Effects of dietary arginine on growth, activity of digestive enzymes, GCN2-ATF4 signalling pathway and nutritional metabolism-related gene expression of large yellow croaker (Larimichthys crocea) larvae
Abstract
A 30-day feeding experiment was conducted to elucidate the effects of dietary arginine on growth performance, activity of digestive enzymes, GCN2-ATF4 signalling pathway and nutritional metabolism-related gene expression of large yellow croaker larvae (initial weight: 10.56 ± 0.26 mg). Four isonitrogenous and isolipidic diets with different arginine levels (19.5, 31.0, 39.6 and 48.6 g kg−1 of dry matter) were formulated. Results showed that no significant differences in survival, growth and whole-body proximate composition were observed among dietary treatments. The whole-body arginine content of fish larvae was significantly increased with increasing contents of dietary arginine, and the maximum was observed in larvae fed the diet with 48.6 g kg−1 arginine. However, the contents of other amino acids were not significantly affected. Larvae fed the diet with 19.5 g kg−1 arginine showed higher activity of alkaline phosphatase (AKP) in the intestinal segments (IS) and brush border membranes (BBM) than other groups. The expression of GCN2-ATF4 signalling pathway-related genes (atf4, chop and asns) in the visceral mass of larvae was significantly upregulated by dietary 39.6–48.6 g kg−1 arginine supplementation. Dietary 48.6 g kg−1 arginine significantly upregulated the expression of proteolytic genes (murf1 and glud) in the visceral mass of larvae. Furthermore, the highest expression of genes related to fatty acid β-oxidation (cpt1α) and glycolysis (gk and pk) was observed in the 39.6 g kg−1-arginine group. In summary, those results suggested that dietary arginine had no significant influence on the growth performance of large yellow croaker larvae. However, the arginine level significantly affected the arginine content of whole larvae, activity of digestive enzymes, lipid metabolism and glycolysis of larvae. The GCN2-ATF4 signalling pathway was stimulated by high-dose (31.0–48.6 g kg−1) arginine, maybe due to the antagonistic effect of arginine and lysine. These results might indicate that 19.5 g kg−1 dietary arginine (37.6 g kg−1 dietary protein) could meet the growth requirement of large yellow croaker larvae.
1 INTRODUCTION
Arginine plays a vital role in the nutrition metabolism and growth of aquatic animals (Luo et al., 2004). Arginine has been shown to be one of the essential amino acids (EAAs) in fish and shrimp due to the very low activity of the urea cycle (Han et al., 2013; NRC, 2011; Oehme et al., 2010). Previous studies have revealed the arginine requirements of various aquatic animals, and the quantitative arginine requirement in fish species ranges from 10.0 to 31.0 g kg−1 of diet (NRC, 2011).
Arginine participates in multiple physiological pathways directly or in the shape of derivatives and can regulate protein synthesis and metabolism through signal pathways such as GCN-ATF4 or mTOR (Ma et al., 2017; Wang et al., 2013). Arginine is the most abundant nitrogen carrier in tissue proteins (Pohlenz et al., 2012). It also plays an essential role in synthesizing critical biological molecules such as proline, ornithine, creatine, polyamines and nitric oxide (Pereira et al., 2017; Wu & Morris, 1998). It has also been established that arginine plays a vital role in modulating immune responses via modulation of T-cell and cytokine production (Bansal & Ochoa, 2003; Pakula et al., 2017).
Large yellow croaker (Larimichthys crocea) is a high-valued marine species with sizeable production in China (Du et al., 2017). Previous studies have investigated the nutrient requirements, histological development of the digestive system and feeding habits of large yellow croaker larvae (Feng et al., 2017; Mai et al., 2005; Xie et al., 2011). However, to our knowledge, no research has been available on the arginine requirements and its relevant effects on nutritional metabolism in large yellow croaker larvae. Hence, this experiment aimed to investigate the effects of dietary arginine on growth performance, activity of digestive enzymes and nutritional metabolism-related gene expression of large yellow croaker larvae.
2 MATERIALS AND METHODS
2.1 Experimental diets
Four diets were formulated with supplementation of different arginine levels, namely, 0 g kg−1 (the control), 10, 20 and 30 g kg−1 (Table 1). The purity of arginine was >98.5% (Guangdong VTR Bio-Tech Co., Ltd). The accurate contents of arginine in diets were 19.5 g kg−1 (the control), 31.0, 39.6 or 48.6 g kg−1 respectively (Table 2).
| Ingredient | Dietary arginine levels (g kg−1) | |||
|---|---|---|---|---|
| 19.5 | 31.0 | 39.6 | 48.6 | |
| White fish meala | 250 | 250 | 250 | 250 |
| Krill mealb | 50 | 50 | 50 | 50 |
| Soybean protein concentrate | 65 | 65 | 65 | 65 |
| Corn gluten meal | 130 | 130 | 130 | 130 |
| Wheat gluten | 150 | 150 | 150 | 150 |
| α-starch | 148.4 | 148.4 | 148.4 | 148.4 |
| Fish oil | 80 | 80 | 80 | 80 |
| Soybean lecithin | 15 | 15 | 15 | 15 |
| Ca(H2PO3)2 | 10 | 10 | 10 | 10 |
| Choline chloride | 10 | 10 | 10 | 10 |
| Vitamin premixc | 10 | 10 | 10 | 10 |
| Mineral premixd | 10 | 10 | 10 | 10 |
| Lysine | 20 | 20 | 20 | 20 |
| Methionine | 5 | 5 | 5 | 5 |
| Threonine | 5 | 5 | 5 | 5 |
| Glycine | 30 | 20 | 10 | 0 |
| Arginine | 0 | 10 | 20 | 30 |
| Alginate sodium | 10 | 10 | 10 | 10 |
| Astaxanthin | 0.1 | 0.1 | 0.1 | 0.1 |
| Antioxidants | 1 | 1 | 1 | 1 |
| Mould inhibitor | 0.5 | 0.5 | 0.5 | 0.5 |
| Proximate analysis | ||||
| Crude protein | 519.3 | 521.2 | 522 | 526.2 |
| Crude lipid | 150.8 | 145.4 | 142.8 | 143.3 |
- a All of these ingredients were supplied by Guangdong VTR Bio-Tech Co., Ltd.
- b All of these ingredients were supplied by Qingdao Bio-ways Ingredients Bio-technology Co., Ltd.
- c Vitamin premix (IU or mg kg−1 dry diet) was supplied by Qingdao Master Biotech Co., Ltd: vitamin A palmitate, 3,000,000 IU; vitamin D3 1,200,000 IU; DL-α-vitamin E 40.0 g kg−1; menadione, 8.0 g kg−1; thiamine-HCl, 5.0 g kg−1; riboflavin, 5.0 g kg−1; D-calcium pantothenate, 16.0 mg kg−1; pyridoxine-HCl, 4.0 mg kg−1; inositol, 200.0 mg kg−1; biotin, 8.0 mg kg−1; folic acid, 1.5 mg kg−1; 4-aminobenzoic acid, 5.0 mg kg−1; niacin, 20.0 mg kg−1; vitamin B12, 0.01 mg kg−1; L-ascorgyl-2-monophosphate-Na (3%), 2000.0 mg kg−1.
- d Mineral premix (mg kg−1 dry diet) was supplied by Qingdao Master Biotech Co., Ltd: Ca(H2PO4)·H2O, 675.0; COSO4·H2O, 0.15; CuSO4·H2O, 5.0; FeSO4·7H2O, 50.0; KCl, 0.1; MgSO4·2H2O, 101.7; MnSO4·2H2O, 18.0; NaCl, 80.0; NaSeO3·H2O, 0.05; ZnSO4·7H2O, 20.0.
| Amino acid | Dietary arginine levels (g kg−1) | |||
|---|---|---|---|---|
| 19.5 | 31.0 | 39.6 | 48.6 | |
| Essential amino acid | ||||
| Arginine | 1.95 ± 0.04d | 3.10 ± 0.09c | 3.96 ± 0.02b | 4.86 ± 0.06a |
| Histidine | 0.97 ± 0.07 | 1.02 ± 0.07 | 0.96 ± 0.01 | 0.98 ± 0.05 |
| Isoleucine | 1.91 ± 0.08 | 2.18 ± 0.13 | 2.07 ± 0.09 | 1.97 ± 0.00 |
| Leucine | 4.50 ± 0.01 | 4.63 ± 0.25 | 4.41 ± 0.09 | 4.50 ± 0.13 |
| Lysine | 3.05 ± 0.12 | 3.33 ± 0.23 | 3.23 ± 0.13 | 3.06 ± 0.01 |
| Methionine | 0.96 ± 0.29 | 1.28 ± 0.06 | 1.35 ± 0.22 | 1.05 ± 0.24 |
| Phenylalanine | 2.69 ± 0.14 | 2.67 ± 0.11 | 2.53 ± 0.06 | 2.81 ± 0.22 |
| Threonine | 1.98 ± 0.03 | 2.15 ± 0.07 | 2.08 ± 0.00 | 1.94 ± 0.05 |
| Valine | 2.00 ± 0.02 | 2.08 ± 0.07 | 2.16 ± 0.06 | 2.04 ± 0.04 |
| Non-essential amino acids | ||||
| Alanine | 2.46 ± 0.16 | 2.79 ± 0.07 | 2.72 ± 0.14 | 2.45 ± 0.21 |
| Aspartic acid | 2.93 ± 0.07 | 2.88 ± 0.05 | 3.17 ± 0.01 | 2.94 ± 0.07 |
| Cysteine | 0.42 ± 0.10 | 0.50 ± 0.01 | 0.57 ± 0.10 | 0.47 ± 0.08 |
| Glutamate | 10.01 ± 0.16 | 9.91 ± 0.24 | 10.08 ± 0.14 | 10.11 ± 0.48 |
| Glycine | 4.31 ± 0.09a | 3.94 ± 0.03b | 2.92 ± 0.02c | 1.89 ± 0.08d |
| Proline | 2.77 ± 0.09 | 3.02 ± 0.05 | 2.98 ± 0.03 | 2.76 ± 0.12 |
| Serine | 2.11 ± 0.03 | 2.34 ± 0.04 | 2.31 ± 0.03 | 2.12 ± 0.07 |
| Tyrosine | 1.65 ± 0.06 | 1.84 ± 0.08 | 1.8 ± 0.06 | 1.70 ± 0.15 |
Note
- Values were Means ± SEM (n = 3). Values in the same row which share a same superscript letter are not significantly different (p > .05).
- a,b,c,d Values in the same row which share a same superscript letter are not significantly different (p > .05).
All kinds of ingredients were ground to a fine powder such that they passed through 100 μm nylon mesh. Experimental diets were manufactured by thoroughly mixing dry ingredients with oil and water. The feed pellets (diameter, 2.5 mm) were produced with a twin-screw pelletizer (F-26 (II), South China University of Technology). The pellets were oven-dried at 55°C for 12 h. The feed pellets were ground into two particle sizes and then stored at −20°C until used. The small size pellets (250 to 380 μm) were fed to larvae from 15 to 25 days after hatch (DAH), and the bigger ones (380 to 500 μm) were fed to larvae after 25 DAH.
2.2 Experimental procedure
Large yellow croaker larvae were bought from the Xiangshan Harbor Aquatic Seeds Company. The feeding experiment was conducted in Marine and Fishery Science and Technology Innovation Base. Fish larvae were fed with rotifers Brachionus plicatilis (0.5–1.5 × 104 individual L−1) from 3 to 8 DAH, Artemia nauplii (1.0–1.5 × 103 individual L−1) from 6 to 11 DAH, and live copepods, Calanus sinicus and the control diet from 10 to 14 DAH. After 14 DAH, large yellow croaker larvae were fed the experimental diets. Larvae (mean body weight, 10.56 ± 0.26 mg) were distributed into 12 blue plastic tanks (water volume 250 L, 3000 larvae per tank). Each diet was randomly assigned to triplicate groups of fish larvae. During the feeding trial, the water temperature fluctuated from 24 to 26°C; dissolved oxygen ≥6.5 mg L−1; pH, 7.8 to 8.2; salinity, 20–24, and photoperiod, 17-h light:7-h dark. The daily water change rate was 150%–200%. Larvae were fed seven times daily (06:30, 08:30, 10:30, 13:30, 15:30, 17:30 and 23:00) to apparent satiation for 30 days.
2.3 Sample collection
At the start of the experiment, 3000 large yellow croaker larvae (15 DAH) were randomly obtained from storage tanks to measure initial body weight and length. At the termination of the feeding experiment, fish were fasted for 24 h before sampling and then anaesthetized (eugenol, 1:10,000). The total number, final body weight and length of large yellow croaker larvae from each tank were measured. Thirty larvae were sampled from each tank on ice to collect the visceral mass, which was immediately frozen in liquid nitrogen and stored at −80°C for gene expression analysis. Pancreatic segments (PS) and intestinal segments (IS) from thirty larvae of each tank were sampled following the methodology described by Cahu and Infante (1994). Other thirty whole larvae were sampled from each tank and immediately frozen in liquid nitrogen and stored at −80°C for amino acid analysis. The remaining fish were collected to measure proximate composition.
2.4 Biochemical analysis
2.4.1 Proximate composition analysis
Samples of experiment diets and larvae were dried in an oven at 105℃ until constant weight to determine the dry matter content. Crude protein was determined by measuring N content (N×6.25) through Kjeldahl method. Crude lipid was quantified with ether extraction by the Soxhlet method.
2.4.2 Amino acid content assay
The amino acid content of experimental diets and whole larvae was analysed according to the method by Xie et al. (2012). The samples were hydrolysed with 6 N HCl at 110℃ for 24 h and then diluted to 5 ml with ultrapure water. The solvent solution was evaporated under nitrogen gas, and then, 0.02 N HCl was added. The hydrolysate was filtered through a 0.22 μm nylon syringe filter and then analysed by an automated amino acid analyzer (L-8900 Amino Acid Analyzer; HITACHI).
2.4.3 Digestive enzyme analysis
About 0.2–0.3 g PS or IS of 45 DAH larvae was homogenized in 2 ml 0°C ultrapure water, then centrifuged at 3300 g for 10 min, and the supernatant was collected for enzyme activities assay. Purified brush border membranes (BBM) were obtained according to Crane et al. (1979) with modifications. Briefly, IS was homogenized with 50 mmol L−1 Mannitol and 2 mmol L−1 Tris; then, 0.1 mol L−1 CaCl2 solution were added, and the solution was centrifuged at 9000 g for 10 min. The supernatant was then taken out. The supernatant was further centrifuged at 20,000 g for 20 min, and the precipitate was dissolved with a formulated 0.1 mol L−1 KCl, 5 mmol L−1 Tris-Hepes and 1 mmol L−1 DTT mixed solution. Substrates used to detect the activity of trypsin and leucine-aminopeptidase (LAP) are BAPNA (Na-benzoyl-DL-arginine-p-nitroanilide) (B4875, Sigma) and leucine-p-nitroanolide (L9125, Sigma) respectively (Holm et al., 1988; Maroux et al., 1973). Alkaline phosphatase (AKP) and amylase activity were measured with commercial kits (Nanjing Jiancheng Bio-Engineering Institute, China), following the manufacturer's instructions.
2.4.4 cDNA synthesis and real-time quantitative polymerase chain reaction
Total RNA was extracted from the visceral mass using RNAiso Plus (Takara). The quality, integrity and concentration of RNA was measured by 1.2% denatured agarose gel and Nano Drop®2000 spectrophotometer (Thermo Fisher Scientific). Then, RNA was reverse-transcribed to cDNA with Prime Script-RT reagent Kit (Takara). The real-time quantitative polymerase chain reaction (RT-qPCR) was carried out in a quantitative thermal cycler (Mastercycler ep realplex; Eppendorf). The amplification was implemented in a total volume of 25 μl (10 mM each primer: 1 μl; cDNA: 1 μl; sterilized double-distilled water: 9.5 μl; SYBR® Premix Ex Taq™ II: 12.5 μl). The procedure of RT-qPCR program was conducted following Tan et al. (2016). At the end of each PCR reaction, a melting curve analysis was performed to confirm that a single PCR product was produced in each one of these reactions. Primers for the RT-qPCR were designed based on the nucleotide sequences and published papers (Table 3). According to Zuo et al. (2013), the fluorescence data obtained were normalized to β-actin via 2−ΔΔCT methods.
| Gene name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Reference |
|---|---|---|---|
| gcn2 | TGAGAAGCGGATTCCCGAGTTAG | CGTTCACATTCATGTTGGTGGTAC | XM_019273183.2 |
| atf4 | GCCGTTATTCTGCTCCATCTTCT | AGACCTTACCCTGAGCCCACAT | Liao et al., 2017 |
| asns | ACCAACTGCTGTTTCGGCTTCC | ACTGCAAGGAATCCATCGTCTGTAA | XM_019261608.2 |
| chop | TCTGGATGTTCTGGAGAGTTGTTC | AGGATGATGATGAGGTGTGATGC | Liao et al., 2017 |
| murf1 | TCCAGGAACCCCTACCACTACTC | GCTCCTTACACTTCGGCTCCTTG | KF527410.1 |
| glud | GCATTGGTGAGATTGATGGAGC | AATGGTGCCGTGTTGCAGTTTAT | XM_010735140.3 |
| fas | CAGCCACAGTGAGGTCATCC | TGAGGACATTGAGCCAGACAC | Yan et al., 2017 |
| srebp1 | TCTCCTTGCAGTCTGAGCCAAC | TCAGCCCTTGGATATGAGCCT | Cai et al., 2016 |
| cpt1α | GATGGAGGGTTGTGCCTTCG | GCGATCGATGCCATCTCCTG | KM624524.1 |
| pparα | GTCAAGCAGATCCACGAAGCC | TGGTCTTTCCAGTGAGTATGAGCC | Cai et al., 2016 |
| dgat1 | GGTATCTTGGTGGACCCCATTCA | TGAGCACCGTGGCTGAAGGAAAGA | XM_019254827.2 |
| gk | GCTTCCTCTGGGTTTCACCTTTTC | CAACAATCATTCCCACTTCACAGC | XM_010735934.3 |
| pk | TGGATGATGCTCACCAGGAAAAC | TCTCAATCTCGCAAATAAGGAAACC | XM_010738573.3 |
| β-actin | CTACGAGGGTTATGCCCTGCC | TGAAGGAGTAACCGCGCTCTGT | Yan et al., 2017 |
- Abbreviations: acyl-CoA, acyl-CoA, diacylglycerol acyltransferase 1; asns, asparagine synthetase; atf4, activating transcription factor 4; chop, C/EBP homology protein; cpt1α, carnitine palmitoyltransferase 1 isoforms A; dgat1; dgat2, diacylglycerol acyltransferase 2; fas, fatty acid synthetase; gcn2, General control nonderepressible kinase 2; gk, glucokinase; glud, glutamate dehydrogenase; murf1, muscle RING-finger 1; pk, pyruvate kinase; srebp1, sterol regulatory element-binding protein 1.
2.5 Calculations and statistical analysis
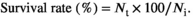
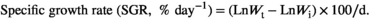
Statistical analysis was performed in SPSS 23.0 (SPSS Inc.), and the results were presented as mean ± SEM (Standard error of means). Homomorphism of the data was verified by Levene tests. All data were subjected to one-way analysis of variance (ANOVA) followed by Tukey's test. The level of significance was set at p < .05.
3 RESULTS
3.1 Survival rate, growth and proximate composition
No significant differences in survival rate and growth performance were observed among dietary treatments (p > .05) (Table 4). Similarly, crude protein, crude lipid or moisture of whole larvae was not significantly different among dietary treatments (p > .05) (Table 5).
| Parameter | Dietary arginine levels (g kg−1) | |||
|---|---|---|---|---|
| 19.5 | 31.0 | 39.6 | 48.6 | |
| Survival rate (%) | 16.24 ± 0.36 | 17.06 ± 0.64 | 15.66 ± 0.58 | 15.43 ± 0.57 |
| Initial body weight (mg) | 10.56 ± 0.26 | 10.56 ± 0.26 | 10.56 ± 0.26 | 10.56 ± 0.26 |
| Final body weight (mg) | 59.73 ± 6.75 | 64.30 ± 2.49 | 61.80 ± 8.28 | 59.12 ± 6.92 |
| Initial body length (mm) | 6.97 ± 0.10 | 6.97 ± 0.10 | 6.97 ± 0.10 | 6.97 ± 0.10 |
| Final body length (mm) | 13.98 ± 0.60 | 14.12 ± 0.21 | 14.33 ± 0.91 | 14.66 ± 0.81 |
| Specific growth rate (%/day) | 5.73 ± 0.40 | 6.02 ± 0.13 | 5.86 ± 0.45 | 5.72 ± 0.39 |
Note
- Values were Means ± SEM (n = 3). Values in the same row which share a same superscript letter are not significantly different (p > .05).
| Parameter | Dietary arginine levels (g kg−1) | |||
|---|---|---|---|---|
| 19.5 | 31.0 | 39.6 | 48.6 | |
| Crude protein | 88.63 ± 3.35 | 88.27 ± 1.47 | 93.00 ± 3.29 | 92.74 ± 2.91 |
| Crude lipid | 22.87 ± 0.58 | 21.92 ± 1.13 | 24.23 ± 0.97 | 23.45 ± 1.64 |
| Moisture | 848.63 ± 4.34 | 851.97 ± 3.75 | 839.70 ± 5.19 | 845.93 ± 6.11 |
Note
- Values were Means ± SEM (n = 3). Values in the same row which share a same superscript letter are not significantly different (p > .05).
3.2 Amino acid profile of whole fish larvae
Larvae fed the diet with 48.6 g kg−1 arginine had significantly higher arginine content compared to those fed the diet with 19.5 g kg−1 arginine (p < .05) (Table 6). However, no statistically significant difference in contents of other amino acid was observed among dietary treatments (p > .05) (Table 6).
| Amino acid | Dietary arginine levels (g kg−1) | |||
|---|---|---|---|---|
| 19.5 | 31.0 | 39.6 | 48.6 | |
| Essential amino acid | ||||
| Arginine | 3.10 ± 0.08b | 3.29 ± 0.07ab | 3.28 ± 0.03ab | 3.40 ± 0.04a |
| Histidine | 1.15 ± 0.08 | 1.23 ± 0.05 | 1.14 ± 0.05 | 1.2 ± 0.03 |
| Isoleucine | 2.4 ± 0.13 | 2.54 ± 0.11 | 2.33 ± 0.1 | 2.48 ± 0.05 |
| Leucine | 3.94 ± 0.23 | 4.13 ± 0.15 | 3.82 ± 0.13 | 4.09 ± 0.04 |
| Lysine | 3.86 ± 0.32 | 4.23 ± 0.15 | 3.72 ± 0.1 | 4.13 ± 0.1 |
| Methionine | 1.39 ± 0.16 | 1.75 ± 0.06 | 1.56 ± 0.07 | 1.6 ± 0.09 |
| Phenylalanine | 2.48 ± 0.18 | 2.57 ± 0.05 | 2.3 ± 0.13 | 2.57 ± 0.15 |
| Threonine | 2.38 ± 0.11 | 2.38 ± 0.08 | 2.29 ± 0.09 | 2.43 ± 0.03 |
| Valine | 2.53 ± 0.12 | 2.62 ± 0.11 | 2.44 ± 0.1 | 2.6 ± 0.01 |
| Non-essential amino acids | ||||
| Alanine | 3.04 ± 0.03 | 2.95 ± 0.1 | 3.01 ± 0.18 | 3.09 ± 0.18 |
| Aspartic acid | 4.94 ± 0.23 | 4.99 ± 0.2 | 4.74 ± 0.13 | 5.03 ± 0.13 |
| Cysteine | 0.48 ± 0.03 | 0.56 ± 0.03 | 0.55 ± 0.02 | 0.53 ± 0.04 |
| Glutamate | 8.17 ± 0.31 | 8.27 ± 0.31 | 8.05 ± 0.36 | 8.39 ± 0.21 |
| Glycine | 3.4 ± 0.1 | 3.53 ± 0.12 | 3.46 ± 0.13 | 3.63 ± 0.18 |
| Proline | 2.18 ± 0.04 | 2.2 ± 0.04 | 2.2 ± 0.05 | 2.23 ± 0.03 |
| Serine | 2.49 ± 0.12 | 2.47 ± 0.08 | 2.42 ± 0.1 | 2.54 ± 0.04 |
| Tyrosine | 1.9 ± 0.13 | 2.05 ± 0.06 | 1.9 ± 0.09 | 2.01 ± 0.02 |
Note
- Values were Means ± SEM (n = 3). Values in the same row which share a same superscript letter are not significantly different (p > .05).
3.3 Activities of digestive enzymes
Larvae fed diets with 31.0 and 39.6 g kg−1 arginine showed significantly higher trypsin activity in IS, compared to those fed the diet with 48.6 g kg−1 arginine (p < .05) (Table 7). However, no significant difference was observed in the activity of trypsin in PS and the ratio of ‘trypsin in the intestinal segment’/‘trypsin in the pancreatic plus intestinal segment’ (Try-IS/[PS + IS]) (p > .05) (Table 7). Larvae fed the diet with 39.6 g kg−1 arginine showed the highest activity of amylase (IS), which was significantly higher than those fed the diet with 31.0 g kg−1 arginine (p < .05) (Table 7). The supplementation of arginine significantly decreased the activities of AKP (IS and BBM) compared to those fed the diet with 19.5 g kg−1 arginine (p < .05) (Table 7).
| Parameter | Dietary arginine levels (g kg−1) | |||
|---|---|---|---|---|
| 19.5 | 31.0 | 39.6 | 48.6 | |
| Trypsin (mU mg−1 protein) | ||||
| PS | 19.36 ± 0.39 | 18.09 ± 1.89 | 22.58 ± 0.81 | 19.35 ± 0.09 |
| IS | 30.83 ± 2.10ab | 32.77 ± 1.35a | 35.33 ± 1.97a | 24.43 ± 2.34b |
| Try-IS/(PS + IS) | 0.61 ± 0.02 | 0.65 ± 0.03 | 0.61 ± 0.02 | 0.56 ± 0.02 |
| Amylase (U mg−1 protein) | ||||
| PS | 0.32 ± 0.06 | 0.38 ± 0.09 | 0.53 ± 0.10 | 0.40 ± 0.07 |
| IS | 1.06 ± 0.02ab | 0.84 ± 0.04b | 1.12 ± 0.11a | 0.90 ± 0.02ab |
| AKP (U g−1 protein) | ||||
| IS | 235.63 ± 10.05a | 175.88 ± 5.05b | 157.55 ± 3.46b | 159.04 ± 1.56b |
| BBM | 1136.33 ± 89.40a | 743.11 ± 43.68b | 776.41 ± 25.14b | 845.43 ± 62.71b |
Note
- Values were Means ± SEM (n = 3). Values in the same row which share a same superscripts letter are not significantly different (p > .05).
- Abbreviations: AKP, alkaline phosphatase; BBM, brush border membrane of intestine; IS, intestinal segments; PS, pancreatic segments; Try-IS/(PS + IS), ‘trypsin in the intestinal segment’/‘trypsin in the pancreatic plus intestinal segment’.
3.4 mRNA expression of GCN2-ATF4 signalling pathway-related genes in the visceral mass of large yellow croaker larvae
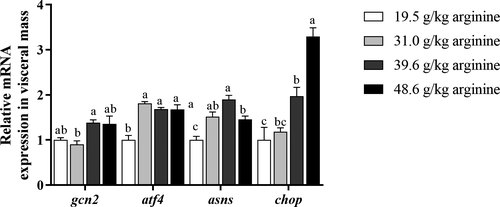
Gcn2 mRNA expression of larvae fed the diet with 39.6 g kg−1 arginine significantly upregulated compared to those fed the diet with 31.0 g kg−1 arginine (p < .05) (Figure 1). Larvae fed diets with 31.0–48.6 g kg−1 arginine showed significantly higher mRNA expression of atf4, compared to those fed the diet with 19.5 g kg−1 arginine (p < .05) (Figure 1). Larvae fed the diet with 39.6 g kg−1 arginine showed significantly higher asns mRNA expression levels, compared to those fed diets with 19.5 g kg−1 and 48.6 g kg−1 arginine (p < .05) (Figure 1). The chop mRNA expression of larvae fed diets with 39.6–48.6 g kg−1 arginine significantly upregulated compared to those fed the diet with 19.5 g kg−1 arginine (p < .05) (Figure 1). Larvae fed the diet with 48.6 g kg−1 arginine showed significantly higher mRNA expression of chop than any other group (p < .05) (Figure 1).
3.5 mRNA expression of amino acid and protein catabolism-related genes in the visceral mass of large yellow croaker larvae
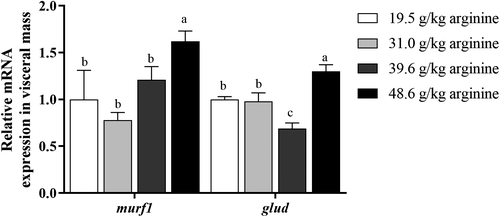
With dietary arginine levels increasing from 19.5 g kg−1 to 48.6 g kg−1, the mRNA expression of protein catabolism key enzyme (murf1) and amino acid catabolism key enzyme (glud) in the visceral mass of larvae was firstly downregulated and then upregulated. Larvae fed the diet with 48.6 g kg−1 arginine showed significantly higher mRNA expression of murf1 and glud than any other group (p < .05) (Figure 2).
3.6 mRNA expression of lipogenesis and lipolysis-related genes in the visceral mass of large yellow croaker larvae
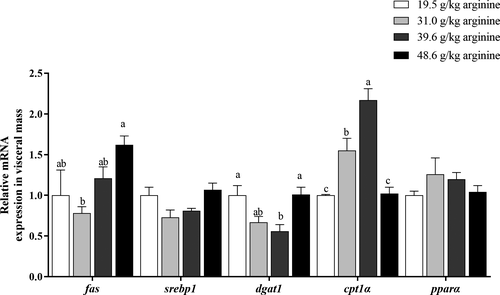
With dietary arginine levels increasing from 19.5 to 48.6 g kg−1, the expression of de novo lipogenesis-related genes (fas and srebp1) and fatty acid esterification-related genes (dgat1) in the visceral mass of larvae firstly downregulated and then upregulated. Larvae fed the diet with 48.6 g kg−1 arginine showed significantly higher mRNA expression of fas compared to those fed the diet with 31.0 g kg−1 arginine (p < .05) (Figure 3). However, the supplementation of arginine did not significantly affect the mRNA expression of srebp1 (p > .05). Larvae fed the diet with 39.6 g kg−1 arginine showed the lowest mRNA expression of dgat1, significantly lower compared to those fed diets with the 19.5 and 48.6 g kg−1 arginine (p < .05) (Figure 3). With increasing arginine levels in diets, the mRNA expression of lipolysis-related genes (cpt1α and pparα) of larvae was first upregulated and then downregulated. The expression of cpt1α of larvae fed the diet with 39.6 g kg−1 arginine was significantly upregulated compared to any other group (p < .05) (Figure 3), while the mRNA expression of pparα was not significantly affected by the dietary arginine (p > .05) (Figure 3).
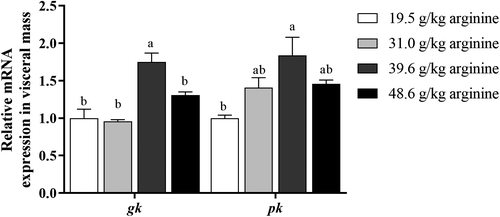
3.7 mRNA expression of glycolysis-related genes in the visceral mass of large yellow croaker larvae
Larvae fed the diet with 39.6 g kg−1 arginine showed significantly higher mRNA expression of gk than any other group (p < .05) (Figure 4). The mRNA expression of pk of larvae fed the diet with 39.6 g kg−1 arginine showed the highest level, which was significantly higher compared to those fed the diet with 19.5 g kg−1 arginine (p < .05) (Figure 4).
4 DISCUSSION
Arginine is an EAA for fish species (Berge et al., 1998; Rahimnejad & Lee, 2014; Zhou et al., 2012). However, results of this study showed that the survival or growth of large yellow croaker larvae was not significantly affected by dietary arginine from 19.5 to 48.6 g kg−1, which was consistent with conclusions on turbot (Zhang et al., 2017) and gilthead seabream (Oliva-Teles et al., 2017). The reason for this phenomenon might be that the arginine content of the control diet (19.5 g kg−1) was able to meet the growing needs of large yellow croaker larvae. Several studies have revealed that there may be an antagonistic effect between arginine and lysine in fish (Berge et al., 1999; Iaccarino et al., 2009; Zhou et al., 2011). Excessive arginine in diets may reduce lysine utilization and reduce growth performance (Ren et al., 2014; Wan et al., 2006). In this study, the growth performance of larvae showed a decreasing trend in diets with 39.6 and 48.6 g kg−1 arginine, while no significant difference was observed among dietary treatments.
Larvae are the early stage of the fish life cycle, and their digestive system has not fully developed (Cahu & Infante, 2001; Yao et al., 2020). Digestive enzyme activity is often used as an indicator to measure the digestive capacity and maturity of larval digestive system (Cahu et al., 1998, 2007; Gisbert et al., 2018). The exocrine level of pancreas and the onset of BBM enzymes could reflect the maturity of larval digestive system (Liu et al., 2015; Segner et al., 1989). The ratio of Try-IS/(PS + IS) and the activity of amylase could reflect the secretion level of pancreas (Cahu et al., 1999; Ma et al., 2005). In the present study, larvae fed the diet with 31.0 g kg−1 arginine showed lower activity of amylase (IS) than larvae fed the diet with 39.6 g kg−1 arginine, while no significant difference was observed in the ratio of Try-IS/(PS + IS). This indicates that arginine levels may affect pancreatic secretion function to some extent. AKP is one of the typical enzymes in the BBM, which could globally reflect the maturation process in the intestinal of fish larvae (Buchet et al., 2000; Cahu & Infante, 1995; Wang et al., 2017). In this study, larvae fed the diet with 19.5 g kg−1 arginine showed the highest activity of AKP (both in IS and BBM), significantly higher compared to the other groups, which might indicate that 19.5 g kg−1 arginine could meet the requirement of larvae for intestinal maturity, and higher-dose arginine contents would inhibit the intestinal development.
The GCN2-ATF4 signalling pathway plays a crucial role in amino acid recognition and protein synthesis. GCN2 is an amino acid sensor activated by uncharged tRNAs when intracellular amino acids are limited (Yuan et al., 2017). Activated GCN2 will phosphorylate the eukaryotic initiation factor-2α (eIF2α), resulting in upregulated expression of various mRNAs, including ATF4. Then, ATF4 can increase the expression of downstream key enzymes (ASNS and CHOP) to improve amino acid biosynthesis and inhibit protein synthesis to adapt the damage caused by intracellular amino acid deficiency (Kilberg et al., 2009; Marion et al., 2011; Vattem & Wek, 2004). The GCN2-ATF4 signalling pathway is stimulated when intracellular amino acids (arginine, lysine and leucine) were limited (Averous et al., 2016; Marion et al., 2011; Wang et al., 2013). However, the mRNA expression of the selected gene markers (gcn2, atf4, chop and asns) for the GCN2-ATF4 signalling pathway was elevated in larvae fed diets with 31.0–48.6 g kg−1 arginine in this study. The different results may be related to antagonistic effects of arginine and lysine in large yellow croaker larvae (Lin et al., 2015; Luzzana et al., 1998; Pereira et al., 2017; Pohlenz et al., 2012; Zhou et al., 2012). In this study, the arginine level of the control group was 19.5 g kg−1, which might have met the requirement for larval survival and growth. Excessive arginine may competitively inhibit the absorption and transport of lysine in larvae, resulting in the lack of lysine, thereby activating GCN2-ATF4 signalling pathway (Averous et al., 2016; Chaveroux et al., 2009).
Intake of amino acids higher than that required for fish will result in additional catabolism of these amino acids (NRC, 2011). In this study, larvae fed the diet with 48.6 g kg−1 arginine showed the highest mRNA expression of glud, significantly higher than the other groups, which might be related to the decomposition of excessive arginine in the diet. Similarly, high dose of arginine (48.6 g kg−1) content upregulated the expression of the key proteolytic gene (murf1), which might be another clue of the antagonistic effect between arginine and lysine in large yellow croaker larvae. Excessive arginine intake may lead to lysine deficiency in the body (Daniel, 2004; Ren et al., 2014; Vilella et al., 1990). Therefore, larvae may tend to decompose protein to meet their requirement for lysine. In another way, decomposition of protein or excessive arginine will convert into fatty acids and/or glycogen for future use (Cowey & Walton, 1989). In this study, high dose of arginine upregulated the expression of de novo lipogenesis-related genes (fas and srebp1), fatty acid esterification gene (dgat1) and rate-limiting enzymes of glycolysis (gk and pk). Similarly, 1.62% dietary arginine could significantly upregulate the fat synthesis and the glycolysis of juvenile blunt snout bream (Liang et al., 2017).
5 CONCLUSION
In summary, this study showed that dietary 19.5–48.6 g kg−1 arginine had no significant influence on the survival or growth performance of large yellow croaker larvae. However, the arginine level significantly affected the arginine content of whole larvae, activity of digestive enzymes, lipid metabolism and glycolysis of larvae. The GCN2-ATF4 signalling pathway was stimulated by high-dose (31.0–48.6 g kg−1) arginine, maybe due to the antagonistic effect of arginine and lysine. These results might indicate that 19.5 g kg−1 dietary arginine (37.6 g kg−1 dietary protein) could meet the growth requirement of large yellow croaker larvae.
ACKNOWLEDGEMENTS
This research was financially supported by China Agriculture Research System of MOF and MARA [grant number: CARS47]. We thank Wei Xu, Houguo Xu, Weiqi Xu, Xiaojun Xiang, Zhaoyang Yin, Wenxing Huang, Xiang Xu, and Yanwen Zhuang for their help during the experiment.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




