Effects of dietary vitamin E on growth performance, antioxidant capacity and lipid metabolism of juvenile golden pompano Trachinotus ovatus
Guanrong Zhang, Chao Xu and Cuihong You Joint first authorship.
Abstract
In order to investigate the dietary requirement of Trachinotus ovatus for vitamin E (VE) and its effects on tissue polyunsaturated fatty acids (PUFA) reservation, a 56-day feeding trial was performed with five diets (D1–D5) supplemented with different levels of VE at 34.56, 47.35, 61.16, 91.06 and 144.88 mg VE/kg diet. A total of 375 fish (initial weight 13.40 ± 0.08 g) were randomly distributed equally into 15 sea cages and each diet in triplicate cages. After 8 weeks of feeding, fish fed diet D3 exhibited significantly higher weight gain (WG), serum superoxide dismutase and glutathione peroxidase activities and also had higher docosahexaenoic acid (DHA) and n-3 PUFA contents in the raw muscle than those of fish fed diets D1 and D5 (p < .05). Moreover, n-6 PUFA and n-3 PUFA contents in cooked muscle of D3 and D4 groups were significantly higher than those of other groups (p < .05). Besides, the contents of hepatic arachidonic acids, eicosapentaenoic acid, DHA, n-6 PUFA and n-3 PUFA in fish of D3 and D4 groups were also significantly higher than those of D1 group (p < .05). Correspondingly, the transcript levels of genes related to PUFA β-oxidation (cpt1 and pparα) and synthesis (fas and elovl5) were lower in D3 and D4 groups. Based on regression analysis for WG, the optimum dietary VE level was 90.75 mg/kg, which was consistent with the above results that fish fed diets D3 and D4 displayed better growth performance, antioxidant capacity and muscle PUFA stability, where the last suggesting an effect of dietary VE against PUFA β-oxidation.
1 INTRODUCTION
Vitamin E (VE), consisting of tocopherols, tocotrienols and the derivatives, serves as a lipid-soluble antioxidant providing nutrients to animals including fish (Bramley et al., 2000). Its primary function is to act as a free radical scavenger that protects cells and tissues against the deleterious effects of free radicals (Liu et al., 2018; Naderi et al., 2019; Wang et al., 2019). Besides, VE can increase antioxidant ability of fish by enhancing the antioxidant enzyme activities containing superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px), such as largemouth bass (Micropterus salmoides) (Li et al., 2018), hybrid snakehead (Channa argus × Channa maculata) (Zhao et al., 2017) and grass carp (Ctenopharyngodon idellus) (Li et al., 2014). Moreover, as an antioxidant, VE supplement could inhibit the oxidation and rancidity of unsaturated fatty acids in animal feed or tissues and improve the growth and feed efficiency of fish (Min et al., 2017; Wassef et al., 2001). Previous studies demonstrated that VE could protect polyunsaturated fatty acids (PUFA) from auto-oxidation, which contributes to increasing PUFA contents in muscle, thereby improving the nutritional quality of meat (Belles et al., 2018; Hamre, 2011; Navarro et al., 2012). Furthermore, VE also has an antioxidant property in the post-mortem tissues and dietary VE supplementation improved lipid stability of cooked meat, which were reported in terrestrial animals such as rabbit (Tres et al., 2008) and chicken (Cortinas et al., 2005). However, similar effects of VE on the amount and type of fatty acid in the cooked flesh have not yet been reported in fish. In addition, several studies have shown that VE can affect fat deposition by regulating genes responsible for lipid metabolism, such as lipogenesis, lipolysis and transport in lambs and broiler chickens (Gonzalez-Calvo et al., 2017; Zhang et al., 2020). Also, VE has been reported to influence hepatic PUFA desaturation and elongation in Zebrafish (Danio rerio) (Lebold et al., 2011). Nevertheless, information on the effects of dietary VE on PUFA β-oxidation and synthesis of fish and other aquatic animals is still limited.
Vitamin E supplementation is considered essential for most fish species, as they cannot synthesize it (Li et al., 2018). Accumulating data indicate that dietary VE deficiency in fish usually results in a number of deficiency symptoms such as muscle degeneration, internal haemorrhaging, retarded growth and depressed immunity (Chen et al., 2004; Head et al., 2021; Kocabas & Gatlin, 1999; Pan et al., 2017). In contrast, overdoes of VE disturbs normal metabolism and consequently resulted in metabolic disorder (Kaewsrithong et al., 2002; Zhang et al., 2016). Considering this, it is necessary to establish an optimal dietary VE level for cultured species. To date, dietary VE requirement has been demonstrated in many fish species, which varying with each other, for example 28 mg/kg diet for hybrid striped bass (Morone chrysops female × Morone saxatilis male) (Kocabas & Gatlin, 1999), 78 to 111 mg/kg for cobia (Rachycentron canadum) (Zhou et al., 2013) and 451 mg/kg for meagre (Argyrosomus regius) (Lozano et al., 2017). So, it is difficult to extrapolate from one fish species to another, and thus, investigating dietary VE requirements for each cultured species is necessary.
Golden pompano (Trachinotus ovatus) has been one of the important cultured marine fish in China because of its fast growth, efficient feed conversion, preferred flesh quality and high economic value (Yin et al., 2015; Zhang, Wang, et al., 2019). To date, some studies have been conducted on the nutrient requirement for this fish, such as optimal dietary carbohydrate, protein and lipid levels (Wang et al., 2013; Zhou et al., 2015; Li et al., 2019; Zhao et al., 2020). However, its requirement on VE remains unknown. Thus, this study was to determine the dietary VE requirement of juvenile T. ovatus and evaluate the effect of dietary VE on PUFA β-oxidation by investigating the effects of dietary VE levels on the growth performance, antioxidant capacity, tissue fatty acid composition, fatty acid stability in muscle during cooking and genes responsible for lipid metabolism of this species. The results obtained here will help to increase the database of the nutritional requirements of T. ovatus and promote the development of nutritionally balanced compound feed.
2 MATERIALS AND METHODS
2.1 Experimental diets
Five isonitrogenous (500 g/kg) and isoclinic (130 g/kg) diets (D1–D5), containing five graded levels of α-tocopheryl acetate (500 g/kg α-tocopherol equivalent, Hangzhou Dehong Biotech Co., Ltd.) resulting from the addition of VE at 0, 20, 40, 80 and 160 mg VE/kg diets, were formulated in this study. High-performance liquid chromatography was used to determine the exact VE content. The results are as follows: 34.56, 47.35, 61.16, 91.06 and 144.88 mg/kg diet in D1–D5, respectively. Fish meal, soy protein concentrate and chicken meal were used as protein sources. Fish oil and soy lecithin were utilized as the lipid sources, α-starch and cassava starch were used as the carbohydrate source. The dry ingredients were finely ground, mixed well and dry pelleted in a laboratory pellet mill (Shanghai, China) through 2 mm dies. Ingredients and proximate composition of the experimental diets are presented in Table 1.
| Diets | |||||
|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | |
| Ingredients (g/kg) | |||||
| Fish meal | 250 | 250 | 250 | 250 | 250 |
| Chicken meal | 150 | 150 | 150 | 150 | 150 |
| Soy protein concentrate | 220 | 220 | 220 | 220 | 220 |
| α-starch | 30 | 30 | 30 | 30 | 30 |
| Cassava starch | 130 | 130 | 130 | 130 | 130 |
| Fish oil | 52 | 52 | 52 | 52 | 52 |
| Premix compounda | 45.17 | 45.17 | 45.17 | 45.17 | 45.17 |
| Lecithin | 20 | 20 | 20 | 20 | 20 |
| Monocalcium phosphate | 10 | 10 | 10 | 10 | 10 |
| Lutein | 2 | 2 | 2 | 2 | 2 |
| α-tocopheryl acetateb (mg/kg) | 0 | 40 | 80 | 160 | 320 |
| Microcrystalline cellulose | 90.83 | 90.79 | 90.75 | 90.67 | 90.51 |
| Proximate composition (% dry weight) | |||||
| Moisture | 8.20 | 8.63 | 8.83 | 8.65 | 8.51 |
| Ash | 8.97 | 8.78 | 8.71 | 9.09 | 8.92 |
| Crude protein | 50.27 | 50.37 | 50.49 | 50.54 | 50.53 |
| Crude lipid | 13.35 | 12.81 | 13.05 | 13.16 | 13.48 |
| Vitamin E (mg/kg) | 34.56 | 47.35 | 61.16 | 91.06 | 144.88 |
| Fatty acid composition (mg FA/g diet, dry weight) | |||||
| 18:2n-6 (LA) | 5.07 | 4.99 | 4.88 | 5.07 | 4.94 |
| 20:4n-6 (ARA) | 0.37 | 0.37 | 0.37 | 0.38 | 0.37 |
| 18:3n-3 (ALA) | 1.37 | 1.37 | 1.46 | 1.47 | 1.47 |
| 20:4n-3 | 0.10 | 0.10 | 0.11 | 0.10 | 0.11 |
| 20:5n-3 (EPA) | 2.87 | 2.85 | 2.89 | 2.95 | 2.84 |
| 22:6n-3 (DHA) | 3.59 | 3.58 | 3.59 | 3.68 | 3.56 |
| ∑SFAc | 14.05 | 13.74 | 13.97 | 14.06 | 13.79 |
| ∑MUFAd | 10.91 | 10.75 | 10.70 | 10.96 | 10.67 |
| ∑n-6 PUFAe | 6.20 | 6.10 | 6.01 | 6.23 | 6.08 |
| ∑n-3 PUFAf | 7.94 | 7.90 | 8.0 | 8.19 | 7.97 |
| n-3/n-6 ratio | 1.28 | 1.30 | 1.34 | 1.32 | 1.31 |
- a Consists of choline chloride, l-ascorbyl-2-polyphosphate, vitamin mixture and mineral compound. Vitamin mixture (per kg mixture): VA: 1,100,000 IU; D3: 320,000 IU; VB12: 8 mg; VK3: 1000 mg; VB1: 1500 mg; VB2: 2800 mg; calcium pantothenate: 2000 mg; nicotinamide: 7800 mg; folic acid:400 mg; inositol: 12,800 mg; VB6: 1000 mg. Mineral compound (per kg mixture): sodium fluoride: 2 mg; potassium iodide: 0.8 mg; cobalt chloride (1%): 50 mg; copper sulphate: 10 mg; copper sulphate: 80 mg; zinc sulphate: 50 mg; manganese sulphate: 60 mg; magnesium sulphate: 1200 mg; common salt: 100 mg; zeolite powder: 15.45 g.
- b α-tocopheryl acetate: contain 50% active ingredient.
- c ∑SFA is the sum of saturated fatty acids and includes 12:0, 13:0, 14:0, 15:0, 16:0, 18:0, 20:0, 21:0, 22:0 and 23:0.
- d ∑MUFA is the sum of monounsaturated fatty acids and includes 14:1, 15:1, 16:1, 17:1, 18:1, 20:1, 22:1n-9 and 24:1.
- e ∑n-6 PUFA is the sum of n-6 polyunsaturated fatty acids and includes 18:2n-6, 18:3n-6, 20:2n-6, 20:3n-6, 20:4n-6 and 22:2n-6.
- f ∑n-3 PUFA is the sum of n-3 polyunsaturated fatty acids and includes 18:3n-3, 20:3n-3, 20:4n-3, 20:5n-3, 22:5n-3 and 22:6n-3.
2.2 Animals and feeding
According to the guide for the use of experimental animals of Shantou University, this study was performed strictly in accordance with the standard operation procedures. All animal care and use procedures in the present study were approved by the Institutional Animal Care and Use Ethics Committee of Shantou University.
Before the experiment, fish were acclimatized to the experimental conditions for two months at the coast near Nan Ao Marine Biology Station of Shantou University. During this time, fish were fed a commercial diet (ash ≤150 g/kg, lipid ≥ 50 g/kg and protein ≥ 400 g/kg; Guangdong Feeds Group Co., Ltd) twice daily. After acclimatization, a total of 375 fish (average weight: 13.40 ± 0.08 g) were randomly distributed among 15 sea cages (1.0 m × 1.0 m × 1.5 m) at 25 fish per cage. Fish were fed to visual satiation twice (7:00 and 17:00 h) a day for 8 weeks. During the feeding trial, water temperature and salinity were fluctuated from 19.96 to 29.63°C and 30‰ to 32‰ respectively. Besides, dissolved oxygen and 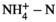 content were maintained at >5.0 and <0.5 mg/L, respectively.
content were maintained at >5.0 and <0.5 mg/L, respectively.
2.3 Sample collection
At the end of the feeding trial, fish were fasted for 24 h and then anaesthetized with 0.1 ml/L 2-phenoxyethanol for sampling. Subsequently, fish weight was recorded to calculate final weight, specific growth rate (SGR), weight gain (WG), feed conversion ratio (FCR) and protein efficiency ratio (PER). Two fish per cage were collected for body composition analysis. Another two fish per cage were used for collecting blood. Blood was collected from the caudal vein, stored at 4°C for 2 h and then centrifuged (3500 g at 4°C for 10 min) to collect serum. The serum samples were stored at −80°C for further analysis. Moreover, liver samples were also collected, flash frozen in liquid nitrogen and stored at −80°C until analysis.
2.4 Analysis of VE content, proximate composition and fatty acid composition
Liver VE content was determined according to the manufacturer’s instruction of the assay kit (Catalog No, A008-1-1; Jiancheng Bioengineering Institute). Moisture, crude lipid, crude protein and ash contents of the experimental diets and tissues were measured using the prescribed methods of AOAC (1995). Fatty acid composition of the experimental diets and liver samples were determined following the methods detailed by Li et al. (2005, 2018). Briefly, 50 µl of C17:0 (1 µg/µl) was added in each sample as the internal standard (Sigma). Total lipid in each sample was extracted with chloroform/methanol (2:1 v/v; 6 ml) containing butylated hydroxytoluene (0.1g/L). Fatty acid methyl esters were prepared with KOH-methanol (0.5 M; 2 ml) and boron trifluoride methanol complex solution (2 ml; 130–150 ml/L BF3 basis, Sigma-Aldrich). Then, the fatty acid methyl esters were separated and analysed using a gas chromatograph (GC-2010; Shimadzu) equipped with an auto-sampler and a hydrogen flame ionization detector (Li et al., 2005, 2008). The detailed gas chromatograph parameters were as depicted before, and each fatty acid was identified by comparing with known commercial standards (Sigma) and quantified relative to the internal standard (Li et al., 2005, 2008).
The main measurement steps of fatty acid change during cooking were as follows: two dorsal muscles samples each fish (six fish per treatment) were cleared with absorbent paper to remove blood, one piece of meat was freeze-dried directly, and the other was put in the clean centrifuge tubes and was cooked at 100°C water for 10 min and then freeze-dried. Then, the fatty acid composition of the raw and cooked muscle was analysed according to the method described above.
2.5 Antioxidant indices analysis
About 0.1 g liver sample per fish was homogenized in 9 volumes of saline buffer (8.6 g/L, m/v) to collect the supernatant by the method described in our previous study (Zhang, Wang, et al., 2019). The protein content of the supernatant was measured by Bradford protein assay kit (Beyotime Biotechnology Co. Ltd). GSH-Px, SOD and CAT activities, as well as total antioxidant capacity (T-AOC) were analysed according to the manufacturer instructions of the assay kits (Nanjing Jiancheng Bioengineering Co. Ltd).
2.6 Total RNA extraction and real-time quantitative PCR analysis
Total RNA of the liver was extracted by using the total RNA extraction kit (BioFlux) (Zhang, Wang, et al., 2019), and the concentration was assessed by spectrophotometrically at 260 nm. Total RNA purity was verified by recording the absorbance at 260 nm (A260) and 280 nm (A280). Then, for each sample, 1 μg of RNA was reverse-transcribed (RT) in a volume of 20 μl using FastKing gDNA Dispelling RT SuperMix (TIANGEN Biotech Co., Ltd.). For real-time quantitative PCR (qPCR), the specific primers (Table 2) were designed according to the published articles (Liu et al., 2017; Tan et al., 2017; Zhang, Chen, et al., 2019), and the sequences of carnitine palmitoyltransferase 1 (cpt1, KP987456.1), liver fatty acid-binding protein (l-fabp, MF034872.1) and peroxisome proliferator-activated receptor α (pparα, KP893147.1) genes in T. ovatus. The qPCR was carried out in a Lightcycler 480 system (Roche) in a total volume of 10 μl contained: 1 μl cDNA, 5 μl SYBR Green Supermix (Biorad), 0.5 μl each primer and 3 μl ddH2O and the qPCR protocol was set as described in Xie et al. (2015). The relative expression of all genes was calculated by the 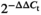 method. In this process, β-actin as the endogenous reference and D1 group as the reference group.
method. In this process, β-actin as the endogenous reference and D1 group as the reference group.
| Primers | qPCR primers, forward/reverse (5′–3′) | Reference |
|---|---|---|
| cpt1 | F: CATTGAGTCAGCTGCATTCTTC | KP987456.1 |
| R: CAGGGACTTGGCGTAACTATC | ||
| apob-100 | F: AAAAGCCACAAGACGAAAGCA | Liu et al. (2017) |
| R: GAAGCAGCAAAAGGCAGAGC | ||
| l-fabp | F: CCAAGGACATCAAGCCAATTAC | MF034872.1 |
| R: TGGTGATTTCAGCCTCCTTAC | ||
| pparα | F: AATCTCAGCGTGTCGTCTT | KP893147 |
| R: GGAAATGCTTCGGATACTTG | ||
| fas | F: GAAGGAGAGGGGGTGGAGTC | Liu et al. (2017) |
| R: GTGTGAAGGTGGAGGGTGTG | ||
| Δ6 fad | F: CATCACCTTCGTCAGGTTTCT | Zhang, Chen, et al. (2019) |
| R: TTAACCAGTCCCGGTGTTTC | ||
| elovl5 | F: TACATGGTCACGCTCATTATCC | Zhang, Chen, et al. (2019) |
| R: CCGTTCTGATGCTCCTTCTTTA | ||
| elovl4 | F: AAACCAGACGACCATCCTTAC | Zhang, Chen, et al. (2019) |
| R: ACAGACACACCCACAAATACA | ||
| β-actin | F: TACGAGCTGCCTGACGGACA | Tan et al. (2017) |
| R: GGCTGTGATCTCCTTCTGC |
Note
- apob-100, apolipoproteinB-100; cpt1, carnitine palmitoyltransferase 1; elovl4, elongation of very-long-chain fatty acids 4; elovl5, elongation of very-long-chain fatty acids 5; fas, fatty acid synthase; l-fabp, liver fatty acid-binding protein; pparα, peroxisome proliferator-activated receptor α; Δ6 fad, delta 6 fatty acid desaturase.
2.7 Statistical analysis
The data were analysed by one-way ANOVA test using the SPSS version 20.0 (SPSS Inc.), taking into account the normality of the data distribution and the homogeneity of variances. If significant (p < .05) differences were found, Duncan's multiple range test was conducted to rank the means. The linear and quadratic effects of dietary VE levels were determined using regression analysis, with dietary VE levels as the independent variable. All data were presented as mean ± pooled SEM (standard error of the mean).
3 RESULTS
3.1 Growth performance and feed utilization
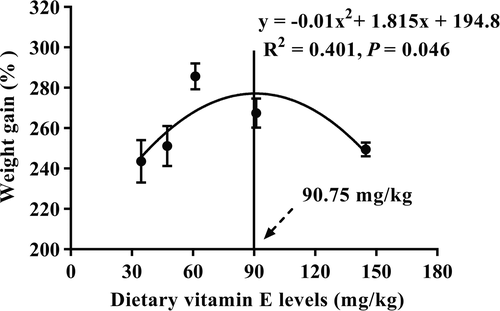
The FCR, PER and survival rate were no significant differences (p > .05) among all groups. However, the final weight, WG and SGR all increased as VE levels increased from 34.56 to 61.16 mg/kg (D1–D3), but decreased significantly (p < .05) with further increasing levels (Table 3). Based on the second-order polynomial regression analysis of WG against VE levels, the optimal VE level for juvenile T. ovatus was 90.75 mg/kg (Figure 1).
| Diets | Pooled SEM | p-Value | Regression (p, r2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | Linear | Quadratic | |||||
| Initial weight (g) | 13.27 | 13.33 | 13.67 | 13.47 | 13.27 | 0.10 | .737 | .820 | .004 | .524 | .102 |
| Final weight (g) | 45.56a | 46.78ab | 52.64c | 49.49b | 46.36a | 0.78 | .002 | .885 | .002 | .019 | .484 |
| WG1 (%) | 243.58a | 251.14a | 285.60b | 267.48ab | 249.44a | 5.07 | .023 | .954 | .000 | .046 | .401 |
| SGR2 (%/day) | 2.20a | 2.24a | 2.41b | 2.32ab | 2.23a | 0.03 | .025 | .983 | .000 | .045 | .403 |
| FCR3 | 1.68 | 1.46 | 1.53 | 1.39 | 1.41 | 0.04 | .221 | .084 | .212 | .105 | .313 |
| PER4 | 30.50 | 34.75 | 32.96 | 36.36 | 36.02 | 0.89 | .205 | .072 | .228 | .104 | .314 |
| SUR5 (%) | 100 | 100 | 100 | 100 | 100 | – | – | – | – | – | – |
Note
- Data represent the means of three biological replicates in each group (n = 3) and SEM is pooled of standard error of mean. Without sharing a common superscript letter in the same row indicate significant differences (p < .05).
- 1 Weight gain (WG, %) = 100 × (final weight − initial weight)/initial weight;
- 2 Specific growth rate (SGR, %/day) = [Ln(final weight (g)) − Ln(initial weight (g))]/days × 100;
- 3 Feed conversion ratio (FCR) = total feed fed/(final weight − initial weight);
- 4 Protein efficiency ratio (PER, %) = wet weight gain (g)/total protein fed (g);
- 5 Survival rate (SUR, %) = survived fish number/total fish number × 100.
3.2 Whole-body and muscle proximate composition
As can be seen from Table 4, whole-body crude protein and ash, as well as muscle moisture and lipid contents of T. ovatus, were not significantly (p > .05) affected by dietary VE levels. Whole-body lipid content increased significantly (p < .05) with increasing VE levels up to 144.88 mg/kg, whereas the opposite was true for whole-body moisture and muscle ash contents. Additionally, fish fed the diet D3 had the highest muscle protein content, but no significant differences (p > .05) with the D1, D2 and D4 groups.
| Diets | Pooled SEM | p-Value | Regression (p, r2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | Linear | Quadratic | |||||
| Whole-body (%) | |||||||||||
| Moisture | 68.57b | 68.32b | 67.43ab | 67.55ab | 66.41a | 0.23 | .009 | .000 | .511 | .002 | .515 |
| Protein | 17.12 | 17.66 | 17.06 | 16.99 | 17.44 | 0.10 | .175 | .780 | .004 | .506 | .077 |
| Lipid | 11.12a | 12.29b | 12.67b | 13.25bc | 13.84c | 0.24 | .000 | .000 | .623 | .000 | .719 |
| Ash | 3.93 | 3.57 | 3.63 | 3.53 | 3.55 | 0.07 | .294 | .175 | .100 | .176 | .185 |
| Muscle tissue (%) | |||||||||||
| Moisture | 73.17 | 72.90 | 71.94 | 72.66 | 72.76 | 0.24 | .616 | .545 | .021 | .419 | .097 |
| Protein | 19.93b | 19.80b | 19.95b | 19.73b | 17.83a | 0.26 | .009 | .001 | .583 | .001 | .704 |
| Lipid | 6.36 | 6.84 | 7.34 | 6.99 | 6.94 | 0.38 | .970 | .652 | .015 | .783 | .037 |
| Ash | 1.04b | 1.19c | 1.16c | 0.92a | 0.94ab | 0.03 | .000 | .006 | .352 | .025 | .353 |
Note
- Data represent the means of three biological replicates in each group (n = 3), and SEM is pooled of standard error of mean. Without sharing a common superscript letter in the same row indicate significant differences (p < .05).
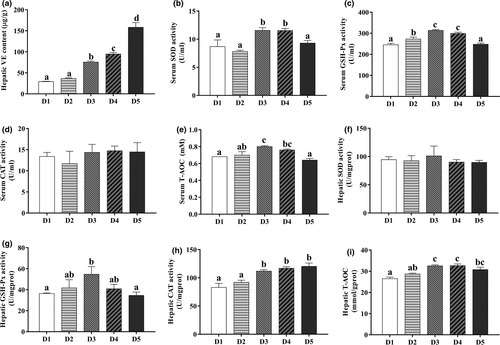
3.3 Vitamin E content and the antioxidant indices
As can be seen from Figure 2, serum CAT and hepatic SOD activities showed no significant differences (p > .05) among the treatments. Hepatic VE content increased significantly (p < .05) with increasing VE levels up to 144.88 mg/kg. The activities of SOD, GSH-Px and T-AOC in serum and GSH-Px, CAT and T-AOC in liver were increased significantly (p < .05) as VE levels increased from 34.56 to 61.16 mg/kg, but decreased with further increasing levels (except for hepatic CAT activities and T-AOC).
3.4 Tissue fatty acid composition
As can be seen from Table 5, hepatic linolenic acid (ALA), saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) contents showed no significant differences (p > .05) among all the treatments. Hepatic linoleic acid (LA), 20:4n-3, arachidonic acid (ARA), docosahexaenoic acid (DHA), n-6 PUFA and n-3 PUFA contents, as well as n-3/n-6 ratio all increased significantly (p < .05) as VE levels increased from 34.56 to 61.16 mg/kg, while decreased with further increasing levels, but no significant difference (except for 20:4n-3 and ARA content and n-3/n-6 ratio). Additionally, hepatic eicosapentaenoic acid (EPA) content increased significantly (p < .05) as VE levels increased from 34.56 to 91.06 mg/kg, but decreased significantly (p < .05) with further increasing levels.
| Fatty acids | Diets | Pooled SEM | p-Value | Regression (p, r2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | Linear | Quadratic | |||||
| 18:2n-6 (LA) | 3.61a | 4.12ab | 4.64b | 4.89b | 4.42ab | 0.15 | .037 | .125 | .171 | .004 | .594 |
| 18:3n-3 (ALA) | 3.16 | 3.17 | 3.64 | 3.38 | 3.19 | 0.14 | .822 | .954 | .000 | .667 | .065 |
| 20:4n-3 | 1.76a | 2.12ab | 2.56b | 2.09ab | 2.02a | 0.09 | .047 | .952 | .000 | .228 | .218 |
| 20:4n-6 (ARA) | 0.34a | 0.45b | 0.60c | 0.50b | 0.48b | 0.02 | .002 | .295 | .084 | .032 | .435 |
| 20:5n-3 (EPA) | 0.21a | 0.26ab | 0.27bc | 0.38d | 0.33c | 0.02 | .000 | .006 | .453 | .000 | .809 |
| 22:6n-3 (DHA) | 1.65a | 3.93b | 4.65b | 4.46b | 4.53b | 0.33 | .001 | .034 | .302 | .003 | .620 |
| ∑SFA | 31.55 | 32.94 | 41.46 | 32.27 | 31.36 | 1.57 | .200 | .565 | .026 | .505 | .108 |
| ∑MUFA | 39.66 | 42.96 | 55.00 | 42.13 | 39.62 | 2.07 | .073 | .507 | .035 | .312 | .177 |
| ∑n-6 PUFA | 5.52a | 6.06ab | 7.07b | 6.94b | 6.51ab | 0.20 | .043 | .192 | .127 | .016 | .500 |
| ∑n-3 PUFA | 7.21a | 10.36b | 13.01c | 11.15bc | 11.10bc | 0.57 | .002 | .177 | .136 | .022 | .472 |
| n-3/n-6 ratio | 0.96a | 1.33b | 1.50c | 1.38bc | 1.31b | 0.05 | .000 | .251 | .100 | .007 | .563 |
Note
- Data represent the means of three biological replicates in each group (n = 3), and SEM is pooled of standard error of mean. Without sharing a common superscript letter in the same row indicate significant differences (ANOVA analysis; p < .05).
- ΣSFA, ΣMUFA, Σn-6 PUFA and Σn-3 PUFA are consistent with Table 2.
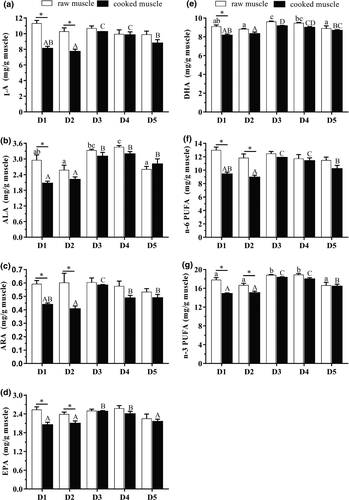
The fatty acid composition of raw and cooked muscle was shown in Figure 3. The contents of LA, ARA, EPA and n-6 PUFA in raw muscle were no significant differences (p > .05) among all groups. The contents of ALA, DHA and n-3 PUFA in raw muscle of fish fed diets with moderate dietary VE level (61.16 and 91.06 mg/kg) were higher than those of other groups. Moreover, fatty acid contents in cooked muscle of fish fed diets with 61.16 mg/kg VE were all significantly (p < .05) higher than those of fish fed diets containing 34.56, 47.35 and 144.88 mg/kg VE. In addition, the contents of LA, ALA, ARA, EPA, DHA, n-6 PUFA and n-3 PUFA in the cooked muscle of fish fed diets with low VE levels (34.56 and 47.35 mg/kg) were significantly lower than those of raw muscle. However, fatty acid contents in raw and cooked muscle were not significantly different (p > .05), as VE levels increased from 61.16 to 144.88 mg/kg.
3.5 Hepatic lipid metabolism-related gene expression
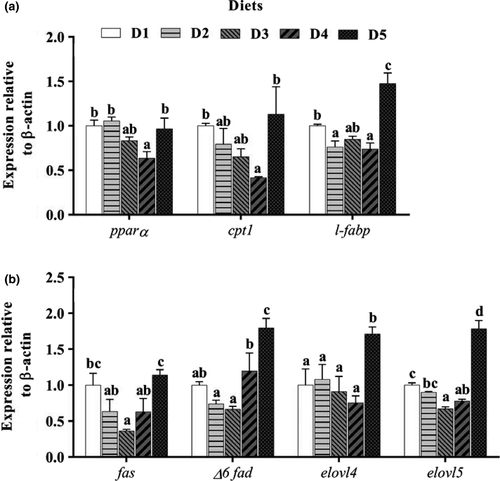
The expression levels of cpt1, l-fabp, pparα, elongation of very-long-chain fatty acids 4 (elovl4) and elongation of very-long-chain fatty acids 5 (elovl5) in liver all decreased as VE levels increased from 34.56 to 91.06 mg/kg, but increased significantly (p < .05) with further increasing levels. In addition, the expression levels of fatty acid synthase (fas) and delta 6 fatty acid desaturase (Δ6 fad) both were decreased as VE levels increased from 34.56 to 61.16 mg/kg, but increased significantly (p < .05) with further increasing levels (Figure 4).
4 DISCUSSION
Nutritional balances have profound influences on fish health and growth, particularly during larval and juvenile stages. In the present study, the final weight, WG and SGR of T. ovatus markedly increased with increasing dietary VE levels from 34.56 to 61.16 mg/kg. Results from the parabola model indicated that VE requirement of T. ovatus juveniles for optimum growth was considered to be 90.75 mg/kg, which is higher than optimum VE requirement of most fish species, for example 55.53 mg/kg for blunt snout bream (Megalobrama amblycephala) (Zhang et al., 2017), 40 mg/kg for parrot fish (Oplegnathus fasciatus) (Galaz et al., 2010) and 31 mg/kg for red drum (Sciaenops ocellatus) (Peng & Gatlin, 2009). However, there was no effect on the growth with the increase in dietary VE levels in Japanese eel (Anguilla japonica) (Shahkar et al., 2018) and meagre (Lozano et al., 2017). This might be because the changes in VE requirements depending on fish species, size, age and culture conditions (Huang et al., 2020; Lozano et al., 2017). In addition, negative effects on growth performance were also observed in T. ovatus, showing that significantly decreased performance in higher VE exceeds 91.06 mg/kg. Similar cases also appeared in several previous studies conducted in cobia (Zhou et al., 2013), yellow catfish (Pelteobagrus fulvidraco) (Lu et al., 2016) and sea cucumber (Apostichopus japonicus) (Wang et al., 2015). This result may be attributed to that high dietary VE level could result in toxic effects, thus inhibiting the growth of fish (Galaz et al., 2010; Kiron et al., 2004).
In the present study, liver VE deposition increased directly in response to the increased level of dietary VE in T. ovatus as reported for other fish containing rainbow trout (Salmo gairdneri) (Puangkaew et al., 2005), coho salmon (Oncorhynchus kisutch) (Huang et al., 2004) and red sea bream (Pagrus major) (Gao et al., 2012). The improvement of VE content in the liver would be expected to increase the antioxidant ability of the biological system (Zhao et al., 2017). Lu et al. (2016) reported that dietary VE can enhance antioxidant ability of organisms by increasing the activities of SOD and CAT. In this study, the activities of serum SOD, GSH-Px and liver GSH-Px, as well as T-AOC in serum and liver of juvenile T. ovatus all increased remarkably as dietary VE levels increased from low-to-moderate levels, then decreased notably with further increasing VE levels. This indicated that optimal VE level might enhance liver antioxidant ability of T. ovatus. This was supported by the following facts: (i) SOD and GSH-Px are important antioxidant enzymes closely involved in the removal of superoxide anions (Dandapat et al., 2000); (ii) T-AOC could directly reflect the antioxidant capacity of fish, and low T-AOC means a decreased antioxidant capacity (Tan et al., 2017). Meanwhile, high VE levels resulted in low activities of liver antioxidant enzymes in fish, which could adversely affect liver antioxidant ability. This might be partly due to that a high dietary VE dose could induce toxic effects, thus inhibiting the activities of liver antioxidant enzymes (Galaz et al., 2010; Kiron et al., 2004).
Previous studies have shown that VE can affect fat deposition (Gonzalez-Calvo et al., 2017; Zhang et al., 2020). In this study, whole-body protein and muscle lipid contents of juvenile T. ovatus showed no significant differences among all the treatments. However, whole-body lipid contents were significantly increased by elevating the VE levels. This might due to that VE functions as a lipid-soluble antioxidant, protecting biological membranes and lipoproteins against oxidation (Zhang et al., 2017), which leads to an increase in lipid contents in fish. In addition, the lowest muscle protein content was found in fish fed the diets with 144.88 mg/kg VE, which was in line with the results obtained in rohu (Labeo rohita), where fish fed with the highest VE levels had the lowest protein content (Sau et al., 2004). Nevertheless, the specific reason for the change caused by excessive doses of dietary VE in muscle protein is unclear and supposed to do further investigations.
Moreover, in this study, the fatty acid composition of the liver was affected by dietary VE levels. Hepatic 20:4n-3, ARA, EPA, DHA, n-6 PUFA and n-3 PUFA contents significantly increased with increasing levels of dietary VE from 34.56 to 91.06 mg/kg. Interestingly, compared with the VE deficiency group (D1), hepatic DHA contents were over twofold increased in VE supplement groups (D2–D5). The positive correlation between liver PUFA and dietary VE levels was also found in channel catfish (Ictalurus punctatus) (Lim et al., 2010) and red sea bream (Gao et al., 2012). According to previous studies, these results might due to the fact that VE can prevent fatty acids against lipid oxidation, thus increasing PUFA contents in the liver (Gao et al., 2012; Hamre & Lie, 1995). With increasing VE levels from 91.06 to 144.88 mg/kg, hepatic 20:4n-3, ARA, DHA, n-6 PUFA and n-3 PUFA contents were slightly reduced. The results may attributed to that high VE levels could result in cell death by increasing cytotoxic activity (Cuesta et al., 2001), as correspondingly increases the degradation of PUFA detached from the cell membrane. Here, in order to characterize the underlying mechanisms related to the regulation of lipid metabolism by VE, molecular investigations were conducted in this study. Hepatic mRNA levels of cpt1, l-fabp, pparα, fas, elovl4 and elovl5 were reduced by the supplementation of dietary VE levels from 34.56 to 91.06 mg/kg. The results indicated a decrease in PUFA β-oxidation and synthesis in the liver of T. ovatus fed the D1–D4 diets. This was supported by the following facts: (i) PPARα activation could accelerate fatty acid oxidation by regulating genes involved in fatty acid transport (fabp), β-oxidation (cpt1) and mitochondrial respiration in various tissues (Lu et al., 2013); (ii) the increased Fas activity could promote body fatty acid biosynthesis (Xu et al., 2018); and (iii) both Elovl4 and Elovl5 are the key elongase involved in PUFA synthesis of fish (Castro et al., 2016; Monroig et al., 2012). The decreased PUFA β-oxidation in liver might be associated with the antioxidant effects of VE (Zhang et al., 2017). It has been proved that dietary VE supplementation could inhibit lipid oxidation in tissues by suppressing the expression of pparα (Zhang et al., 2017). In addition, the decrease in PUFA synthesis is also not surprising since optimal VE supplementation in diets has been demonstrated to inhibit liver fatty acid synthesis by down-regulating the expression of pparγ, which plays an important role in lipid accumulation (Stumvoll & Haring, 2002; Zhang et al., 2017). Zhang et al. (2020) also reported that VE could restrain fatty acid synthesis by down-regulating the expression of fas and acc in broiler chickens. Then, hepatic mRNA levels of pparα, cpt1, l-fabp, fas, Δ6 fad, elovl4 and elovl5 were all significantly increased as VE levels increased from 91.06 to 144.88 mg/kg, suggesting an enhancement of PUFA β-oxidation and synthesis in the liver of T. ovatus. This may be due to the fact that high dietary VE levels can cause cell death by increasing cytotoxic activity (Cuesta et al., 2001), as might reduce lipid stability on the cell membrane, thus increasing PUFA β-oxidation and auto-oxidation. As a result, when PUFA oxidation products increase, they would inevitably promote PUFA synthesis, which may be reflected by the up-regulation of fas, Δ6 fad, elovl4 and elovl5 mRNA levels.
Fish contains high n-3 PUFA levels, especially in EPA and DHA, which are beneficial to human health (Gogus & Smith, 2010). However, PUFA is potentially susceptible to lipid peroxidation (Hamre, 2011), which results in the compromise of the nutritional values of fish. The present results found that suitable dietary VE level (61.16–91.06 mg/kg) could improve the contents of ALA, DHA and n-3 PUFA in raw muscle. This finding was in line with the results obtained by Lozano et al. (2017) and Xu et al. (2019), who reported optimum dietary VE supplements could improve PUFA content in muscle of meagre and cobia, respectively. In addition, the EPA and DHA contents of the cooked muscle in fish fed the diet with low VE levels were significantly lower than those of the raw muscle. However, no significant differences were observed between the raw and cooked muscle, when VE levels increased from 61.16 to 144.88 mg/kg. The results might be attributed to that VE could protect biological membranes containing PUFA against free radicals formation and effectively minimize lipid peroxidation, thus increasing PUFA content and stability in fish (Mourente et al., 2000; Tocher et al., 2003). In general, dietary supplementation of more than 47.35 mg/kg could avoid the reduction in muscle PUFA content and stability, thereby elevating the flesh quality of juvenile T. ovatus.
The results in this study indicated that dietary VE supplementation could improve growth performance in T. ovatus. Based on the second-order polynomial regression analysis of WG against VE levels, the optimal VE levels for juvenile T. ovatus were 90.75 mg/kg in diet. Moreover, increasing dietary VE (from 34.56 to 91.06 mg/kg) increased the antioxidant ability and muscle DHA and n-3 PUFA contents. Besides, supplementation of more than 47.35 mg/kg VE could protect muscle PUFA content from decreasing during cooking. In addition, the optimal dietary VE may inhibit PUFA β-oxidation by suppressing the expression of cpt1, l-fabp and pparα to increase the PUFA contents and restrain PUFA synthesis by down-regulating the expression of fas, Δ6 fad, elovl4 and elovl5 in liver of juvenile T. ovatus.
ACKNOWLEDGEMENTS
This work was financially supported by the China Agriculture Research System of MOF and MARA (CARS-47).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data to support the findings of this study are included in this paper.




