abnormally high TG levels reduce the intensity of oxidative reactions in fish, thus weakening the fatty acid oxidative energy supply pathway (Park and Harris, 2002). Also serum TC can be used as an indicator of the body's lipid metabolism capacity (Wang et al., 2010). The S00 exhibited high serum TG and TC levels indicating that CPC would reduce lipid metabolism and antioxidant capacity in hybrid grouper. In addition, the serum levels of TG, TC, GPT and GOT all showed a decrease followed by an increase with dietary tributyrin, indicating that dietary tributyrin could effectively alleviate hepatic damage of hybrid grouper. The 0.32% tributyrin addition level led to an increase in TC and TG content, which may be closely related to the fact that butyrate can be involved in the synthesis of TG and TC as a substrate (Singh et al., 2015). The addition of 0.2% tributyrin can alleviate hyperlipidemia caused by high levels of soybean oil by reducing serum TG and TC levels in juvenile large yellow croaker (Larimichthys crocea; Xu et al., 2020). After the addition of 0.05%–0.2% of tributyrin to the feed of crucian carp, the serum GPT and GOT also showed a trend of first decreasing and then increasing (Jiang, 2019), both of which were consistent with the results of this experiment. From the point of view of aquatic animal health, these results indicate that the use of tributyrin in aquatic animal feeds needs to be controlled within a reasonable range.
After the animal has been fed for a long time, the feed will have a profound effect on various organs. The fish intestine is not only an important digestive and absorptive organ but also has immune, endocrine and metabolic functions (Bakke et al., 2014; Feng et al., ). Damage to the intestinal tract can directly affect the uptake of nutrients in the feed, as well as reduce the immunity of the fish, thus negatively impacting growth (Bakke et al., 2010; Salinas and Parra, 2017; Tran-Ngoc et al., 2019). The increase in the height of the intestinal folds reflects, to some extent, the increase in the area of the intestine for nutrient absorption (Ologhobo et al., 1995). However, measuring fold height alone does not take into account changes in surface area due to changes in intestinal diameter (Wang et al., 2017). Therefore, we used the Fh/Id ratio to measure the overall development of the intestine. From the results of this experiment, CPC significantly reduced the distal intestinal Fh/Id ratio, indicating that CPC substitution for fishmeal inhibited the nutrient absorption capacity of the intestine of hybrid grouper, and similar results were also found on juvenile turbot (Scophthalmus maximus L.; Bian et al., 2017) and blunt snout bream (Megalobrama amblycephala; Yuan et al., 2020). We found that dietary tributyrin increased the Fh/Id ratio, suggesting that tributyrin has a significant promotion effect on distal intestinal development, thus improving the nutrient absorption capacity of hybrid grouper. The addition of tributyrin to juvenile yellow drum (Nibea albiflora) feed can effectively alleviate intestinal dysplasia caused by soybean oil (Zhu et al., 2020) and soybean meal (Tan et al., 2020) and can also alleviate intestinal damage caused by high soybean meal in black sea bream (Volatiana et al., 2020), similar to the results of this experiment.
The main function of goblet cells is to synthesize and secrete mucin, which forms a mucosal barrier to protect epithelial cells from pathogenic invasion (Chieng et al., 2020). The replacement of fishmeal by CPC led to a decrease in the number of distal intestinal goblet cells, whilst the number of distal intestinal goblet cells showed a trend of first increase and then decrease after supplementing tributyrin, suggesting that tributyrin may affect the immune function of the distal intestine of hybrid grouper through regulating goblet cell. Previous studies have shown that butyrate produced by intestinal microbiota can promote the differentiation of intestinal goblet cells, which is mainly associated with butyrate root (Liang et al., 2019). Combined with the results of this experiment, it appears that exogenous intake of butyrate and butyrate produced by intestinal microorganisms has similar effects. Meanwhile, excessive exogenous intake of butyric acid may have the opposite effect. At present, there are few studies on the effect of tributyrin on intestinal goblet cells in fish. Similar to the results of this experiment, supplementation of tributyrin in piglet diets was also able to increase the number of goblet cells in the colon, thus modulating the intestinal immune system of piglets (Hou et al., 2014).
To gain further insight into what response occurs in the distal intestine of hybrid grouper, we further analysed the expression of cytokines mRNAs associated with intestinal health. The inflammatory responses of the host are usually accompanied by upregulation of pro-inflammatory factors and downregulation of anti-inflammatory factors. After CPC substitution of fishmeal, we observed significant upregulation of the pro-inflammatory factors TNF-α, IL-1β, IFN-γ and IL-8, and downregulation of the anti-inflammatory factors TGF-β1 and hepcidin. This indicated that inflammation occurred in the distal intestine of hybrid grouper at this time. The mRNA levels of TNF-α, IL-1β and IL-8 were significantly reduced after tributyrin supplementation, indicating that tributyrin could effectively inhibit the inflammatory process caused by high level CPC in the distal intestine of hybrid grouper. Similar results were found in grass carp, where supplementation of 1.00% sodium butyrate, a compound that also has butyric acid root, in the diet significantly reduced the expression of TNF-α and IL-1β in the distal intestine of grass carp, whilst supplementation of 1.50% sodium butyrate significantly reduced the expression of IL-8 in the distal intestine (Tian et al., 2017), in addition to repairing common carp distal intestinal damage caused by oxidized soybean oil by reducing TNF-α and IL-1β (Liu et al., 2014). Tributyrin can also reduce TNF-α and IL-1β levels in macrophages from mice on a high-fat diet (Vinolo et al., 2012), suggesting that tributyrin might have similar immunomodulatory mechanisms for TNF-α and IL-1β in mice and fish. However, there were few research on tributyrin and fish intestinal cytokines, and it may need to be further investigated whether it is a direct association or an indirect role between them.
In addition, the anti-inflammatory factors TGF-β1 and hepcidin showed a gradual increase with dietary tributyrin. Similar results were also observed in blunt snout bream, where the expression of intestinal TGF-β was significantly increased by dietary 0.03% tributyrin (Liang et al., 2021). Currently, there is no report on the association of tributyrin with hepcidin in aquatic animals, while sodium butyrate enhanced distal intestinal immune function in grass carp was closely associated with the upregulation of hepcidin expression (Tian et al., 2017). However, IFN-γ did not vary with dietary tributyrin levels and consistently showed high expression, which may imply that the anti-inflammatory effects of tributyrin on the distal intestine of hybrid grouper are incomplete.
Studies have shown that upregulation of pro-inflammatory factors usually leads to disruption of the tight junction barrier of epithelial cells (Al-Sadi et al., 2009; Pan et al., 2017). In addition to cytokines, the structural integrity of the intestine is also crucial for fish. Altered intestinal permeability may be a key mechanism leading to the formation of intestinal inflammation (Penn et al., 2011). Intestinal tight junctions are mainly maintained by the membrane proteins occludin, claudin, the cytoplasmic protein ZO-1 and the intercellular adhesion molecule jam, which prevent the spread of pathogenic bacteria and certain antigens between epithelial cells (Zhao et al., 2014). From the expressions of tight junction protein mRNA levels in this experiment, CPC substitution for fishmeal disrupted distal intestinal tight junction, indicating that CPC, similar to soybean meal, also would cause the disruption of tight junctions in the distal intestine of hybrid grouper after high level replacement of fishmeal (Zhang, 2020).
In this experiment, the expression of jam1, claudin3, claudin12, claudin15 and ZO-1 all showed a gradual upregulation with the addition of tributyrin. This indicated that supplementation of tributyrin can significantly improve the tight junctions in the distal intestine of hybrid grouper. Dietary 0.05%–0.40% tributyrin to feed promoted tight junctions in the distal intestine of common carp by upregulating the expression of occludin and ZO-1 (Xie et al., 2020). It can also attenuate chronic alcohol overdose induced intestinal barrier damage in mice by upregulating the protein expression of claudin3, occludin and ZO-1 (Cresci et al., 2017). The intestinal protections of tributyrin in livestock animals such as broiler chicken (Hansen et al., 2021) and weaning pig (Tugnoli et al., 2014) were also mainly associated with improving intestinal tight junctions. This might suggest that tributyrin promotion of growth in aquatic and livestock animals is associated with improved intestinal tight junctions. Moreover, this improved tight junctions may enhance the barrier function of the intestinal epithelium in hybrid grouper.
Apoptosis plays an important role in maintaining homeostasis in the organism. Intestinal inflammation is usually accompanied by an increase in apoptosis (Wang, 2019). Apoptosis is mainly mediated by activated cystease caspases, including initiator proteins caspase-8 and caspase-9 and effector proteins caspase-2, caspase-3 and caspase-7. Therefore, we further used TUNEL staining to observe apoptosis in the distal intestine of hybrid grouper. Interestingly, we did not observe any significant apoptotic changes from microscopy among the seven groups. We speculate that apoptosis is not a necessary condition for enteritis caused by CPC in hybrid grouper. When we analysed the expression of genes closely related to apoptosis at the transcriptional level, we found that the promoter caspase-2 and effector caspase-8 were significantly upregulated after the replacement of fishmeal by CPC, and both showed a decreasing and then increasing trend with the level of tributyrin. However, caspase-3, caspase-6, FOXO-1 and FasL were not affected by CPC and tributyrin. This may indicate that the apoptotic signal is blocked at the time of efference from the effector. Since there are few studies on the mechanism of apoptosis of tributyrin on aquatic animal intestinal cells, and no significant apoptosis occurred in the S00 group, further in-depth studies are needed to determine whether tributyrin has positive or negative effects on apoptosis in the intestinal epithelial cells of hybrid grouper.
5 CONCLUSION
In conclusion, the results of this study demonstrated that the optimum dietary tributyrin requirement for maximum SGR of hybrid grouper was estimated to be 790.0 mg/kg diet after CPC replaced 45% fishmeal protein. Tributyrin produced positive effects on growth performance, disease resistance, and serum biochemical parameters in hybrid grouper and alleviated intestinal inflammation caused by CPC by modulating inflammatory factors and upregulating tight junction protein expression. However, tributyrin had no ameliorating effect on hepatic lipid deposition. In addition, no apoptosis occurred when CPC induced intestinal inflammation in hybrid grouper.
ACKNOWLEDGEMENTS
This work was supported by the National Key R&D Program of China (2019YFD0900200), National Science Foundation of China (No. 31772864) and Natural Science Foundation of Guangdong Province (2018A030313154 and 2020A1515011129). We would like to thank the Key Laboratory of Control for Disease of Aquatic Economic Animals of Guangdong Higher Education Institutes (Zhanjiang, China) for kindly providing Vibrio alginolyticus strain in this experiment.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.




