Fishmeal substitution by Iberian pig meal and vegetable proteins blend and inclusion of Isochrysis aff. galbana (T-Iso) in diets for gilthead seabream (Sparus aurata L.): Effects on growth and feed utilization efficiency
Funding information
The research was supported by a grant financed by Generalitat Valenciana, IDIFEDER/2020/029. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. It was additionally granted by Contrato Predoctoral para la Formación de Profesorado Universitario from Subprogramas de Formación y Movilidad within the Programa Estatal de Promoción del Talento y su Empleabilidad of the Ministerio de Educación, Cultura y Deporte of Spain.
Abstract
Research was carried out into the effect of partial and total fishmeal (FM) replacement by a vegetable and animal proteins blend, as well as the inclusion of microalgae in diets for gilthead seabream (Sparus aurata L.). Fish of 64 g on initial weight were fed until apparent satiation for 88 days. The control diet (FM100) contained FM as the main protein source, whilst in FM25, FM10 and FM0 diets, the FM was replaced 75%, 90% and 100%, respectively, by a proteins blend consisting of Iberian pig meal (IPM) and vegetable protein meals. FM0+ was similar to FM0 diet but included 50 g/kg of Isochrysis aff. galbana (T-Iso). Results obtained in the final bodyweight and the specific growth rate indicate that the FM25 and FM100 diets achieved similar performances. An improvement in growth performance and nutrient utilization was observed in the FM0+ diet with respect to the FM0 diet. The highest retention efficiencies of protein, energy and essential amino acids were found in FM100 and FM25 diets. In conclusion, up to 75% FM substitution by a vegetable and animal proteins blend in on-growing gilthead seabream is feasible, in addition, the inclusion of Isochrysis aff. galbana (T-Iso) improves the growth and retention efficiencies in a non-FM diet.
1 INTRODUCTION
Aquaculture of gilthead seabream (Sparus aurata L.) is carried out in 20 countries, and it is the principal species in production in the Mediterranean Sea. The aquaculture seabream harvest in Spain in 2019 was 13.521 t, and the total aquaculture production in Europe and the rest of the Mediterranean reached 252.406 t (Asociación Empresarial de Acuicultura de España [APROMAR], 2020), positioning it as a species of great economic importance for the aquaculture industry.
With the rapid intensification of aquaculture production in the world, the demand for aquafeeds and their main protein ingredient, fishmeal (FM), is increasing exponentially, given that this raw material still remain the principal sources of high-quality protein utilized in feed for carnivorous fish. This continuous increase in demand, together with the decrease in the supply of FM, has led the aquaculture sector to the need to find new alternatives for partial or total FM replacement in fish diets, which should be economic, environmentally friendly, safe, sustainable and palatable for fish species (Shafique et al., 2021). Consequently, the aquaculture industry and academia have been focussed on the search for alternative raw ingredients, in order to reduce the dependency on this ingredient, seeking to become as economically sustainable as possible. Currently, plant-based proteins together with processed animal proteins (PAPs) from non-ruminant animals (poultry and pigs) are used as ingredients in formulated fish feeds, to meet the fish's nutritional requirements for their good digestibility and palatability, lower carbon footprint and reduced levels of antinutritional factors (ANFs) than vegetable products, which improves fish health and welfare (Lanes et al., 2021).
Studies with high replacement of FM by mixture of plant-proteins or plant and animal proteins have produced good results in growth performance and feed utilization, but other important parameters such as survival (Estruch et al., 2018) and quality have been affected. In the case of survival, the impact is generally attributed to the presence of ANFs present in plant sources (Francis et al., 2001), hence the current study of new alternatives, try to minimize this effect by the use of a mix of animal and plant protein (PP), as well as food additives.
Studies carried out with animal protein sources in diets for cultured marine fish are scarce. Animal by-products are potential alternative ingredients for FM and are largely available, such as meat and bone meal (MBM), poultry by-product meal (PBM), feather meal and blood meal. A provisional solution to reduce production costs lies in the identification of low-price food items, easily affordable and with no interest for human markets. The quality of the proteins in the meals from animal by-products will vary according to the origin of the raw materials; meat protein would have better quality than other tissues such as tendon or skin; therefore, it is necessary to measure protein quality in animal by-products meals. In addition, animal by-product meal contains a reasonable amount of phosphorus, an important nutrient for aquatic animals (Tangendjaja, 2015).
The use of PAP in aquafeeds is widely varied depending on the region in which they are utilized. In the European Union (EU), its use was prohibited from 1990 to 2000 (Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001), due to the appearance of bovine spongiform encephalopathy in ruminants in the 1980s–1990s. Despite this, in 2013 this restriction was partially lifted authorizing the use of PAP derived from non-ruminant animals for feeding aquaculture animals (Commission Regulation (EU) No 56/2013 of 16 January 2013). This allowed access to a new range of ingredients that can be widely used in aquafeeds (Moutinho, Martínez-Llorens, et al., 2017). The quality of these terrestrial animal protein sources is highly dependent on the quality of the raw material, as well as the processing to which it has been exposed. Use of more suitable processing technologies, in particular drying techniques, has made it possible to produce more specific and selected products for the formulation of fish diets (Bureau et al., 1999, 2000). For example, co-extrusion and flash drying are currently used to produce superior quality meat and bone, and poultry by-products (Hernández et al., 2008). However, the technological process for the production of PAP was reviewed (EC No. 94/449; pressure, 3 bar by steam for 20 min; maximum particle size, 50 mm; temperature higher than 133℃), which could lead to compromising their nutritional quality. Accordingly, it is necessary to thoroughly assess these new ingredients (Moutinho, Martínez-Llorens, et al., 2017).
The Iberian pig is considered to be the most valuable Mediterranean breed of pig due to its considerable population size, as well as its economic importance (Álvarez et al., 2014; Juárez et al., 2009). Pig meat production in Spain amounted to more than 52.9 million slaughtered animals and about 4.64 million tonnes of meat produced in 2019, figures that keep Spain in the fourth position in the world. The Iberian pig census represented 10.8% (3.3 million animals in December 2019) of the total pig census in Spain (31.2 million animals in December 2019). Since 2015, production in Spain has grown by 20%, giving an idea of the huge growth that the pig sector is experiencing at the national level (Spanish Ministry of Agriculture [MAPA], 2020). According to the above, it could be estimated that the large volumes of slaughterhouse waste would allow a constant availability of Iberian pig by-products for the production of Iberian pig meal for aquafeeds, which would help reduce the production costs, as well as ensuring sustainability of the sector. The Iberian pig is an autochthonous variety from the Iberian Peninsula pig whose particularity is based on its high quality of fat and flavour (Lopez-Bote, 1998), as well as in its high rusticity (hereditary resistance to non-optimal conditions of the environment; Martinez-Macipe et al., 2016). Furthermore, the Iberian pig carcass is highly prized in the market, based on an outstanding balance of fatty acids in its lipid deposits—intramuscular and subcutaneous fat—especially, subcutaneous fat. Indeed, in the Iberian pig sector, a lower proportion of palmitic acids (C16:0), stearic (C18:0) and linoleic (C18:2 n-6) and a high proportion of oleic acid (C18:1 n-9) in the carcasses are utilized as quality indicators (De Pedro, 2001; Tejerina et al., 2012). Iberian pig by-product meal could position itself as an emerging ingredient for aquaculture feeds in Spain, due to its nutritional characteristics, availability and ease of use.
A wide variety of additives are used in aquaculture that have great beneficial effects on the host, such as fighting disease, improving growth and, in some cases, acting as alternative antimicrobial compounds (Irianto & Austin, 2002), as well as stimulating the immune response of the host. Moreover, the amount of research into the development of new strategies in food supplementation has increased, which can be evaluated in the introduction of various compounds that promote health and growth, such as probiotics, prebiotics, symbiotics, phytobiotics and other functional food supplements (Akhter et al., 2015; Denev, 2008).
Microalgae comprise an extensive group of photosynthetic heterotrophic organisms, many of which are rich in protein, lipids and bioactive compounds (Yarnold et al., 2019), which are classified according to certain characteristics, such as cell structure, pigments and substances (Cerezuela et al., 2012). Depending on the algal species and their growth conditions, they can contain up to 60% protein, 60% carbohydrates or 70% oils (Draaisma et al., 2013) and produce valuable pigments, growth-promoting substances and hormones, as well as secondary metabolites that provide natural antioxidant, antimicrobial, anti-inflammatory and immunostimulant benefits to aquatic animals (García-Chavarría & Lara-Flores, 2013; Michalak & Chojnacka, 2015). In addition, they have the ability to synthesize all amino acids (thus providing those which are essential to animals and humans); existence of carbohydrates in the form of starch, cellulose, sugars and other polysaccharides; lipids in the form of fatty acids of the n-3 and n-6 families and glycerol; and an important content of many essential vitamins (A, B1, B2, B6, B12, C, E, biotin, pantothenic acid and folic acid), minerals (iron, selenium, zinc, magnesium, calcium, phosphorus) and antioxidant substances (Borowitzka, 1997; Cerezuela et al., 2012; Duerr et al., 1998). Currently, microalgae may play important roles in feed (for cattle, poultry, shellfish and fish), food additives, FM and oil replacement, colouring of salmonids, inducers of biological activities, and enhancers of nutritional value of zooplankton fed to fish larvae and fry (Camacho et al., 2019; Dineshbabu et al., 2019; Guedes et al., 2015; Valente et al., 2021; Yarnold et al., 2019). All these particularities have led to further exploration of new functional ingredients from microalgae with the purpose of providing an additional health benefit in addition to the energy and nutritional aspects of food (Christaki et al., 2011; Plaza et al., 2009; Spolaore et al., 2006). The microalgae-derived materials are made up of bioactive compounds. Their bioactivity can be selected from one or more of immune-enhancement, growth promotion, disease resistance, antiviral and antibacterial action, improved gut function, probiotic colonization stimulation, as well as enhanced feed conversion, reproductive performance and weight control (Harel et al., 2007; Madeira et al., 2017; Yarnold et al., 2019). The reports of anti-inflammatory effects on rats due to I. galbana (Nuño et al., 2013) may correspond to the action of bioactive compounds in I. galbana, including eicosapentaenoic acid (EPA) and other than EPA (Bonfanti et al., 2018). These bioactive compounds may be protein, polyunsaturated fatty acids, carotenoids, vitamins and minerals (Camacho et al., 2019). The content of vitamin C (ascorbic acid), present in Isochrysis aff. galbana (T-Iso) as a bioactive compound, amounts to 885 mg per kg DW (Bandarra et al., 2003). The properties of this bioactive compound benefit gastrointestinal physiology and lipid metabolics (Nuño et al., 2013), hypocholesterolaemic potential (Dvir et al., 2009) and antioxidant action (Matos et al., 2017). Likewise, other studies confirm that I. galbana result highly digestible and its nutrients support the growth of gilthead seabream (Palmegiano et al., 2009), and its inclusion in the diets for European sea bass does not adversely affect feed intake and growth performance (Tibaldi et al., 2015).
For these reasons, the aim of this present work was to evaluate the effect of FM substitution by a vegetable and animal proteins blend, as well as the inclusion of the microalgae I. aff. galbana (T-Iso) on the growth performance, feed utilization efficiency and protein efficiency (protein and amino acids retention) of gilthead seabream (S. aurata).
2 MATERIALS AND METHODS
The experimental protocol implemented in this trial was reviewed and approved by the Committee of Ethics and Animal Welfare of the Universitat Politècnica de València (code: P4-04-05-2017). All experiments were carried out in an accredited animal care facility (code: ES462500001091) in accordance with the Spanish Animal Protection Regulations RD 53/2013, which complies with European Union Directive 2010/63 with regard to the protection of animals used for experimental and other scientific purposes.
2.1 Experimental diets
Four isonitrogenous (450 g/kg crude protein) and isolipidic (200 g/kg crude lipid) experimental diets were formulated with different levels of FM replacement and were named as FM25, FM10, FM0 and FM0+. In addition, a control diet (FM100), whose ingredients were FM (as the protein source), wheat, fish and soy oils and a complex of vitamins and minerals was used. In the FM25, FM10, FM0 and FM0+ diets, FM was replaced at a proportion of 75%, 90% and 100%, respectively, by an animal and vegetable proteins blend consisting in Iberian pig meal (IPM), pea, sunflower and soybean meal. Additionally, microalgae I. aff. galbana (T-Iso), provided by Marine Microalgae Biotechnology Research Group of the University of Almeria (Spain), was included at 50 g/kg in the FM0 + diet. To cover the essential amino acids (EAA) needs, methionine (Met) was added using the reference of amino acids (AA) requirements of S. aurata reported by Peres and Oliva-Teles (2009). Ingredients and chemical composition of the experimental diets are presented in Table 1.
| Experimental diets | |||||
|---|---|---|---|---|---|
| FM100 | FM25 | FM10 | FM0 | FM0+ | |
| Ingredients (g kg−1) | |||||
| Fishmeala | 590 | 150 | 60 | ||
| Wheat mealb | 259 | 56 | 14 | ||
| Soybean mealc | 171 | 206 | 220 | 206 | |
| Pea meald | 101 | 122 | 129 | 111 | |
| Sunflower meale | 101 | 122 | 129 | 111 | |
| Iberian pig mealf | 237 | 288 | 328 | 328 | |
| Microalgae I. aff. galbana (T-Iso)g | 50 | ||||
| Soybean oil | 96 | 56 | 50 | 41 | 41 |
| Fish oil | 45 | 85 | 90 | 100 | 100 |
| Mono calcium phosphate | 28 | 33 | 38 | 38 | |
| L-Methionineh | 5 | 5 | 5 | 5 | |
| Multivitamin and minerals mixi | 10 | 10 | 10 | 10 | 10 |
| Ratio FM:PP:IPM | 0.94:0.06:0 | 0.24:0.33:0.43 | 0.1:0.38:0.53 | 0:0.4:0.6 | 0:0.38:0.62 |
| Analyzed composition (g per kg dry weight) | |||||
| Dry matter (DM) | 908.6 | 916.6 | 905.0 | 908.8 | 902.6 |
| Crude protein (CP) | 472.0 | 465.1 | 471.4 | 470.4 | 459.8 |
| Crude lipid (CL) | 198.9 | 190.6 | 185.6 | 186.7 | 195.3 |
| Ash | 111.1 | 83.6 | 76.6 | 89.1 | 89.1 |
| Calculated values | |||||
| Crude fiber (CF, g kg−1)j | 8.0 | 33.0 | 38.1 | 39.9 | 34.9 |
| Energy (kJ g−1)k | 21.78 | 23.34 | 23.26 | 23.68 | 23.45 |
| NFE (g kg−1)l | 210.0 | 227.7 | 228.3 | 213.9 | 220.9 |
- a Fishmeal (g kg−1): (932 DM, 707 CP, 89 CL, 151 Ash); Vicens I Batllori S.L.
- b Wheat meal (g kg−1): (890 DM, 116 CP, 15 CL, 18 Ash); DESCO.
- c Soybean meal (g kg−1): (882 DM, 499 CP, 22 CL, 71 Ash); DESCO.
- d Pea meal (g kg−1): (866 DM, 216 CP, 10 CL, 39 Ash); DESCO.
- e Sunflower meal (g kg−1): (896 DM, 291 CP, 15 CL, 67 Ash); DESCO.
- f Iberian pig meal (g kg−1): (959 DM, 804 CP, 163 CL, 19 Ash); Slaughterhouse Guijuelo S.A. – Maguisa.
- g Microalgae I. aff. galbana (T-Iso; g kg−1): (889.8 DM, 350 CP, 10.9 CL, 29.7 Ash); Biotechnology research group of the University of Almeria, Spain.
- h L-Methionine: Guinama®.
- i Multivitamin and minerals mix (values are g kg−1): Premix: 25; Choline, 10; DL-α-tocopherol, 5; ascorbic acid, 5; (PO4)2Ca3, 5. Premix composition: retinol acetate, 1,000,000 IU kg−1; calciferol, 500 IU kg−1; DL-α-tocopherol, 10; menadione sodium bisulphite, 0.8; thiamine hydrochloride, 2.3; riboflavin, 2.3; pyridoxine hydrochloride, 15; cyanocobalamin, 25; nicotinamide, 15; pantothenic acid, 6; folic acid, 0.65; biotin, 0.07; ascorbic acid, 75; inositol, 15; betaine, 100; polypeptides, 12.
- j Crude Fibre, CF (g kg−1) was calculated by FEDNA tables (Fundación Española para el Desarrollo de la Nutrición Animal [FEDNA], 2010).
- k Energy (kJ g−1) = (51.8 × (%C/100)) − (19.4 × (%N/100)). Calculated according to Brouwer (1965).
- l NFE, Nitrogen-free extract (g kg−1) = 100 − CP − CL − CF − Ash.
Before formulating the diets, a chemical analysis of each of the ingredients was carried out, they were weighed individually and then mixed to homogenize the mixture. Subsequently, the diets were prepared using a cooking-extrusion process with a semi-industrial twin screw extruder (CLEXTRAL BC-45) at the UPV facilities. The processing conditions were as follows: a pressure of 4–5 Mpa, a temperature of 110℃ and a screw speed of 100 rpm. All feed ingredients and the experimental diets were analysed in triplicate.
2.2 Growth trial and fish sampling
Gilthead seabream (S. aurata) juveniles were provided by a local fish farm (Alevines del Mediterráneo, S. L. (Blaumar), Sagunto, Spain) and transported to the Fish Nutrition Laboratory of the UPV, Spain. Before starting the feeding test, all fish were acclimated to indoor rearing conditions for four weeks and fed a standard diet for seabream (480 g/kg crude protein, CP; 230 g/kg crude lipid, CL; 110 g/kg ash; 22 g/kg crude fibre, CF and 140 g/kg nitrogen free-extract, NFE). After the acclimation period, gilthead seabream juveniles (initial average weight: 64 ± 1.3 g, mean ± standard error of the mean) were redistributed in 15 cylindrical fibreglass tanks (three per treatment) in groups of 24 fish per tank. The capacity of each tank was 1750 L.
The duration of the experiment was 88 days. The experiment was carried out in a seawater recirculation system (65 m3 capacity) that had a rotary mechanical filter and a gravity biofilter (approximately 6 m3). The water temperature was kept at 21 ± 0.82℃, dissolved oxygen was 7.1 ± 0.73 mg L−1, salinity was 33 ± 2.15 g L−1, and pH fluctuated between 8.0 and 8.5 during the experiment. All tanks had aeration supply. The water temperature was kept constant with the help of a heat/cold specific pump installed in the system. The photoperiod was natural and all tanks maintained similar lighting conditions.
Fish were observed daily and were weighed at 28-day intervals to determine growth parameters. Before weighing, all fish were, fasted for 41 h and anesthetized with 30 mg L−1 of clove oil (Guinama®) that contain 87% of eugenol. Fish were fed by hand twice a day (09:00 and 16:00 hours) until apparent satiation from Monday to Saturday, with fasting on Sunday. Pellets were distributed slowly, allowing all fish to eat. Feed intake (FI) was recorded daily. The uneaten diet was collected and dried to determine FI.
At the end of the feeding trial, all the fish were individually weighted. Five fish from each tank, as well as five fish from the initial stock, were randomly slaughtered using a lethal bath of clove oil (150 mg L−1), for the determination of biometric parameters and whole-body proximate composition. The samples from each tank were pooled and stored at −30℃. Fish total weight and length, as well as viscera, visceral fat and liver weights were recorded for determination of condition factor (CF), viscerosomatic (VSI), visceral fat (VFI) and hepatosomatic (HSI) indexes.
The growth performance indicators and retention efficiencies of ingested protein (PIR), energy (EIR) and essential amino acids (AAIRE) were determined at the end of the experiment and the tank was used as an experimental unit. The specific growth rate (SGR), FI, feed conversion ratio (FCR) and protein efficiency ratio (PER) were obtained taking into account the monthly reported biomass of dead fish. The biometric parameters were obtained at the end of the growth trial, using five fish per tank, 15 per treatment.
2.3 Chemical analyses
Fish diets, feed ingredients and proximate composition of whole fish were analysed in accordance with the Association of Official Analytical Chemists (AOAC, 2002) procedures: dry matter, official method 934.01 (105℃ to constant weight); crude protein, official method 990.03 (analysed by direct combustion method DUMAS using LECO CN628); crude lipid, official method 920.39 (extracted with methyl-ether using ANKOMXT10 Extractor) and ash, official method 942.05 (incinerated at 550℃ for 5 h). All analyses were performed in triplicate.
2.4 Amino acids analyses
Based on the method described by Bosch et al. (2006), the AA contents of the ingredients, diets and fish carcasses, were determined using a Waters HPLC system (Waters 474, Waters) consisting of an auto sampler (Model 717, Waters), a fluorescence detector (Model 474, Waters), two pumps (Model 515, Waters) and a temperature control module. Aminobutyric acid was added as an internal standard prior to hydrolysation. Amino acids were derivatized with AQC (6-aminoquinolyl-N-hydroxysuccinimidyl carbamate). Cysteine and Met were determined separately as cysteic acid and methionine sulphone following oxidation with performic acid. Amino acids were separated with a C-18 reverse-phase column Waters Acc. Tag (150 mm × 3.9 mm) and then converted to Cys and Met. The AA composition of the diets and main protein sources used can be seen in Table 2.
| Ingredients | Experimental diets | ||||||
|---|---|---|---|---|---|---|---|
| FM | IPM | FM100 | FM25 | FM10 | FM0 | FM0+ | |
| EAA (g per kg dry weight) | |||||||
| Arginine | 56.0 | 51.1 | 30.4 | 30.4 | 29.1 | 27.8 | 29.0 |
| Histidine | 24.2 | 11.5 | 9.10 | 6.90 | 6.30 | 6.90 | 6.80 |
| Isoleucine | 32.5 | 24.1 | 18.6 | 15.8 | 15.4 | 15.0 | 15.4 |
| Leucine | 62.6 | 46.5 | 31.3 | 27.7 | 27.0 | 26.4 | 27.2 |
| Lysine | 57.4 | 43.0 | 28.1 | 20.7 | 21.2 | 20.8 | 19.2 |
| Methionine | 22.0 | 9.80 | 11.1 | 11.5 | 9.30 | 9.20 | 10.0 |
| Phenylalanine | 35.6 | 26.0 | 16.1 | 15.3 | 14.5 | 14.6 | 15.9 |
| Threonine | 33.9 | 17.6 | 16.1 | 12.8 | 12.4 | 11.5 | 12.7 |
| Valine | 37.0 | 38.2 | 21.8 | 19.8 | 19.7 | 19.7 | 19.6 |
| NEAA (g per kg dry weight) | |||||||
| Alanine | 41.3 | 64.4 | 22.9 | 24.3 | 25.9 | 25.4 | 24.8 |
| Aspartate | 66.5 | 65.5 | 32.7 | 36.9 | 39.0 | 38.7 | 35.9 |
| Cysteine | 5.3 | 2.3 | 5.1 | 4.1 | 3.2 | 3.6 | 3.7 |
| Glutamate | 95.5 | 119.2 | 56.8 | 60.5 | 64.8 | 62.2 | 59.1 |
| Glycine | 40.7 | 149.1 | 24.8 | 43.8 | 46.0 | 46.6 | 48.1 |
| Proline | 27.4 | 87.6 | 17.8 | 29.0 | 31.3 | 31.3 | 33.3 |
| Serine | 32.6 | 25.5 | 16.1 | 15.3 | 14.7 | 15.1 | 15.4 |
| Tyrosine | 25.5 | 16.8 | 11.7 | 9.40 | 9.60 | 8.70 | 9.40 |
| EAA | 361.2 | 267.7 | 182.6 | 160.9 | 154.9 | 151.9 | 155.9 |
| NEAA | 334.8 | 530.4 | 188.0 | 223.4 | 234.6 | 231.5 | 229.7 |
| EAA/NEAA | 1.08 | 0.50 | 0.97 | 0.72 | 0.66 | 0.66 | 0.68 |
- Abbreviations: EAA, essential amino acids; FM, fishmeal; IPM, Iberian pig meal; NEAA, non-essential amino acids.
2.5 Statistical analyses
Prior to analysis, all variables were checked for normal distribution with the Kolmogorov–Smirnov test and homogeneity of variances by the Levene test. Growth data, nutrient utilization, biometric parameters, body composition and amino acid composition and retention were treated using multifactor analysis of variance (ANOVA). Student–Newman–Keuls test was used to assess specific differences amongst diets. Data were considered statistically significant when p < .05 and the data are shown as the mean ± pooled standard error of the mean (SEM). Mean values for each tank were the units of observation for statistical evaluation (five fish per tank, three tanks per treatment). Statistical data analyses were performed using Statgraphics, Statistical Graphics System, Version Centurion XVI.
3 RESULTS
3.1 Fish growth and feed utilization efficiency
The results obtained on growth performance and feed utilization efficiency are shown in Table 3. At the end of the growth period, gilthead seabream fed with the non-fishmeal diet (FM0) presented by some margin the lowest final bodyweight, WG and SGR (137.4 g, 110.6% and 0.8% day−1, respectively), whereas fish fed the FM25 diet and fish fed the control diet (FM100) showed the highest growth (SGR, 1.2 and 1.4% day−1, respectively). Fish fed the FM0+ and FM10 diets attained a higher weight and SGR than those fed the FM0 diet. All diets were well accepted and no significant differences between groups for FI were detected. The FCR was higher (2.1) in fish fed the FM0 diet, and the FM0+ diet did not show a difference with the FM100, FM25 or FM10 diet (1.6 and 1.5, 1.5, 1.7, respectively). Significant differences were found in PER, being the lowest in fish fed the FM0 diet, whilst the FM0+ diet showed no difference with the FM100 and FM25 diets.
| Experimental diets | |||||
|---|---|---|---|---|---|
| FM100 | FM25 | FM10 | FM0 | FM0+ | |
| Initial weight (g) | 63.1 ± 1.84 | 64.1 ± 1.84 | 64.1 ± 1.84 | 65.4± 1.84 | 63.4 ± 1.84 |
| Final weight (g) | 214.7 ± 12.39a | 193.2 ± 12.39a | 162.9 ± 12.39ab | 137.4 ± 12.39b | 175.3 ± 12.39ab |
| WG (%)1 | 239.5 ± 14.54a | 200.1 ± 14.54ab | 153.7 ± 14.54bc | 110.6 ± 14.54c | 176.7 ± 14.54b |
| SGR (% day−1)2 | 1.4 ± 0.06a | 1.2 ± 0.06ab | 1.1 ± 0.06b | 0.8 ± 0.06c | 1.2 ± 0.06b |
| FI (g 100 g fish−1 day−1)3 | 1.8 ± 0.07 | 1.6 ± 0.07 | 1.7 ± 0.07 | 1.7 ± 0.07 | 1.7 ± 0.07 |
| FCR4 | 1.5 ± 0.12b | 1.5 ± 0.12b | 1.7 ± 0.12ab | 2.1 ± 0.12a | 1.6 ± 0.12b |
| PER5 | 1.6 ± 0.10a | 1.6 ± 0.10a | 1.4 ± 0.10ab | 1.1 ± 0.10b | 1.5 ± 0.10a |
| CF (g cm−3)6 | 2.1 ± 0.05a | 1.9 ± 0.05bc | 1.8 ± 0.05bc | 1.7 ± 0.05c | 1.9 ± 0.05b |
| VSI (%)7 | 7.7 ± 0.27 | 7.9 ± 0.27 | 8.0 ± 0.27 | 8.3 ± 0.27 | 8.8 ± 0.27 |
| HSI (%)8 | 1.3 ± 0.07 | 1.0 ± 0.07 | 1.0 ± 0.07 | 1.0 ± 0.07 | 1.0 ± 0.07 |
| VFI (%)9 | 1.8 ± 0.20 | 1.4 ± 0.20 | 1.4 ± 0.20 | 2.1 ± 0.20 | 1.9 ± 0.20 |
Note
- Data are presented as mean ± SEM (n = 3, growth performance and nutrient utilization; n = 15, biometric parameters). Different superscript letters indicate significant differences amongst treatments (p < .05).
- 1 Weight gain (WG, %) = 100 × (final weight − initial weight)/initial weight.
- 2 Specific growth rate (SGR, % day−1) = 100 × ln (final weight/initial weight)/days.
- 3 Feed intake (FI, g 100 g fish−1 day−1) = 100 × feed consumption (g)/average biomass (g) × days.
- 4 Feed conversion ratio (FCR) = feed offered (g)/weight gain (g).
- 5 Protein efficiency ratio (PER) = weight gain (g)/protein offered (g).
- 6 Condition factor (CF, g cm−3) = 100 × total weight (g)/total length3 (cm).
- 7 Viscerosomatic index (VSI, %) = 100 × visceral weight (g)/fish weight (g).
- 8 Hepatosomatic index (HIS, %) = 100 × liver weight (g)/fish weight (g).
- 9 Visceral fat index (VFI, %) = 100 × visceral fat weight (g)/fish weight (g).
3.2 Biometric indexes and body composition
Regarding biometric parameters (Table 3), statistical differences were detected in condition factor (CF), fish fed the FM0+ diet obtained a higher value (1.9 g cm−3) than fish fed the FM0 diet (1.7 g cm−3), similar to those fed the FM10 and FM25 diets, and however, lower than the FM100 (2.1 g cm−3). No differences were observed in the viscerosomatic index (VSI), hepatosomatic index (HSI), and visceral fat index (VFI).
The proximate composition of the whole body, expressed as g kg−1 of the wet weight, is shown in Table 4. Fish fed the FM100 and FM0 diets exhibited the lowest moisture content (664.8 and 674.1 g kg−1, respectively), and accordingly, the lipid content of those fish were the highest (127.1 and 130.2 g kg−1, respectively). No significant differences for whole-body protein and ash contents were found.
| Initial | Experimental diets | |||||
|---|---|---|---|---|---|---|
| FM100 | FM25 | FM10 | FM0 | FM0+ | ||
| Analysed composition (g per kg wet weight) | ||||||
| Moisture | 665.0 | 664.8 ± 0.32c | 687.7 ± 0.32a | 680.5 ± 0.32ab | 674.1 ± 0.32bc | 688.1 ± 0.32a |
| Crude protein (CP) | 169.0 | 175.0 ± 0.28 | 170.4 ± 0.28 | 171.4 ± 0.28 | 168.9 ± 0.28 | 167.9 ± 0.28 |
| Crude lipid (CL) | 123.8 | 127.1 ± 0.28a | 110.2 ± 0.28b | 117.7 ± 0.28b | 130.2 ± 0.28a | 112.9± 0.28b |
| Ash | 33.0 | 31.7 ± 0.14 | 30.4 ± 0.14 | 28.4 ± 0.14 | 27.9 ± 0.14 | 29.2 ± 0.14 |
Note
- Data are presented as mean ± SEM (n = 3). Different superscript letters indicate significant differences amongst treatments (p < .05).
3.3 Amino acids composition and retention efficiencies
No significant differences were observed in the whole-body AA content of the fish as shown in Table 5, except for the non-essential amino acid aspartate, which had the highest value in fish fed the FM100 diet (14.1 g kg−1) and the lowest value in fish fed the FM0 diet (12.2 g kg−1).
| Initial | Experimental diets | |||||
|---|---|---|---|---|---|---|
| FM100 | FM25 | FM10 | FM0 | FM0+ | ||
| EAA (g per kg wet weight) | ||||||
| Arginine | 14.5 | 12.9 ± 0.04 | 12.1 ± 0.15 | 11.7 ± 0.08 | 11.8 ± 0.06 | 11.7 ± 0.09 |
| Histidine | 3.30 | 2.80 ± 0.01 | 2.80 ± 0.07 | 3.30 ± 0.14 | 2.50 ± 0.04 | 2.60 ± 0.02 |
| Isoleucine | 4.90 | 6.80 ± 0.02 | 6.70± 0.09 | 6.50 ± 0.04 | 6.20 ± 0.04 | 6.20 ± 0.02 |
| Leucine | 12.9 | 11.7 ± 0.04 | 11.6 ± 0.12 | 11.5 ± 0.07 | 11.0 ± 0.04 | 11.1 ± 0.03 |
| Lysine | 12.7 | 10.8 ± 0.16 | 10.5 ± 0.05 | 10.4 ± 0.15 | 9.20 ± 0.02 | 9.90 ± 0.06 |
| Methionine | 4.30 | 3.90 ± 0.005 | 3.80 ± 0.02 | 3.60 ± 0.03 | 3.70 ± 0.03 | 3.70 ± 0.02 |
| Phenylalanine | 5.40 | 6.00 ± 0.07 | 5.90 ± 0.12 | 5.70 ± 0.04 | 5.80 ± 0.08 | 5.90 ± 0.10 |
| Threonine | 7.00 | 6.20 ± 0.01 | 6.30 ± 0.11 | 6.30 ± 0.03 | 5.90 ± 0.03 | 6.10 ± 0.03 |
| Valine | 7.10 | 8.10 ± 0.02 | 7.90 ± 0.09 | 7.70 ± 0.04 | 7.40 ± 0.03 | 7.30 ± 0.01 |
| NEAA (g per kg wet weight) | ||||||
| Alanine | 13.1 | 8.70 ± 0.07 | 8.80 ± 0.07 | 8.60 ± 0.05 | 8.40 ± 0.05 | 8.50 ± 0.01 |
| Aspartate | 18.4 | 14.1 ± 0.11a | 13.4 ± 0.13ab | 13.5 ± 0.12ab | 12.2 ± 0.04b | 12.7 ± 0.09ab |
| Cysteine | 1.40 | 1.40 ± 0.01 | 1.30 ± 0.02 | 1.10 ± 0.01 | 1.30 ± 0.02 | 1.30 ± 0.01 |
| Glutamate | 27.2 | 20.4 ± 0.14 | 20.2 ± 0.17 | 20.4 ± 0.13 | 18.9 ± 0.04 | 19.7 ± 0.13 |
| Glycine | 15.5 | 11.0 ± 0.04 | 11.6 ± 0.09 | 10.7 ± 0.04 | 11.5 ± 0.01 | 11.3 ± 0.20 |
| Proline | 8.90 | 6.20 ± 0.04 | 6.20 ± 0.02 | 5.80 ± 0.01 | 6.30 ± 0.02 | 6.00 ± 0.05 |
| Serine | 8.10 | 6.10 ± 0.02 | 6.70 ± 0.12 | 6.00 ± 0.01 | 6.00 ± 0.04 | 5.90 ± 0.03 |
| Tyrosine | 5.10 | 5.30 ± 0.08 | 4.80 ± 0.09 | 4.90 ± 0.005 | 4.70 ± 0.05 | 4.80 ± 0.06 |
| EAA/NEAA | 0.74 | 0.95 ± 0.08 | 0.93 ± 0.005 | 0.94 ± 0.06 | 0.92 ± 0.05 | 0.92 ± 0.008 |
Note
- Data are presented as mean ± SEM (n = 3). Different superscripts letters indicate significant differences amongst treatments (p < .05).
- Abbreviations: EAA, essential amino acids; NEAA, non-essential amino acids.
The retention efficiency of protein (PIR) and energy intake (EIR) were lowest (19.0 and 48.9%, respectively) in fish fed the FM0 diet (Table 6); whereas fish fed the FM100 diet presented the highest efficiencies (28.7 and 76.3%, respectively). In fish fed the FM10 and FM0+ diets, the PIR values did not show differences (23.8% and 25.8%, respectively) as was the case with the EIR values (59.4% and 61.5%, respectively).
| Experimental diets | |||||
|---|---|---|---|---|---|
| FM100 | FM25 | FM10 | FM0 | FM0+ | |
| PIR (%)1 | 28.7 ± 2.00a | 28.0 ± 2.00a | 23.8 ± 2.00ab | 19.0 ± 2.00b | 25.8 ± 2.00ab |
| EIR (%)2 | 76.3 ± 5.34a | 68.1 ± 5.34ab | 59.4 ± 5.34ab | 48.9 ± 5.34b | 61.5 ± 5.34ab |
| AAIRE (%)3 | |||||
| Arginine | 30.8 ± 2.26a | 26.8 ± 2.26ab | 22.3 ± 2.26ab | 18.0 ± 2.26b | 24.8 ± 2.26ab |
| Histidine | 21.8 ± 8.71 | 26.7 ± 8.71 | 35.2 ± 8.71 | 14.1 ± 8.71 | 22.9 ± 8.71 |
| Isoleucine | 31.4 ± 2.27 | 36.0 ± 2.27 | 32.1 ± 2.27 | 26.3 ± 2.27 | 32.3 ± 2.27 |
| Leucine | 27.3 ± 2.23ab | 29.6 ± 2.23a | 25.8 ± 2.23ab | 18.8 ± 2.23b | 26.1 ± 2.23ab |
| Lysine | 25.1 ± 3.88ab | 31.2 ± 3.88a | 23.1 ± 3.88ab | 10.8 ± 3.88b | 26.6 ± 3.88ab |
| Methionine | 26.1 ± 2.52 | 23.2 ± 2.52 | 22.6 ± 2.52 | 18.6 ± 2.52 | 23.9 ± 2.52 |
| Phenylalanine | 29.7 ± 3.24 | 30.3 ± 3.24 | 26.9 ± 3.24 | 22.6 ± 3.24 | 27.3 ± 3.24 |
| Threonine | 28.0 ± 2.74 | 35.1 ± 2.74 | 30.8 ± 2.74 | 23.2 ± 2.74 | 31.3 ± 2.74 |
| Valine | 30.1 ± 1.85a | 31.5 ± 1.85a | 26.7 ± 1.85ab | 21.1 ± 1.85b | 27.2 ± 1.85ab |
Note
- Data are presented as mean ± SEM (n = 3). Different superscript letters indicate significant differences amongst treatments (p < .05).
- 1 Retention efficiency of protein intake (PIR, %) = 100 × protein fish gain (g)/protein intake (g).
- 2 Retention efficiency of energy intake (EIR, %) = 100 × energy fish gain (g)/energy intake (g).
- 3 Retention efficiency of amino acid (AAIRE, %) = 100 × AA fish gain (g)/AA ingested (g).
There were significant differences in EAA retention efficiency for arginine (Arg), leucine (Leu), lysine (Lys) and valine (Val) in gilthead seabream fed the different experimental diets (Table 5). Fish fed the FM25 diet showed the highest retention for Leu, Lys and Val, and fish fed the FM100 diet showed the highest retention for Arg and Val. The retention efficiency of Lys and Leu did not show differences in the FM100, FM10 and FM0+ diets, as was the case with Arg in the FM25, FM10 and FM0+ diets, and Val in the FM10 and FM0+ diets. Fish fed the FM0 diet presented the lowest values of EAA retention efficiency.
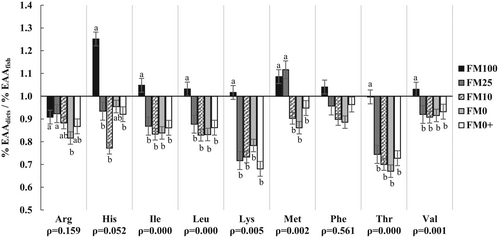
Significant differences were observed in the ratio between the ingested EEA of the experimental diets and the EAA of whole fish, except for EAA phenylalanine (Phe) where no significant differences were observed (Figure 1). Fish fed the FM100 diet showed the highest values in almost all EAA. Arg and Met showed similar values in fish fed the FM100 and FM25 diets. Values of isoleucine (Ile), Leu, Lys, threonine (Thr) and Val followed the same trend for fish fed the FM25, FM10, FM0 and FM0+ diets. Except for Lys in the group fed the FM0+ diet and Thr in the group fed the FM0 diet, the ratio % EAAdiet/% EAAfish were all higher than 0.7.
4 DISCUSSION
One of the main purposes of replacing FM is to improve sustainability of carnivorous fish production, as well as provide more economical alternatives compared with the high cost of FM. The results of the present study indicate that up to 75% of FM (FM25) can be replaced by an animal and vegetable proteins blend in gilthead seabream (S. aurata) diets without compromising feed utilization and growth performance. In a similar way to these results, other studies corroborate the feasibility of high FM substitutions. Dietary FM can be replaced at least up to 83% with PBM, along with a constant mixture of both animal and plant protein sources, in diets for gilthead seabream juveniles without affecting growth performance and feed utilization (Fontinha et al., 2021). The FE showed considerable enhancement and SGR was unchanged or moderately reduced in gilthead seabream fed a with 75% FM replacement diet by PP sources (Sitjà-Bobadilla et al., 2005). Both the FM diet and the diet with a 75% mixture of PP sources in seabream, had similar weight gain and SGR, whilst FE and PER were significantly higher in the PP diet (De Francesco et al., 2007). No statistical differences were found in the final weight and the nutritional efficiency indexes in seabream, between the FM diet and the diet with a 75% FM substitution with a mixture of plant meals (Estruch et al., 2018).
The diet with total FM replacement and no addition of I. aff. galbana T-Iso (FM0) showed the lowest values on growth performance. Total FM replacement is widely shown to cause low growth rates (Lunger et al., 2007), poor feed efficiency (Gómez-Requeni et al., 2004), even immunosuppression (Sitjà-Bobadilla et al., 2005), and mortality (Estruch et al., 2015). Even when amino acid is balanced with the blend of sources, not good results are achieved usually especially in aquafeeds designed for high-level carnivores. This effect is mainly explained cause a ‘small inclusion of FM in the former diet (8% of dietary protein) must have provided some essential nutrients that aided in keeping the fish alive’ (Lunger et al., 2007). Contrary to the above, the fishmeal-free diet and addition of the microalgae I. aff. galbana T-Iso (FM0+) presented a notable improvement in growth performance in relation to the results obtained the FM0 diet. This clearly indicates that the inclusion of I. aff. galbana (T-Iso) has a positive effect at the same level of substitution, perhaps due it, marine provenance may provide something that is needed by fish, provided usually by FM. This positive effect of the microalgae addition in the FM0+ diet is a relevant finding. The use of microalgae as an additive in aquaculture has received a lot of attention due to the positive effect on weight gain, increased triglyceride and protein deposition in muscle, improved resistance to disease, improved taste and consistency of flesh, decreased nitrogen output into the environment, increased omega-3 fatty acid content, physiological activity, starvation tolerance, carcass quality and increase in the rate of growth of aquatic species due to better digestibility (Becker, 2004; Fleurence et al., 2012). As is known, microalgae contain compounds such as carbohydrates, proteins (from 300 to 550 g per kg DM; González López et al., 2010), minerals, oil, fats, polyunsaturated fatty acids (40% PUFAs, (Batista et al., 2013)), as well as bioactive compounds such as antioxidants (polyphenols, tocopherols [vitamin E], vitamin C, mycosporine-like amino acids) and pigments, such as carotenoids (carotene xanthophyll), chlorophylls and phycobilins (phycocyanin, phycoerythrin), which possess antibacterial, antiviral, antifungal, antioxidative, anti-inflammatory and antitumor properties (Michalak & Chojnacka, 2015). According to the above, the improvement in fish growth observed in the FM0+ diet could be related to these properties. Our results did not show significant differences with respect to FI, indicating that palatability was not affected by the inclusion of a vegetable and animal proteins blend, and may evidence an attempt by fish to adjust the digestible energy intake. In fact, it is assumed that, up to a certain level, animals can adjust FI to meet their digestible energy needs (Boujard & Médale, 1994; Cho & Kaushik, 1985; Peres & Oliva-Teles, 1999; Yamamoto et al., 2000). On some occasions, animal protein sources can give fish palatability problems, as is the case of Laporte (2007) who evidenced that palatability of poultry meat meal, could be one of the principal factors that restricts the inclusion of this product in the diet of gilthead seabream. In contrast, in this study, the inclusion of IPM did not appear to affect negatively on the diets’ palatability, similar to previous findings (Moutinho, Peres, et al., 2017) with the inclusion of MBM. Animal by-products have a positive effect on animal performance because they contain short peptides and certain AA (taurine, glycine, Arg, glutamic acid and alanine) that are stimulants for feeding and enhancers of palatability and increase acceptance of artificial diets (Martínez-Alvarez et al., 2015).
Significant differences were found in the CF between the control diet (FM100) and the FM0 diet, but no significant differences were found for the other biometric indexes (VSI, HIS and VFI). Similar results were obtained by Sánchez-Lozano et al. (2009), where even without having significant differences, there has been a slight increase in visceral fat in seabream fed the diets that contained a greater substitution of FM. However, Kaushik et al. (2004) found a considerable increment in the amount of fat with increasing levels of FM substitution in diets for European seabass, Dicentrarchus labrax. Accordingly, this resulted in a similar increase in content energy of the whole body. Kaushik et al. (2004) pointed out that the high fat and energy retention values in fish fed diets with PP sources, clearly suggest that there was increased lipogenesis with increasing levels of FM replacement, without any effect on nitrogen utilization. In higher vertebrates, it is known that the level and source of dietary protein, such as soybean proteins, can affect lipid deposition, influence the pattern and potential of fatty acid bioconversion, and alter the serum and liver lipids (Aoyama et al., 2000; Dias et al., 2005; Lindholm & Eklúnd, 1991; Potter, 1995; Terasawa et al., 1994).
When the FM is substituted for alternative raw materials many factors can influence the growth and FE results. Ingredients derived from agricultural products can contain ANFs that may affect animal performance. On the contrary, animal-derived meals are exempt from these ANFs, which make their use possible because they do not pose any problem, especially in carnivorous fish. Nevertheless, the AA profile of the muscle or the efficiency in PIR and EIR can be affected by substitution, as a result of a lower efficiency in apparent retention of EAA. The results show that most of the EAA apparent retention values are affected by the substitution. Essential amino acid deficiency is one of the most important issues regarding FM substitution with alternative ingredients (Kaushik & Seiliez, 2010) and unbalanced EAA levels in the diets have been reported as one of the main causes for growth depression in fish fed animal by-products based diets (García-Gallego et al., 1998; Millamena, 2002; Moutinho, Martínez-Llorens, et al., 2017; Xavier et al., 2014). FM replacement affects not only the relative abundance of EAA but also NEAA, and an insufficiency in NEAA results in a reduced growth rate in fish (Schuhmacher et al., 1995). It follows that there must be an optimum dietary ratio of essential to non-essential amino acids (EAA:NEAA ratio), which will achieve maximum protein utilization for growth. Few studies have been carried out to determine the potential of some of the NEAAs and the ratios between essential and non-essential amino acids in the diet (EAA/NEAA ratio; Hughes, 1985; Mambrini & Kaushik, 1994). Gómez-Requeni et al. (2003) found that the best growth performance in seabream occurs with a diet that resembles the EAA profile and EAA/NEAA muscle ratio, when FM has been 35% replaced by plant ingredients. In this study, the FM100 diet had an EAA/NEAA ratio of 0.97 and the fish a mean value of 0.93. In the FM0 diet the EAA/NEAA ratio decreases to 0.66 and also fish fed with this diet showed the lowest values for retention efficiency of ingested EAA, which is related to its low final body weight gain. Other authors corroborated that gilthead seabream fed with an EAA/NEAA ratio of 1:1 have better zootechnical results than with a dietary ratio of 0.8 (Gómez-Requeni et al., 2003; Kaushik & Seiliez, 2010).
Significant differences were detected in protein ingested, energy and EAA retention efficiencies in fish fed with the assessed diets. The diets with higher percentages of retention efficiency were FM100 and FM25, whose values are similar to other research (Moutinho, Peres, et al., 2017). This shows that a FM replacement up to 75% can be achieved according to the growth and retention results. In the present study, the results obtained in the retention efficiency for Met and Arg in the FM100 diet, are quite similar (close to 30%) to those presented by Martínez-Llorens et al. (2012). The FM0 diet presented the lowest retention efficiency for all AA, which is in accordance with the growth results obtained with this diet. This detriment in retention efficiencies may be due to lower nutrient availability due to higher fibre content in FM10 and FM0 diets reducing digestibility in diets with less FM, which has already been proven in several species, including gilthead seabream (Lupatsch et al., 1997).
The EAAdiet/EAAfish ratio of gilthead seabream fed the FM25, FM10, FM0+ and FM0 diets presented the lowest values for all the essential amino acid. In general terms, the EAAdiet/EAAfish values are similar to those obtained by Moutinho, Martínez-Llorens, et al. (2017) with the replacement of FM for MBM in diets for seabream. In both studies, the values are lower than those of Sánchez-Lozano et al. (2011) and Martínez-Llorens et al. (2012), because the ratio has been calculated with digestible EAA. If the ratio between EAAdiet/EAAfish is lower than 1.0 for any AA, this may signify that the EAA is ‘deficient’ in the diet, and, in contrast, if the ratio is higher than 1.0, it may signify that this AA is ‘in excess’ (Sánchez-Lozano et al., 2011). In present work, the differences between EAAdiet/EAAfish ratio only justified the worst FM0 diet growth, this fact must be explained by amino acid efficiency, that in general was lower with this diet, possibility due to by a poor amino acid availability in fish fed with the FM0 diet, probably caused by the inflammatory effects of vegetable diets in gut fish. Isochrysis may have anti-inflammatory properties because the improvement showed in growth and nutritional parameters of FM0+ diet.
5 CONCLUSIONS
Findings from this study revealed that the up to the 75% of FM replacement is possible with a vegetable and animal (IPM) proteins blend without affect to the growth performance, feed utilization efficiency and protein metabolism of gilthead seabream (Sparus aurata L.). In addition, the inclusion of the microalgae I. aff. galbana (T-Iso) as additive in non-fishmeal diet (FM0+) improve the growth performance and protein efficiency of seabream.
ACKNOWLEDGMENTS
The authors acknowledge Marine Microalgae Biotechnology Research Group of the University of Almeria (Spain) for supplying the microalgae Isochrysis aff. galbana (T-Iso), as part of the research supported by the SABANA project of the European Union’s Horizon 2020 Research and Innovation Programme (grant # 727874).
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article




