Optimal dietary L-ascorbyl-2-monophosphate inclusion improved the growth performance and nonspecific immunity of red swamp crayfish (Procambarus clarkii)
Abstract
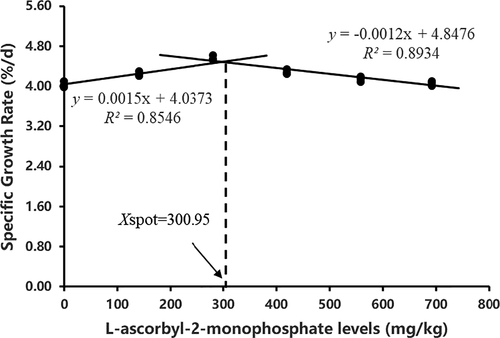
L-ascorbyl-2-monophosphate (AMP) was used as a vitamin C source to investigate its effects on growth performance and nonspecific immunity of red swamp crayfish (Procambarus clarkii). Six experimental diets (AMP0, AMP15, AMP30, AMP45, AMP60 and AMP75) with different levels of AMP (.00, 141.01, 280.44, 419.06, 558.13 and 692.09 mg kg−1 diet, respectively) were fed to juvenile crayfish (average weight: .70 ± .01 g) for 56 days. At the end of the 56-day trial, the highest final body weight, specific growth rate (SGR), phenoloxidase activity, lysozyme activity, superoxide dismutase activity and anti-superoxide anion activity all occurred in the AMP30 group (p < .05). Compared with the AMP0 group, crayfish fed with AMP-supplemented diets showed significantly higher hepatosomatic index value (p < .05). Conversely, the feed conversion ratio value of crayfish fed with AMP-supplemented diets was significantly reduced relative to that of the AMP0 group (p < .05). AMP15 to AMP30 diets showed significantly higher muscle rate value, crude protein content, total haemocyte count and blood cell respiratory burst activity (p < .05). Broken-line regression analysis on SGR indicated that the optimal dietary AMP level of red swamp crayfish was estimated to be 300.95 mg kg−1 diet.
1 INTRODUCTION
Vitamin C (ascorbic acid) is an essential micronutrient for normal growth and physiological function of most aquatic animals (Dawood & Koshio, 2018), which plays a vital role in promoting growth and wound recovery (anti-inflammatory), enhancing immune response and antioxidant capacity (Darias et al., 2011; Ellulu, 2017). Most crustaceans are extremely sensitive to vitamin C deficiency (Lee & Shiau, 2002). Therefore, these animals are dependent on constant dietary supply of vitamin C. It has been proven that vitamin C-supplemented diets can improve the survival, weight gain and feed utilization of crustaceans (Asaikkutti et al., 2016; Celada et al., 2013; Hou et al., 2015). Also, dietary supplementation of vitamin C showed beneficial effects on the serum bactericidal activity, phagocytosis, lysozyme activity, antibody levels and the activities of superoxide dismutase and catalase in shrimps (Asaikkutti et al., 2016; Zhou et al., 2004). The aforementioned studies have revealed the regulatory roles of vitamin C on growth performance and immune response of crustaceans.
Furthermore, vitamin C is extremely unstable, and its activity in formulated diets is easily lost during processing and storage (Shiau & Hsu, 1993). More stable and bioavailable forms of vitamin C are currently available. Thus, various attempts of incorporating stable forms of vitamin C derivatives into shrimp feeds have been conducted (Chen & Chang, 1994; Hsu & Shiau, 1998). A phosphate derivative of vitamin C, L-ascorbyl-2-monophosphate (AMP), was reported to be more stable and of higher bioactivity compared with L-ascorbic acid and other derivatives (Moe et al., 2004; Shiau & Hsu, 1994).
Red swamp crayfish, Procambarus clarkii, a member of Crustacea that originates from the south-central United States and northern Mexico, was introduced into China in the 1930s (Liu et al., 2020). This crayfish, a vital source of proteinaceous food that contains various essential amino acids required for the human body (Kong et al., 2021), vitamin B and minerals (Dai et al., 2017) are deeply favoured by Chinese consumers throughout the country and are considered as an important resource of freshwater crayfish (Cheng & Wu, 2019). However, because of the expansion of farming scale and environmental pollution, red swamp crayfish are easily suffered from severe viral and bacterial infections, which have restricted the production of this crayfish (Gao et al., 2020). Therefore, it is particularly important to determine the nutrient requirements of crayfish and to develop the high-efficiency compound feed, so as to promote the growth and health status of crayfish. The dietary protein and lipid requirements for red swamp crayfish have been determined to be 280–320 g kg−1 diet (Hubbard et al., 1986; Xu et al., 2013) and 40–70 g kg−1 diet (Xu et al., 2013), respectively. Besides, optimal animal protein to vegetable protein ratio in the diet (Tan et al., 2018; Xu et al., 2010), growth and immune improvement effect of dietary polysaccharide supplementation (Liu et al., 2020) have also been investigated in red swamp crayfish. However, few nutritional studies on vitamins for crayfish except the effects of dietary vitamin C on the growth performance, nonspecific immunity and antioxidant capacity were reported (Kong et al., 2021), which indicated that the retention rate of the coated vitamin C after processing is very low. Therefore, it is particularly important to explore the more stable dietary vitamin C forms for red swamp crayfish.
The aims of the present work were to determine the stability and bioactivity of AMP in red swamp crayfish diet and to investigate the effect of dietary AMP on the growth performance, antioxidant capacity and nonspecific immunity of red swamp crayfish. The results of the present study would provide a direct guideline to the incorporation of proper vitamin C source in the diets for the healthy culture of this species.
2 MATERIALS AND METHODS
2.1 Experimental diets
Six experimental diets with different AMP (vitamin C concentration: 330 g kg−1, Shandong Tianli Pharmaceutical Co., Ltd.) contents, designated as AMP0, AMP15, AMP30, AMP45, AMP60 and AMP75, were formulated by supplementing AMP to a basal diet to obtain the dietary vitamin C level at 0, 150, 300, 450, 600 and 750 mg kg−1 diet, respectively. The basal diet was designed to contain crude protein (300 g kg−1) and lipid (70 g kg−1), according to our previous study (Tan et al., 2018). Fish meal (American seafood, provided by Coland Feed Industry Co., Ltd, Wuhan, China) was used as animal protein, and distillers dried grains with solubles, soybean meal and rapeseed meal (COFCO Xiangrui Oils & Grains Industries [Jingmen] Co., Ltd) were used as the vegetable protein sources. Formulations and proximate composition of the experimental diets for red swamp crayfish are shown in Table 1. The analysed ascorbic acid concentrations of the test diets were 0.00, 141.01, 280.44, 419.06, 558.13 and 692.09 mg kg−1 diet in AMP0, AMP15, AMP30, AMP45, AMP60 and AMP75 diets, respectively. The raw materials were thoroughly crushed and then sieved through a 60-mesh sieve. The components with an inclusion level of no more than 1% in Table 1, including 2% astaxanthin premix, sodium chloride, choline chloride (50%) and cholesterol, were premixed first and then thoroughly mixed with the other components. Among them, AMP and microcrystalline cellulose were premixed first. Oil was later added and thoroughly mixed. Before pelleting, water was added for the gelatinization of the dietary starch during processing. Pellets (2.0 mm length and 1.5 mm diameter) were obtained using a laboratory pellet presser (YR008; Jiedong Gangmei Yongren Foodstuff Machinery Factory, Jiedong City, Guangdong Province, China), air dried at 30°C for 36 h and then stored at −20°C till using.
| Ingredients | AMP0 | AMP15 | AMP30 | AMP45 | AMP60 | AMP75 |
|---|---|---|---|---|---|---|
| Fish meal | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Soybean meal | 270.0 | 270.0 | 270.0 | 270.0 | 270.0 | 270.0 |
| Rapeseed meal | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
| DDGS | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Wheat flour | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
| Soya oil | 39.5 | 39.5 | 39.5 | 39.5 | 39.5 | 39.5 |
| Calcium biphosphate | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Choline chloride | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Cholesterol | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Astaxanthin premix | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Premixa | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| AMPb | 0.00 | 0.45 | 0.91 | 1.36 | 1.82 | 2.27 |
| Sodium chloride | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Carboxymethyl cellulose | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Cellulose | 32.50 | 32.05 | 31.59 | 31.14 | 30.68 | 30.23 |
| Proximate composition (%) | ||||||
| Moisture | 109.8 | 110.2 | 108.7 | 109.0 | 110.6 | 111.2 |
| Crude protein | 323.2 | 321.9 | 319.8 | 320.9 | 322.4 | 324.9 |
| Crude lipid | 65.2 | 66.0 | 67.3 | 67.7 | 66.2 | 65.6 |
| Ash | 73.1 | 72.9 | 75.4 | 74.3 | 74.2 | 72.4 |
| Vitamin C (mg kg−1) | ND | 141.01 | 280.44 | 419.06 | 558.13 | 692.09 |
- Abbreviations: AMP, L-ascorbyl-2-monophosphate; DDGS, distillers dried grains with solubles; ND, not determined.
- a Premix for crayfish provided (kg−1 diet): vitamin A 2000 IU, vitamin D3 2400 IU, vitamin K3 15 mg, vitamin E 100 mg, vitamin B1 5 mg, vitamin B2 15 mg, vitamin B6 10 mg, VB12.02 mg, niacin 150 mg, folic acid 3 mg, D-calcium pantothenate 50 mg, biotin 1 mg, inositol 200 mg, magnesium 200 mg, manganese 80 mg, iron 35 mg, zinc 50 mg, copper 20 mg, iodine 1 mg, cobalt.4 mg and selenium 0.225 mg.
- b L-ascorbyl-2-monophosphate, purity: 33% active ingredient.
2.2 Feeding trial
Juvenile red swamp crayfish were purchased from Shouyu Aquaculture Technology Co., Ltd. (Wuhan, China). Prior to the feeding trial, the crayfishes were acclimated to experimental conditions and fed with a commercial diet for 2 weeks.
After acclimation, 540 crayfish with an initial body weight of 0.70 ± 0.01 g were pooled, bulk weighed and then randomly distributed into an indoor system consisted of 18 circular fiberglass tanks (diameter: 80 cm; height: 60 cm) with 30 crayfish per tank after 1 day of food deprivation. Fifteen PVC water pipes with 5 cm internal diameter and 15 cm length per each were put into each tank so that the crayfish could seek shelter to avoid cannibalism. During the feeding trial, each diet was considered as one treatment and was assigned to three randomly selected tanks (replications). Crayfish were hand fed to apparent satiation twice daily (08:00 and 18:00). The amount of feed consumed by the crayfish in each aquarium was recorded daily. Uneaten feed was collected 2 h after feeding by siphoning and oven dried at 60°C to correct the feed intake. A continuous flow of dechlorinated tap water was supplied to each tank at the rate of 0.2 L min−1. The water in each tank was kept at a depth of 25 cm with continuous aeration to keep the water quality conditions acceptable for crayfish. The growth trial lasted for 8 weeks, during which the water temperature ranged from 24.5 ± 1.0°C. Ammonia-N was monitored once a week by the hypobromite method and was less than 0.5 mg L−1; dissolved oxygen was monitored once a week by the Winkler titration method and was above 5 mg L−1; pH was 7.0–7.6. A natural light/dark regime was applied in the trial. Besides, the crayfish in the molting stage were individually raised in 30 cm (length) ×18 cm (width) ×20 cm (height) plastic cubic aquaria with a water depth of 10 cm to avoid a predator and were put back into the culture tank after 24 h.
2.3 Sample collection
The crayfish from each tank were counted and collectively weighed to calculate the final body weight (FBW), survival rate (SR), specific growth rate (SGR), feeding rate (FR) and feed conversion ratio (FCR) at the end of the feeding trial after 24 h starvation. Five crayfish from each tank were randomly selected to measure their total length and body weight individually for calculating the condition factor (CF). Then, hemolymph samples were collected from these five crayfish: 1 ml sterile syringe was used to pierce the heart at the back of the crayfish breastplate, and.5 ml of hemolymph was drawn and put into a 1.5-ml centrifuge tube containing the same amount of cooled anticoagulant (glucose 20.5 g L−1, sodium citrate 8 g L−1, sodium chloride 4.2 g L−1, pH 7.5) for total haemocyte count (THC) and respiratory burst (RB). Another.5 ml of hemolymph was for serum preparation: serum was obtained by centrifugation (3000 × g, 10 min, 4°C), and an equal volume of serum from each of the five crayfish was mixed as a sample and then stored at −80°C for enzyme activity assays. Next, the hepatopancreas and muscle were dissected out on the ice and weighed to calculate hepatosomatic index (HSI) and muscle rate (MR), then the length of the intestine was measured to calculate intestine–body length ratio. Another five crayfish from each tank were randomly selected and stored at −20°C for whole-body composition analysis.
2.4 Analytical methods
2.5 Proximate composition assays
Analyses of moisture (105°C, 24 h), crude protein (Kjeldahl nitrogen ×6.25), crude lipid (ether extraction by Soxhlet method) and ash (550°C, 18 h) in diets and whole-body samples were performed following the standard AOAC procedures (AOAC, 1995). The dietary vitamin C concentrations were determined by o-phenylenediamine fluorometry as previously described (Wu et al., 2003).
2.6 Nonspecific immune response assays
The phenoloxidase (PO) activity in serum was determined by spectrophotometry, according to Liu et al. (2020). Ten μl of dihydroxyphenylalanine solution (.01 mol L−1), 300 μl of phosphate buffer (.1 mol L−1, pH 6.0) and 10 μl of serum were added to a 96-well microtitration plate, and the optical density at 490 nm was measured every 2 min. An increase of.001 min−1 in the OD490 value was considered to be an active unit.
The serum activities of lysozyme (LZM, turbidimetric method, Product No. A050-1–1), superoxide dismutase (SOD, WST-1 method, Product No. A001-3–2), anti-superoxide anion (ASA) capacity (colorimetric method, Product No. A052-1–1), nitric oxide synthase (NOS, colorimetric method, Product No. A014-2–1), alkaline phosphatase (microwell plate method, Product No. A059-2–2), acid phosphatase (ACP, microwell plate method, Product No. A060-2–2) and nitric oxide concentration (microwell plate method, Product No. A013-2–1) were detected by commercially available kits from Nanjing Jiancheng Bioengineering Institute, China, according to the producer's manual.
The THC was observed and counted by an optical microscope (Mshot ML31-B, Guangzhou, China) following that 20 µl of collected anticoagulant-treated hemolymph was placed on a blood cell counting plate.
The haemocyte RB activity was quantified according to Song and Hsieh (1994) with some modification. Briefly, 0.1 ml of hemolymph diluted with 0.4 ml of anticoagulant was resuspended to 106 cells ml−1 in a modified complete Hank's balanced salt solution (MCHBSS) containing 10 mM CaCl2, 3 mM MgCl2, 5 mM MgSO4 and 24 mg ml−1 HBSS (Sigma). Haemocyte suspension (100 µl) was added to a flat-bottomed 96-well microtitre plate (105 haemocytes per well) and cytocentrifuged at 800 × g for 10 min at 4℃. After removing the supernatant, 100 µl of zymosan (2 mg ml−1, Sigma) was added and allowed to react for 30 min at 37℃, and MCHBSS was added as a control. NBT (100 µl 0.3% in MCHBSS) was added to the haemocytes and incubated for 30 min at 37℃. The staining reaction was terminated by removing the NBT solution and adding absolute methanol. The haemocytes were then air dried and coated with a solution of 120 µl of 2 M KOH, and 140 µl of dimethyl sulphoxide was added to dissolve the cytoplasmic formazan; the dissolved cytoplasmic formazan was measured at 630 nm with a precision microplate reader.
2.7 Statistical analysis
Data were expressed as means of three replicates. Statistical differences between treatments were firstly determined by one-way ANOVA and then by Tukey's post hoc test. All statistical analyses were performed using the SPSS computer program, version 19 (IBM, Armonk, NY, USA). The difference was considered significant at p <.05. Data resulting from the dose–response trial were also subjected to orthogonal polynomial contrasts and then were further subjected to a regression analysis to fit the best model if a statistical significance was detected (linear, quadratic or cubic). When the significance for a quadratic or cubic regression was noted, the broken-line model was examined as well and chosen if the model best represented the data. The R square (R2) was used for optimal regression selection to detect optimal AMP inclusion for dependent variables. The broken-line regression was used to estimate the optimum dietary AMP level for red swamp crayfish based on SGR.
3 RESULTS
3.1 Growth performance, feed intake and morphology parameters
After 8 weeks of the feeding trial, the results of growth performance, feed intake and morphology parameters were summarized in Table 2. As the dietary AMP content increased gradually, the FBW and SGR exhibited an increasing first and then decreasing trend in a quadratic model (p <.05), and both of which showed the highest values in the AMP30 group, followed by the AMP15 and AMP45 groups and the lowest in the AMP0, AMP60 and AMP75 groups. The FCR decreased first and then rose with increasing AMP content in a quadratic model, which was the lowest in the AMP30 group (p <.05). With the gradual increase of dietary AMP level, the HSI and MR showed cubic and quadratic trends, respectively (p <.05). The HSI was significantly higher in the AMP15, AMP30, AMP45, AMP60 and AMP75 groups than in the AMP0 group (p <.05). The MR reached a peak value in the AMP15 and AMP30 groups (p <.05). The regression value (R2) of all aforementioned parameters ranged from 0.42 to 0.94. Graded levels of dietary AMP exhibited no significant effects on the SR, FR, intestine–body length ratio and CF of juvenile crayfish.
| Items | Diet treatments | PSE1 | Orthogonal contrast2 | Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP0 | AMP15 | AMP30 | AMP45 | AMP60 | AMP75 | Linear | Quadratic | Cubic | Model3 | (Pr > F)4 | R 2 | ||
| IBW (g) | .70 | .70 | .69 | .69 | .69 | .69 | .005 | .0172 | .4818 | .2040 | Ln | .0104 | .35 |
| FBW (g) | 6.69a | 7.54bc | 8.93d | 7.62c | 7.02ab | 6.72a | .214 | .0218 | .0000 | .0000 | Qd | .0006 | .63 |
| SR (%) | 87.78 | 93.33 | 94.45 | 92.22 | 90.00 | 90.00 | 3.042 | .9401 | .0253 | .0907 | Ns | — | — |
| SGR (%/d) | 4.04a | 4.25bc | 4.56d | 4.29c | 4.14ab | 4.06a | .052 | .0885 | .0000 | .0003 | Qd | .0003 | .67 |
| FR (%/d) | 3.65 | 3.54 | 3.56 | 3.66 | 3.63 | 3.61 | .062 | .5261 | .4517 | .0250 | Ns | — | — |
| FCR | 1.26c | 1.20ab | 1.16a | 1.23bc | 1.24bc | 1.24bc | .019 | .2892 | .0003 | .0012 | Qd | .0170 | .42 |
| HSI (%) | 6.08a | 7.96b | 8.07b | 7.85b | 7.78b | 7.91b | .163 | .0000 | .0000 | .0000 | Cu | .0000 | .94 |
| MR (%) | 14.57a | 17.88d | 18.33d | 16.68c | 16.01bc | 15.31ab | .284 | .0232 | .0000 | .0000 | Qd | .0003 | .67 |
| ILR (%) | 62.10 | 60.45 | 63.40 | 61.37 | 60.93 | 60.14 | 1.696 | .0687 | .2322 | .5092 | Ns | — | — |
| CF (g cm−3) | 3.47 | 3.47 | 3.59 | 3.53 | 3.49 | 3.18 | .232 | .2261 | .0983 | .4681 | Ns | — | — |
- Abbreviations: IBW (initial body weight, g); FBW (final body weight, g); SR (survival rate, %) =100 × (number of survival / total number); SGR (specific growth rate, %/d) =100 × {ln [final body weight (g) − ln initial body weight (g)]} / 56(d); FR (feed rate, %/d) =100 × total dry matter intake (g) / {56 (d) × [initial body weight (g) + final body weight (g)] / 2}; FCR (feed conversion ratio) = feed intake (g) / [final body weight (g) − initial body weight (g)]; HSI (hepatosomatic index, %) =100 × [final hepatopancreas weight (g) / final body weight (g)]; MR (muscle rate, %) =100 × [final muscle weight (g) / final body weight (g)]; ILR (intestine–body length ratio, %) =100 × [final intestinal length (cm) / final body length (cm)]; CF (condition factor, g cm−3) =100 × [final body weight (g) / final body length (cm)3].
- 1 Pooled standard error of treatment means.
- 2 If statistical significance (p <.05) was detected, the model that fits best with the data was selected.
- 3 Ns, no structure (p >.05); Ln, linear; Qd, quadratic; and Cu, cubic.
- 4 Probability associated with the F statistic test.
- * All data were means of three parallel tanks (n = 3, except that n = 15 for HISI, MR, ILR and CF). Mean values within the same row with different superscripts are significantly different (p <.05).
The broken-line regression analysis between the SGR and the AMP levels indicated that the optimum dietary AMP level was 300.95 mg kg−1 diet for juvenile crayfish (Figure 1).
3.2 Body proximate composition of crayfish fed with different levels of AMP
As dietary AMP content increased gradually, the crude protein content of the whole crayfish showed a significant quadratic trend (p <.05, R2 =.45), which was the highest in the AMP15 and AMP30 groups, followed by the AMP45 and AMP60 groups and the lowest in the AMP0 and AMP75 groups (Table 3). In contrast, moisture, crude lipid and ash contents in the whole body were not significantly different among all treatments.
| Items | Diet treatments | PSE1 | Orthogonal contrast2 | Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP0 | AMP15 | AMP30 | AMP45 | AMP60 | AMP75 | Linear | Quadratic | Cubic | Model3 | (Pr > F)4 | R 2 | ||
| Moisture | 729.9 | 710.1 | 708.7 | 713.8 | 718.5 | 721.3 | 48.0 | .9575 | .5982 | .7485 | Ns | — | — |
| Crude protein | 167.5a | 188.1c | 189.2c | 177.1b | 176.0b | 174.4ab | 3.0 | .3484 | .0000 | .0000 | Qd | 0.0115 | 0.45 |
| Crude lipid | 78.4 | 77.6 | 81.1 | 75.6 | 78.1 | 79.7 | 1.8 | .2019 | .0454 | .5744 | Ns | — | — |
| Ash | 14.7 | 15.0 | 14.1 | 15.5 | 14.8 | 15.2 | .5 | .2420 | .5846 | .6959 | Ns | — | — |
Note
- *All data were means of three parallel tanks, and five crayfish in each tank were pooled as one sample for analysis (n = 3). Mean values within the same row with different superscripts are significantly different (p <.05).
- 1 Pooled standard error of treatment means.
- 2 If statistical significance (p <.05) was detected, the model that fits best with the data was selected.
- 3 Ns, no structure (p >.05); Qd, quadratic.
- 4 Probability associated with the F statistic test.
3.3 Blood immune index of crayfish fed with different levels of AMP
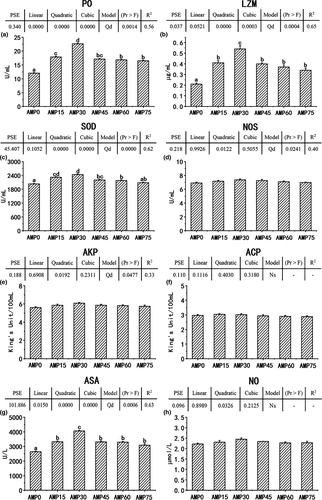
As dietary AMP level increased, the serum activities of PO, LZM, SOD and ASA all increased first and then decreased in quadratic trends (Figure 2; R2 =.56,.65,.62 and.63, respectively). The PO activity was highest in the AMP30 group (p <.05), followed by the AMP15 and AMP45 groups, then the AMP60 and AMP75 groups and lowest in the AMP0 group. The LZM and ASA activities reached peak values in the AMP30 group and showed the medium values in the AMP15, AMP45, AMP60 and AMP75 groups and were lowest in the AMP0 group (p <.05). The SOD activity was the highest in the AMP30 group, but there was no significant difference from the AMP15 group, followed by the AMP45 to AMP75 groups and the lowest in the AMP0 group. The serum NOS and alkaline phosphatase activities showed a quadratic trend responding to the increasing AMP level, while showing no significant difference among groups. Graded levels of dietary AMP exhibited no significant effects on the serum ACP activity and nitric oxide content.
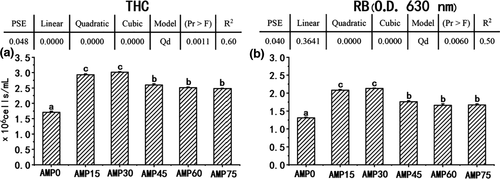
As shown in Figure 3, the THC and RB both increased first and then decreased in quadratic models (R2 =.60 and.50, respectively) as dietary AMP level increased. The foregoing parameters showed the highest values in the AMP15 and AMP30 groups, followed by the AMP45, AMP60 and AMP75 groups and the lowest in the AMP0 group (p <.05).
4 DISCUSSION
The positive effect of vitamin C on growth performance has been reported for crustaceans such as juvenile swimming crab (Portunus trituberculatus) (Hou et al., 2015), Pacific white shrimp (Litopenaeus vannamei) (Niu et al., 2009) and red swamp crayfish (Kong et al., 2021). The present work supported the aforementioned results that the growth performance of crayfish was improved by the dietary supplementation of vitamin C when the AMP was used as vitamin C source. The highest values of FBW and SGR both occurred in the AMP30 group, and MR appeared in the AMP15 and AMP30 groups, which indicated that optimal AMP inclusion could obtain the best growth of crayfish in this study. The vitamin C requirement for red swamp crayfish (300.95 mg kg−1 diet, based on SGR) in the present study was higher than that for freshwater Monsoon River prawn, Macrobrachium malcolmsonii (94.24 mg kg−1 determined by broken-line regression analysis on SGR) (Asaikkutti et al., 2016). This indicates that different shrimp/crayfish species and vitamin sources caused different vitamin C requirements. Compared with our previous study (Kong et al., 2021), different vitamin sources were used in this study to explore whether AMP had a better stability and bioactivity. The results showed that the optimal vitamin C level in this study did not apparently vary, which suggests that AMP might show similar bioactivity with L-ascorbic acid in red swamp crayfish. In contrast, it is suggested that AMP showed higher bioactivity than L-ascorbic acid in grass shrimp (Shiau & Hsu, 1994). However, this study had its limitation: the size of crayfish in this study is smaller than that of our previous study, whether this difference in size caused the somewhat higher vitamin C requirement in this study needs direct proof, although it has been suggested that the nutrient requirements of larval crayfish are higher than those of the larger crayfish (Niu et al., 2009).
In the current study, the FCR value was decreased by the dietary supplementation of AMP, and the lowest FCR was observed in the AMP15 and AMP30 groups. Similar results were also observed in signal crayfish Pacifastacus leniusculus (Celada et al., 2013) and Monsoon River prawn (Asaikkutti et al., 2016). These results indicated that the improvement on growth by feeding vitamin C might be due to an increase in feed efficiency of the diet as the feeding rate was not affected in this study (Dawood & Koshio, 2018). HSI is an indicator of organism health and energy reserve (Khan et al., 2015). The present study demonstrated that an elevated level of AMP significantly increased the HSI of crayfish, which indicated that the nutritional status of crayfish was better than that of the control. Similar results were reported in this crayfish feeding on coated vitamin C-supplemented diets (Kong et al., 2021). Previous research also showed that the dietary supplementation of vitamin C enhanced the synthesis of egg yolk precursors in the hepatopancreas of crustaceans (Cavalli et al., 2003). Whether the increase in HSI is related to the accumulation of large amounts of lipids in the hepatopancreas for the mature process deserves further investigation.
In the present work, dietary supplementation of AMP significantly increased the whole-body composition of crude protein but did not affect crude lipid, moisture and ash contents. Such crude protein improvement was also observed in Monsoon River prawn (Asaikkutti et al., 2016). High levels of vitamin C could stimulate protein production through promotion on protein synthesis in aquatic animals (Chagas & Val, 2003). The increased crude protein content in our study might suggest that AMP enhanced the protein synthesis and storage of crayfish. However, inconsistent results regarding the effect of vitamin C on body composition were reported in crustaceans. Apart from protein, body crude lipid, moisture and ash contents were all enhanced in prawns fed on supplemented vitamin C (Asaikkutti et al., 2016). In contrast, dietary vitamin C levels did not influence the crude protein content for Pacific white shrimp (Chen et al., 2017).
Crayfish usually live in water environments containing different parasites and pathogens. They depend totally on their nonspecific immune system (such as PO, LZM, SOD and ACP activities) to fight against the infections (Shi et al., 2014). Changes in haematological indices are widely used to assess the health and nutritional status of aquatic animals (Cheng & Wu, 2019). PO, a key enzyme in the melanin synthesis pathway, plays a vital innate immune defense of crustaceans (Liu et al., 2021). LZM is an enzyme that exerts activity and antibacterial activity in the body, which shows a positive correlation to the immunity of aquatic animals (Liu et al., 2021). The present study showed that supplementation of AMP could increase the serum PO and LZM activities for crayfish, and both of which had the strongest enzyme activity in the AMP30 group. These were consistent with the serum immune results of Pacific white shrimp (Zhou et al., 2004) and red swamp crayfish (Kong et al., 2021). Previous studies have shown that AMP as a source of vitamin C significantly affected the serum PO activity of grass shrimp (Lee & Shiau, 2002). However, comparing the two studies, the optimal dietary AMP levels for the highest PO activity are different, which may be due to the different species. The SOD is the primary enzyme for scavenging free radicals to defend against oxidative stress (Wang et al., 2012). Most of these free radicals are reactive oxygen species (ROS), such as superoxide anions (O2−) (Dawood & Koshio, 2018). The ASA activity reflects the total scavenging capacity against O2− radical (Nyska & Kohen, 2002). In our study, both SOD and ASA had the best activity in the AMP30 group as dietary AMP level increased, which indicated that optimal dietary AMP could enhance the clearance ability of crayfish serum O2−. This was consistent with the results regarding the immune response of crayfish to the coated vitamin C (Kong et al., 2021) and that of freshwater prawn to dietary AMP (Asaikkutti et al., 2016). The PO, LZM, SOD and ASA activities in the serum of crayfish fed with AMP-supplemented diets were considerably improved and showed the highest value in the AMP30 group as SGR did, which may suggest that a better status of nonspecific immunity of crayfish at the optimal AMP level resulted in the maximum growth. THC, a sensitive index that reflects the immune status in pathogen resistance (Sunish et al., 2020), can be increased by dietary vitamins in crustaceans (Lee & Shiau, 2003). In this study, the dietary supplementation of AMP significantly increased THC, and the AMP15 and AMP30 groups had the strongest activity. Similarly, the THC of grass shrimp fed with different forms of vitamin C increased significantly (Lee & Shiau, 2002, 2003). Hemolymph cells eliminate foreign particles, such as bacteria by phagocytosis, during which hemolymph cells produce a series of microbicidal substances including ROS, in a process known as the RB (Song & Hsieh, 1994). Therefore, RB activity can be used as an indicator to reflect the innate immune response. Our results showed that dietary AMP supplementation increased the RB activity of crayfish. Similar results were also observed in juvenile crayfish fed with coated vitamin C (Kong et al., 2021). However, the PO, LZM, SOD, ASA and RB activities and THC decreased at high dietary levels of AMP compared with the optimal dietary VC group. These results indicated that excessive dietary AMP might show reverse effect, which should be avoided.
Comparing the retention rates of coated vitamin C in the previous study (Kong et al., 2021) and AMP in this study after feed processing, ascorbic acid in the form of AMP (93%) is more stable than coated vitamin C (21%) during feed processing. Similar results were reported by Shiau and Hsu (1994). Based on the results in the current study, we strongly encourage the use of AMP rather than coated vitamin C in crayfish diets, because of the less actual addition amount of AMP to ensure the optimal retained ascorbic acid dosage and the much lower cost at the current market prices of coated vitamin C and AMP, which are roughly the same.
5 CONCLUSIONS
Dietary AMP inclusion significantly improved the growth performance and body protein contents and also enhanced the nonspecific immune response of red swamp crayfish. Broken-line regression analysis on the SGR indicated that the optimal dietary AMP requirement for red swamp crayfish was 300.95 mg kg−1 diet. In addition, AMP, as a source of vitamin C, is highly stable during feed process and storage compared and is suitable for crayfish feed. These results provide a theoretical basis and reference data for diet preparation and health regulation of crayfish in the future culture.
ACKNOWLEDGEMENTS
This work was financially supported by Guangdong Haida Group Co., Ltd (Grant No. 0220180505) and National Natural Science Foundation of China (Grant No. 32072950). Thanks to everyone who contributed to this experiment.
CONFLICT OF INTEREST
All listed authors have accepted the attached manuscript. There is no conflict of interest that goes out in submitting this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




