An evaluation on the selenium yeast supplementation in the practical diets of early juvenile sea cucumber (Apostichopus japonicus): Growth performance, digestive enzyme activities, immune and antioxidant capacity, and body composition
Yanchang Ning and Xiangying Wu contributed equally to this work.
Abstract
A 45-day feeding trial was carried out to investigate the effects of dietary selenium yeast (Se-yeast) on the survival, growth performance, activities of digestive enzymes and antioxidant enzymes, and body composition of early juvenile Apostichopus japonicus. Five isoprotic (15.6%) and isolipic (1.5%) feeds with graded levels of Se-yeast (0, 0.5, 1.0, 1.5 and 2.0 mg/kg) were formulated and randomly allocated to the early juvenile sea cucumbers (initial weight: 0.11 ± 0.01 g). The results showed that the weight gain rate (WGR) and relative visceral weight ratio (RVW) significantly increased as the Se-yeast supplementation increased from 0 to 1.0 mg/kg, and then reached a plateau with further increase in Se-yeast, while survival rate (SR) increased as the supplementation level of Se-yeast increased, and the lipase and amylase activities first significantly increased as the Se-yeast increased from 0 to 1.0 mg/kg, and then significantly decreased with the continuous supplementation of Se-yeast. The antioxidant capacity and nonspecific immunity were significantly elevated by the moderate level (0.5 and 1.0 mg/kg) of Se-yeast. Besides, the activities of immune-related enzymes and transcription of antioxidation-related genes were significantly elevated by the supplementation of Se-yeast. However, malonaldehyde contents were significantly reduced in the treatments with 0.5 and 1.0 mg/kg Se-yeast. The selenium content in the body wall of the sea cucumber showed a markedly increasing trend with increasing Se-yeast supplementation levels. Results above indicated that a moderate level (0.5–1.0 mg/kg) of Se-yeast enhanced the growth performance, digestive enzyme activities, antioxidant capacity and nonspecific immunity of early juvenile A. japonicus.
1 INTRODUCTION
Sea cucumber (Apostichopus japonicus) is a marine animal species naturally distributed in the coast of North China (Li et al., 2012). Due to the nutritional, medicinal and healthcare values, A. japonicus has been accepted as a delicacy by the Chinese for thousands of years (Halder & Pahari, 2020; Shikov et al., 2020). In the last decades, market demand for sea cucumbers has increased rapidly especially in some Asian countries. Increasing demand by consumers has thereafter led to overfishing and depletion of the natural stock of sea cucumbers including A. japonicus (Kazanidis et al., 2010; Purcell et al., 2013). As the wild populations decline, aquaculture of A. japonicus has increasingly expanded in the provinces of Liaoning, Shandong, Hebei and Fujian of China since the 1990s (Robinson & Lovatelli, 2015). Now, it has become an important economical culture species in China with an annual yield amounting to 180 thousand tons (Han et al., 2016). The early juvenile sea cucumbers were fragile and susceptible to complicated aquatic environments (Xi et al., 2016). Thus, it is imperative to improve the nursery technologies to improve their survival and nonspecific immunity especially during the summer season. Immune stimulants have been verified to be an effective and efficient strategy to promote immunity and disease resistance in a variety of aquatic animal species (Hana et al., 2021; Vidya & Vijay, 2021; Zhang et al., 2014). However, little information is available about the effects of selenium (Se) supplementation on nonspecific immune responses of early juvenile sea cucumbers.
Se is the active centre of glutathione peroxidase (GPx), which is principally in charge of protection against oxidative damage (Godin et al., 2015). The catalase (CAT) could scavenge free radicals to avoid hyperoxidation of membrane phospholipids, exerting an important role in the inhibition of ROS formation (Zuo et al., 2017), and the major role of heat shock protein (HSP) is to manage protein folding and extensively participate in important processes such as cell cycle and signal transduction (Pratt, 2004). Under stress conditions, such as heat, anoxia, pathogen infection and oxidation, Hsp70 and Hsp90 are among the most abundant cellular proteins that help protect proteins from denaturation and stress-induced damage (Meng et al., 2014; Qian et al., 2012).
As an essential trace element, Se participates in several major metabolic pathways and physiological functions of almost all organisms (Wang et al., 2018). In aquatic animals, the importance of Se nutrition has received due to attention and has been comprehensively discussed by many researchers (Khan et al., 2017; Kumar et al., 2018). Generally, proper Se supplementation (0.4–2.0 mg/kg) in the diets increased the growth performance and antioxidant capacities of most aquatic animals, such as juvenile meagre (Argyrosomus regius) (Mansour et al., 2017), grouper (Epinephelus malabaricus) (Lin & Shiau, 2003) and marron (Cherax cainii) (Nugroho & Fotedar, 2013). Insufficient Se intake may cause a high death rate, slow growth rate and reduce feed efficiency of juvenile grouper (Lin & Shiau, 2005). On the opposite, excessive Se intake will impair health and decrease cognitive function in several fish species, including loach (Paramisgurnus dabryanus) (Hao et al., 2014), white sturgeon (Acipenser transmontanus) (Zee et al., 2016), meagre (Argyrosomus regius) (Khalil et al., 2018) and Wuchang bream (Megalobrama amblycephala) (Guo et al., 2018).
Inorganic and organic Se are the main forms of additives for aquatic animals. Compared with the inorganic Se, organic Se is more and more widely used in animal husbandry due to its safety and low toxicity (Payne & Southern, 2005). Among the organic Se, selenium methionine (Se-met), nano selenium (nano-Se) and selenium yeast (Se-yeast) have been previously reported for utilization in a variety of aquatic animals (Rider et al., 2009; Wang & Lovell, 1997; Wang et al., 2011). It was found that Se-met (0.4–0.6 mg/kg) in the diets could have beneficial effects on the growth performance and immune response of A. japonicus (Lu et al., 2015; Wang, Wang, et al., 2012; Zhou et al., 2015). Hu et al. (2019) found that selenium methionine (Se-met) showed better effects than bio-fermenting Selenium (Se-bio) when considering its accumulation efficiency and immune promoting function in A. japonicus. However, Se-yeast is a green feed additive which the safety record was excellent. Se-yeast can provide nature form of Se and is a recognized source of organic Se food (Schrauzer, 2006). It has been widely used as a nutritive antioxidant in the feeds of terrestrial livestock (He et al., 2013). Furthermore, the beneficial effects of Se-yeast supplementation on growth performance have been reported on several aquatic animals including hybrid striped bass (Morone saxatili) (Cotter et al., 2008) and rainbow trout (Oncorhynchus mykiss) (Robinson & Lovatelli, 2015). However, little information was available about the effects of Se-yeast supplementation in early juvenile A. japonicus. Thus, this study was performed to evaluate the effects of Se-yeast supplementation on the growth, immunity, digestive enzyme activities, antioxidant capacity and related gene expression in early juvenile A. japonicus. It was expected to quantify the optimal supplementation level of Se-yeast in formulated feeds and elucidating underlying mechanisms about the positive effects on the early juvenile A. japonicus.
2 MATERIALS AND METHODS
2.1 Ethics statement
All experimental procedures on the experimental animals in this study strictly followed the relevant national guidelines of China and Dalian Ocean University.
2.2 Experimental diets and feeding experiment
Five iso-proteinic (15.6%) and isolipidic (1.5%) experimental diets were formulated by including graded levels (0, 0.5, 1.0, 1.5 and 2.0 mg/kg) of Se-yeast. The Se-yeast was provided by FUBON (Angel Yeast Co., Ltd.) with an effective content of 2000 mg/kg. The feed ingredients and proximate composition were shown in Table 1. The solid ingredients in the diets were accurately weighed and mixed evenly. Finally, the feed pellets were produced by an automatic pellet-making machine (Jinan Dingrun Machinery Company). After drying (60℃ for 48 h), the pellets were broken up and sieved into convenient pellet sizes (100 μm) and stored at −20℃.
| Ingredient | Selenium yeast supplementation level (mg/kg) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Sea mud | 410 | 410 | 410 | 410 | 410 |
| Sargassum fusiforme meala | 340 | 340 | 340 | 340 | 340 |
| Sargassum thunbergii mealb | 120 | 120 | 120 | 120 | 120 |
| Mussel mealc | 80 | 80 | 80 | 80 | 80 |
| Premixd | 30 | 30 | 30 | 30 | 30 |
| Selenium-free yeast | 20 | 19.75 | 19.5 | 18.7 | 19 |
| Selenium yeast | - | 0.25 | 0.5 | 1.3 | 1 |
| Proximate composition | |||||
| Crude protein (%) | 15.60 | 15.60 | 15.61 | 15.60 | 15.63 |
| Crude lipid (%) | 1.52 | 1.53 | 1.52 | 1.51 | 1.54 |
- a Sargassum fusiforme meal: crude protein 11% dry matter, Dalian Xinyulong Marine Biological seed Technology Co., Ltd. (Dalian, Liaoning Province, China).
- b Sargassum thunbergii meal: crude protein 16.8% dry matter, Dalian Xinyulong Marine Biological seed Technology Co., Ltd. (Dalian, Liaoning Province, China).
- c Mussel powder: crude protein 57.5% dry matter, crude lipid 7.2% dry matter, Dalian Xinyulong Marine Biological seed Technology Co., Ltd. (Dalian, Liaoning Province, China).
- d Premix: vitamin premix and mineral premix, provided by Dalian Xinyulong Marine Biological seed Technology Co., Ltd. (Dalian, Liaoning Province, China).
The feeding experiment was carried out in the nursery room of Dalian Xinyulong Marine Biological Seed Technology Co., Ltd. Healthy early juvenile sea cucumbers (initial weight: 0.11 ± 0.01g) were purchased from a local farm (Xinyulong Marine Biological Seed Technology Co., Ltd.). After 2 weeks of acclimation, 570 g of healthy sea cucumbers of a similar size were selected and randomly distributed into 15 tanks (500 L), with each tank stocked with 38g (300 ± 30 individuals). Each experimental diet was randomly allocated to three replicates of tanks. The experimental animals were fed up to 3% of their body weight at 7:00 and 16:00 every day. During the experiment, the water temperature was 19℃ to 21℃, salinity was 30 ± 1 ‰, pH was 8.0 ± 0.1, dissolved oxygen was above 8.0 mg L−1, ammonia concentrations were below 0.2 mg L−1, and nitrite concentrations were below 0.1 mg L−1. About 30% of seawater was exchanged daily, and the experiment period was lasting for 45 days.
2.3 Sample collection
When the feeding experiment ended, sea cucumbers were deprived of food for 48 h. The body weight and the number of sea cucumbers in each diet were monitored to calculate the weight gain rate (WGR) and survival rate (SR). Then, 15 sea cucumbers were randomly sampled for the detection of body length (BL), body width (BW), digestive tract length (DL), digestive tract diameter (Dd) and digestive tract weight (DW). After that, the coelomic fluid was collected from 15 sea cucumbers individuals in each tank, dissected for supernatant after centrifugation (3,500 rpm/min, 4℃, 10 min) and stored at −80℃ for subsequent analysis of activities of immune-related enzymes, including superoxide dismutase, malonaldehyde, glutathione peroxidase, catalase, alkaline phosphatase and total nitric oxide synthase. At last, the body wall and digestive tract were separately sampled, placed in a 1.5 ml tube (RNAase-Free; Axygen) and store at −80℃ for proximate analysis.
2.4 Determination of digestive enzyme activities and antioxidant capacity
Commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to analyse the activities of digestive enzymes in the digestive tract and antioxidant capacity and nonspecific immunological parameters in the coelomic fluid of sea cucumbers. The digestive tract homogenate was prepared with 0.9% NaCl in an ice-water bath and then centrifuged at 2500 rpm at 4℃ for 10 min. Thereafter, the supernatant was dissected for the determination of protease, amylase, cellulase, lipase and lysozyme. A unit of pepsin activity was defined as the pepsin in one-milligram protein to produce one microgram of tyrosine in 1 min at 25℃. A unit of amylase activity was defined as the amount of enzyme in one-milligram protein to hydrolyse ten-milligram starch in 30 min. A unit of lipase activity was defined as the amount of enzyme in one gram protein to hydrolyse one micromole triglyceride at 25℃. A unit of cellulase activity was defined as the cellulase in one microgram protein to produce one microgram reducing sugar from the substrate cellulose in 1 min (550 nm). Lysozyme activity unit was defined as the amount of sample causing a decrease in absorbance of 0.001 in 1 min (530 nm).
A unit of superoxide dismutase (SOD) was defined as the amount of enzyme in one-milligram protein necessary to produce 50% inhibition of the ferricytochrome C reduction rate measured at 25℃ (550 nm). Malonaldehyde (MDA) was defined as the MDA amount in one-milligram protein to react with thiobarbituric in 1min at 25℃. Glutathione peroxidase (GSH-Px) unit was defined as the GSH-Px amount in one-milligram protein to reduce GSH concentration by one micromole per litre reaction solution in 1 min at 25℃. Catalase (CAT) unit was defined as the amount of enzyme in one-milligram protein to hydrolyse one micro mole of H2O2 in one second at 25℃. Alkaline phosphatase (AKP) unit was defined as one-milligram phenol generated in 15 min catalysed by enzymes in one gram tissue proteins at 25℃, and the amount of enzyme to produce nanomole NO in one millilitre coelomic fluid in 1 min was considered as a unit of total nitric oxide synthase (T-NOS).
2.5 Nutrients and selenium determination in tissues
Proximate analysis of the feeds and sea cucumber was assayed according to AOAC (1995). Crude lipid and crude protein were determined by using the Soxhlet method and the Kjeldahl method. Carbohydrates were measured by the phenol-sulfuric acid colorimetry method (470 nm). At 105℃, the samples were dried to constant weight to determine the moisture content. For the determination of Se content, about 1.5 g dry sample was accurately weighed and added with 10 ml of the mixed acid (perchloric acid: nitric acid, 1:9) for cold digestion overnight. Then, 2 ml of nitric acid and 5 ml HCl were successively added for heat digestion until the appearance of white smoke. After cooled, the solution was transferred to a bottle tube with 10 ml volume and mixed with 2.5 ml K3Fe(CN)6 (100 g/L). Finally, the Se concentrations in the sample were tested by using the method of hydride atomic fluorescence spectrometry (Zeng et al., 2020).
2.6 Real-time PCR (RT-PCR) of related genes
The expression pattern of immune-related genes in the digestive tract was detected with RT-PCR using the specific primers. The sequences of the specific primers were present in Table 2. The detailed procedures are described in the study by Zuo et al. (2017). Briefly, total RNA was extracted and examined for concentration and integrity. Then, Total RNA was reverse transcribed using a Commercial kit (TaKaRa). The transcription of related genes was performed by using the LightCycler® 96 real-time PCR system. The reaction conditions were as follows: 95℃ for 10 min; followed by 45 cycles of 95℃ for 15 s and 60℃ for 60 s; 95℃ for 10 s; 65℃ for 60 s; and 97℃ for 1 s. The relative gene expression was analysed following the formula of the 2−ΔΔCT (Kenneth & Thomas, 2002).
| Gene | Primer sequences (5’−3’) | Annealing temperature (℃) | Reference |
|---|---|---|---|
| β-actin | F:TTATGCTCTTCCTCACGCTATCC | 60 | Wang et al. (2011) |
| R:TTGTGGTAAAGGTGTAGCCTCTCTC | |||
| Cat | F:TAACTACCAGAGAGACGGACCT | 60 | Wang et al. (2011) |
| R:ACATCACCGGAGTAGTGAATCTG | |||
| Gpx | F:CCTTCAGGAACCGGGAGCAA | 60 | Zeng et al. (2020) |
| R:CGTTAGCGCCATTGACGTCACA | |||
| hsp70 | F:AAGAGCACAGGCAAAGAG | 60 | Zeng et al. (2020) |
| R:TGATGATGGGTTGGCACA | |||
| hsp90 | F:TGGTGTTGGCTTTTACTCTGCTT | 60 | Zeng et al. (2020) |
| R:CACCTGTAGCATTCGTCATCGT |
2.7 Calculations and statistical analysis

Where Ni, Nf, Wi and Wf were the initial number, final number, initial average weight and final weight of sea cucumbers in each tank; DL and BL were the length of the digestive tract and whole body length of each individual selected from each tank; DW, VW and W were the wet weight of digestive tract, viscera and the whole body of each individual selected from each tank, respectively.
The data were indicated as means ±S.E.M. The data were analysed by one-way analysis of variance (ANOVA) with the software of SPSS 22.0. When a significance (p < .05) was detected, Duncan multiple comparisons test was used to identify significant differences between different treatments.
3 RESULTS
3.1 Growth Performance of A. japonicus in response to graded levels of Se-yeast
The body width (BW) of sea cucumber first increased and then decreased as the supplementation level increased, with the highest value observed in the dietary treatment with 1.0 mg/kg Se-yeast. The weight gain rate (WGR) of sea cucumbers significantly increased from 171% to 280% as the Se-yeast supplementation increased from 0 to 1.0 mg/kg (p < .05), and then reached a plateau with further increase in dietary Se-yeast. As the supplementation level increased from 0.5 to 2.0 mg/kg, the survival rate (SR) of sea cucumbers significantly increased from 31.34% to 43.37% (p < .05) (Table 3).
| Se-yeast supplementation level (mg/kg) | |||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Wi (g) | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.01 |
| Wf (g) | 0.26 ± 0.01b | 0.28 ± 0.00b | 0.41 ± 0.02a | 0.38 ± 0.02a | 0.40 ± 0.01a |
| Ni (head) | 329.00 ± 4.00 | 277.00 ± 7.00 | 257.00 ± 21.00 | 257.00 ± 8.00 | 265.00 ± 22.00 |
| Nf (head) | 127.00 ± 1.00a | 87.00 ± 2.00b | 84.00 ± 4.00b | 97.00 ± 3.00b | 113.00 ± 4.00a |
| BL (cm) | 2.57 ± 0.04 | 2.53 ± 0.16 | 2.94 ± 0.05 | 2.63 ± 0.12 | 3.09 ± 0.49 |
| BW (cm) | 0.81 ± 0.07ab | 0.79 ± 0.03ab | 0.96 ± 0.06a | 0.68 ± 0.09b | 0.68 ± 0.00b |
| WGR (%) | 171.79 ± 4.19b | 185.23 ± 5.69b | 280.77 ± 6.07a | 253.27 ± 24.49a | 263.45 ± 20.16a |
| SR (%) | 38.63 ± 0.43ab | 31.34 ± 2.35b | 33.08 ± 3.56b | 37.65 ± 4.71ab | 43.37 ± 2.03a |
- Data with different lowercase letters in the same row are significantly different (p < 0.05).
- Abbreviations: BL, body length; BW, body width; Nf, final number of experiment sea cucumber; Ni, initial number of experiment sea cucumber; SR, survival rate; Wf, final body weight; WGR, weight gain rate; Wi, initial body weight.
3.2 Digestive tract trait index of A. japonicus in response to graded levels of Se-yeast
The digestive tract length (DL) of sea cucumbers increased as the Se-yeast supplementation level increased from 0 mg/kg to 0.5 mg/kg (p < .05), and then decreased with further increase in the supplementation level (p > .05). The digestive tract diameter (Dd) of sea cucumbers significantly increased from 0.20 cm to 0.23 cm as the supplementation level of Se-yeast increased from 0 to 1.0 mg/kg (p < .05), and then significantly decreased to 0.20 cm with further increase in the supplementation level. The digestive tract weight (Dw) increased from 0.10 g to 0.17 g as the supplementation level increased although no significance was detected among dietary treatments (p > .05). The relative digestive tract length ratio (RDL) fluctuates among different dietary treatments with the highest valuation observed in the treatment with 0.5 and 2.0 mg/kg Se-yeast. The relative digestive tract weight ratio (RDW) and relative visceral weight ratio (RVW) first significantly increased and then reached a plateau as the Se-yeast supplementation level increased. The highest value of RDW and RVW was observed in the treatments with 0.5 and 1.0 mg/kg Se-yeast, which were significantly higher than that of the control group (p < .05) (Table 4).
| Se-yeast supplementation level (mg/kg) | |||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| DL (cm) | 7.26 ± 0.65c | 9.09 ± 0.74ab | 8.32 ± 0.38abc | 7.70 ± 0.08bc | 9.44 ± 0.42a |
| Dd (cm) | 0.20 ± 0.00b | 0.19 ± 0.00b | 0.23 ± 0.01a | 0.21 ± 0.01ab | 0.20 ± 0.02b |
| DW (g) | 0.10 ± 0.02 | 0.13 ± 0.01 | 0.14 ± 0.02 | 0.14 ± 0.03 | 0.17 ± 0.02 |
| RDL | 2.83 ± 0.29 | 3.62 ± 0.40 | 2.83 ± 0.15 | 2.94 ± 0.12 | 3.24 ± 0.62 |
| RDW | 0.09 ± 0.01b | 0.12 ± 0.01a | 0.11 ± 0.00ab | 0.13 ± 0.01a | 0.12 ± 0.01a |
| RVW | 0.52 ± 0.00b | 0.62 ± 0.03ab | 0.66 ± 0.05a | 0.57 ± 0.01ab | 0.58 ± 0.01ab |
- Data with different lowercase letters in the same row are significantly different (p < .05).
- Abbreviations: Dd, digestive tract diameter; DL, digestive tract length; DW, digestive tract weight; RDL, relative digestive tract length ratio; RDW, relative digestive tract weight ratio; RVW, relative visceral weight ratio.
3.3 Digestive enzyme activities of A. japonicus in response to graded levels of Se-yeast
The pepsin activity of sea cucumber first increased and then decreased as the Se-yeast supplementation level increased with the highest value observed in the treatment with 0.5 mg/kg Se-yeast (p < .05). The pepsin activity of sea cucumber fed diets with 1.5 and 2.0 mg/kg Se-yeast was comparable to that fed the control diet, but was significantly lower than that fed diets with moderate level (0.5 and 1.0 mg/kg) of the Se-yeast. The lipase and amylase activities of sea cucumber first significantly increased as the Se-yeast supplementation level increased from 0 to 1.0 mg/kg (p < .05), and then significantly decreased with further increase in the supplementation of Se-yeast (p < .05). As the supplementation level of Se-yeast increased, the cellulase activity of sea cucumber in the digestive tract significantly increased from 8.93 U/mg prot to 11.44 U/mg prot (p < .05) (Table 5).
| Se-yeast supplementation level (mg/kg) | |||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Pepsin (U/mg prot) | 1.19 ± 0.03c | 2.14 ± 0.05a | 1.74 ± 0.05b | 1.25 ± 0.05c | 1.25 ± 0.07c |
| Lipase (U/g prot) | 2.94 ± 0.07d | 3.21 ± 0.07d | 4.90 ± 0.09a | 4.52 ± 0.14b | 3.81 ± 0.11c |
| Amylase (U/mg prot) | 1.66 ± 0.03d | 2.13 ± 0.04b | 2.47 ± 0.05a | 2.25 ± 0.02b | 1.83 ± 0.06c |
| Cellulose (U/mg prot) | 8.93 ± 0.32c | 9.68 ± 0.36bc | 10.50 ± 0.22ab | 10.77 ± 0.32a | 11.44 ± 0.25a |
- Data with different lowercase letters in the same row are significantly different (p < .05).
3.4 Immune and antioxidant capacity of A. japonicus in response to graded levels of Se-yeast
The superoxide dismutase (SOD) activity in the coelomic fluid of sea cucumber increased as the supplementation level of Se-yeast increased from 0 to 0.5 mg/kg (p < .05), and then reached a plateau with further increase in the Se-yeast (p > .05). The significantly promoting effects on catalase (CAT) were also achieved by the supplementation of 0.5 mg/kg Se-yeast, but these positive effects were eliminated when the supplementation level was equal to or above 1.0 mg/kg. The activities of glutathione peroxidase (GSH-Px) and total nitric oxide synthase (T-NOS) increased in a stepwise way as the Se-yeast level increased from 0 mg/kg to 1.0 mg/kg (p < .05), and then decreased with further increase in this level. The activities of GSH-Px and T-NOS increased significantly as the supplementation level increased from 0 to 1.5 mg/kg (p < .05), and then decreased sharply (p < .05) with further increase in the Se-yeast. As for malonaldehyde (MDA) content, it was first decreased and then increased as the Se-yeast supplementation increased. The lowest MDA content was observed in the treatment with 0.5 mg/kg and 1.0 mg/kg, which were significantly lower than that in the other groups (p < .05) (Table 6).
| Se-yeast supplementation level (mg/kg) | |||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| SOD (U/ml) | 137.70 ± 0.76b | 145.75 ± 1.89a | 147.08 ± 1.15a | 146.29 ± 0.21a | 144.85 ± 0.38a |
| MDA (nmol/ml) | 0.74 ± 0.01a | 0.60 ± 0.01c | 0.64 ± 0.00b | 0.75 ± 0.01a | 0.75 ± 0.00a |
| GSH-Px (U/ml) | 15.23 ± 0.31d | 17.18 ± 0.27c | 19.97 ± 0.28ab | 19.03 ± 0.13b | 21.08 ± 0.74a |
| CAT (U/ml) | 0.37 ± 0.01c | 0.51 ± 0.01a | 0.40 ± 0.00b | 0.38 ± 0.00bc | 0.39 ± 0.01bc |
| T-NOS (U/ml) | 5.44 ± 0.04c | 5.73 ± 0.09c | 6.81 ± 0.05a | 6.12 ± 0.01b | 6.05 ± 0.18b |
| AKP (U/100ml) | 1.04 ± 0.03b | 1.05 ± 0.03b | 1.06 ± 0.02b | 1.34 ± 0.06a | 1.05 ± 0.07b |
| LZM (U/mg prot) | 230.91 ± 0.82d | 244.93 ± 0.38c | 293.88 ± 2.05b | 304.63±3.24a | 209.63 ± 0.16e |
- Data with different lowercase letters in the same row are significantly different (p < .05).
- Abbreviations: AKP, alkaline phosphatase; CAT, catalase; GSH-PX, glutathione peroxidase; LZM, lysozyme; MDA, malonaldehyde; SOD, superoxide dismutase; T-NOS, total nitricoxide synthase.
3.5 Proximate compositions of A. japonicus body wall in response to graded levels of Se-yeast
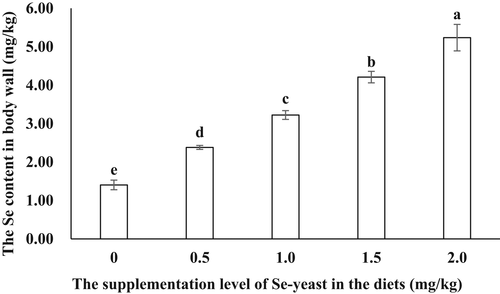
There were no significant effects of Se-yeast supplementation on the contents of crude protein and moisture in the body wall of sea cucumbers (p > .05). As the supplementation level increased, the carbohydrates in the body wall significantly decreased, with the significantly lower values observed in the treatments with moderate or higher levels (1.0 to 2.0 mg/kg) of Se-yeast. The promoting effects of Se-yeast on the lipid content were observed in the treatment with 0.5 mg/kg Se-yeast and were then eliminated as the Se-yeast level reached equal to or above 1.0 mg/kg (Table 7). Se accumulation in the body wall increased in a stepwise way as the Se-yeast supplementation level increased (p < .05) (Figure 1).
| Selenium yeast supplementation level (mg/kg) | |||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Crude protein (%) | 4.01 ± 0.02 | 4.01 ± 0.02 | 4.01 ± 0.01 | 4.03 ± 0.03 | 4.06 ± 0.03 |
| Crude lipid (%) | 0.10 ± 0.00b | 0.14 ± 0.02a | 0.08 ± 0.00b | 0.09 ± 0.01b | 0.09 ± 0.01b |
| Carbohydrate (mg/kg) | 1.54 ± 0.02a | 1.49 ± 0.09a | 1.14 ± 0.14b | 1.09 ± 0.06b | 1.15 ± 0.02b |
| Moisture (%) | 92.03 ± 0.6 | 92.33 ± 0.10 | 92.59 ± 0.09 | 92.41 ± 0.04 | 92.41 ± 0.25 |
- Data with different lowercase letters in the same row are significantly different (p < .05).
3.6 Expression of immune-related genes of A. japonicus in response to graded levels of Se-yeast
The transcription of cat overall showed an increasing tendency (p < .05) as the Se-yeast supplementation increased. The transcription of cat in the digestive tract of sea cucumbers fed diets with relatively higher supplementation of Se-yeast (1.5 mg/kg and 2.0 mg/kg) was about 3.13-fold and 3.86-fold higher than that of the control group (p < .05) (Figure 2A). On the contrary, the transcription of gpx decreased significantly as the supplementation level of Se-yeast increased (p < .05). The lowest value was observed in the treatments with a relatively higher level (1.5 mg/kg and 2.0 mg/kg) of Se-yeast, which were about 4.0-fold lower than that in the control treatment (Figure 2B).
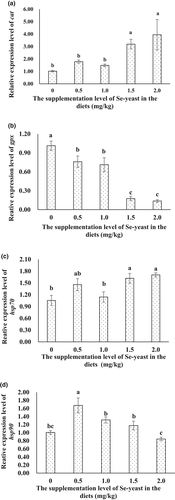
The transcription of hsp70 overall showed an increasing tendency (p < .05) as the Se-yeast supplementation increased. Transcription of hsp70 in the digestive tract of sea cucumbers fed diets with the relatively higher level (1.5 and 2.0 mg/kg) of Se-yeast was about 1.54-fold and 1.62-fold higher than that in the control treatment (p < .05) (Figure 2C). The transcription of hsp90 first significantly increased by about 0.5-fold as the supplementation level of Se-yeast increased from 0 to 0.5 mg/kg (p < .05), and then significantly decreased to the comparable level with that in the control treatment as Se-yeast further increased to 2.0 mg/kg (Figure 2D).
4 DISCUSSION
Several studies have reported the positive effects of Se as a feed additive (0.4–2.0 mg/kg) on the nonspecific immunity and growth performance of several aquatic animals, such as sea cucumber (Lu et al., 2015), abalone (Haliotis discus hannai) (Wang, Mai, et al., 2012), kingfish (Seriola lalandi) (Le & Fotedar, 2014), Nile tilapia (Oreochromis niloticus) (Iqbal et al., 2020; Lee et al., 2016) and grouper (Epinephelus malabaricus) (Lin, 2014). In the present study, sea cucumbers fed diets with the supplementation of 0.5–1.5 mg/kg Se-yeast also showed a significant increase in the growth performance, digestive tract index, digestive enzyme activities as well as immune and antioxidant capacity. The beneficial effects of Se-yeast supplementation on growth performance have been previously reported on hybrid striped bass (Morone saxatili) (Cotter et al., 2008), rainbow trout (Oncorhynchus mykiss) (Robinson & Lovatelli, 2015) and Wuchang bream (Megalobrama amblycephala) (Long et al., 2017). It has been speculated that the increased bioavailability of organic Se occurs because it is transported through the intestine in an intact form to the target tissues (Ashmead & Zunino, 1992). Se was an essential micronutrient responsible for the wonted steady growth of aquatic animals (Durigon et al., 2018). However, it was proven to be highly toxic if at a slightly higher concentration (Iqbal et al., 2017). The higher supplementation level (above 2 mg/kg) of Se was found to be detrimental to the amylase and lipase activities of tilapia (Oreochromis niloticus) (Iqbal et al., 2020). Similarly, the positive effects of Se-yeast on the activities of main digestive enzymes (pepsin, lipase and amylase) were eliminated when the supplementation level was above 1.0 mg/kg. However, inconsistent with the results reviewed above, the digestive enzyme activities of the pacu juveniles (Piaractus mesopotamicus) were not significantly affected by the supplementation of Se-yeast (0.72–2.51 mg/kg) (Takahashi et al., 2017). This could be attributed to the differences in the animal species, growth stage and feed formulation. Based on the growth performance, digestive tract development and digestive enzyme activities, the optimal supplementation level of Se-yeast was estimated to be 1.0 mg/kg for early juvenile sea cucumbers.
Se can be incorporated into a series of selenoproteins (glutathione peroxidase, selenocysteine and selenomethionine) that has been reported to be vital for exerting antioxidant properties, anti-inflammation roles and anti-diabetic effects (Abdel et al., 2007; Bosello-Travain et al., 2013; Lin & Shiau, 2007; Rayman, 2012). Generally, an increase in the activity of relevant antioxidant enzymes in the body may represent the elevated antioxidant capacity (Zhang et al., 2015). In this study, the supplementation of Se-yeast at a level of 0.5–1.5 mg/kg significantly increased the activities of immune and antioxidant-related enzymes (SOD, GSH-Px, CAT, T-NOS, ALP and LZM). This result was consistent with the findings of Long et al. (2017) who found that the supplementation of 0.50 mg/kg Se-yeast could effectively improve nitrite resistance in Wuchang bream (Megalobrama amblycephala). Additionally, the high level of selenium (5 mg/kg) combined with medium level VC (5 g/kg) had positive effects on improving the digestive ability, immune and antioxidant capacity of sea cucumber (Zeng et al., 2020). Dietary Se (Na2SeO3) at a supplementation level of 0.86 mg/kg improved the antioxidant system of tilapia juveniles (Durigon et al., 2018). Chen et al. (2019) found that the activity of the antioxidant enzymes, the transcription of IL-1β, IFN-γ, TNF-α and Hsp70 and metallothionein (MT) were enhanced by adding selenite and se-yeast (3–12 mg/kg) in the diets of the tilapia, and the MDA was the oxidized product of lipid membrane cells used as a marker of tissue cell injury, respectively (Jia et al., 2018; Monteiro et al., 2010). In this study, Se-yeast supplementation (0.5–1.0 mg/kg) significantly reduced the content of MDA in the digestive tract of early juvenile sea cucumbers. This further verified the positive effects of Se-yeast on the antioxidant-related enzyme activities. However, the gpx transcription level decreased as the Se-yeast supplementation level increased. This discrepancy could be due to the fact that the enzyme activities were modulated at both pretransitional and post-transitional levels (Alberto Burgos-Aceves et al., 2018).
In this study, the water temperature increased gradually from 19℃ to 21℃. The high temperature was found to be a critical factor in inducing oxidative stress and therefore increasing the mortality of A. japonicus. It has been proven that hsp70 protein can reduce the damage of H2O2 to the cell membrane (Gullo & Teoh, 2004), while hsp90 participates in the protein folding which is important processes during the cell cycle and signal transduction (Pratt, 2004). The results found that adding Se-yeast to the feeds significantly increased the expression levels of hsp70 and hsp90 genes in the digestive tract of sea cucumber. The upregulation of HSP expression is a self-protection mechanism, which can reduce the damage caused by environmental stress such as relatively high water temperature (Wu et al., 2014). In Atlantic salmon (Salmo salar) and juvenile lake whitefish (Coregonus clupeaformis), transcription of Hsp70 increased after heat stress (Stefanovic et al., 2016; Takle et al., 2005). A previous study has also found that starvation can protect trout larvae from fatal heat shock by inducing the expression of hsp70 and hsp90 (Cara et al., 2005). In this experiment, transcription of hsp70 and hsp90 was significantly increased by the supplementation of Se-yeast in the diets. In addition, some studies have found that dietary supplementation of Se-yeast (0.5mg/kg) could effectively improve nitrite resistance in Wuchang bream (Megalobrama amblycephala) (Long et al., 2017) and Chinese mitten crab (Eriocheir sinensis) (Wang et al., 2019). Guo et al. (2018) found that single and combined supplementation of selenium yeast and green tea-derived polyphenols improved the antioxidant capacity and immune response in juvenile Wuchang bream under ammonia stress. This finding possible implies the possibility of using Se-yeast as an additive to enhance the immune response and antioxidant capacity during the summer season when the water temperature is usually at a range of 28℃ to 31℃.
Dietary Se can effectively improve the flesh quality of common carp (Saffari et al., 2017) and rainbow trout (Liu et al., 2011). The improved fish flesh quality was mainly associated with the inhibited protein degradation in the muscle. The body wall is the main edible part of sea cucumbers, which is rich in proteins, peptides, polysaccharides and a variety of active substances (Cheng et al., 2018). However, the crude protein of the body wall of sea cucumber in the present study was not significantly affected by the supplementation level of Se-yeast. In this study, 0.5 mg/kg Se-yeast significantly increased crude lipids in the body wall of sea cucumbers. Similarly, dietary Se (3 mg Se/kg) has been found to increase the contents of total triglyceride, total cholesterol and nonesterified fatty acids in the liver and adipose tissues up to threefold compared with control pigs. (Zhao et al., 2016), but the contents of carbohydrates in the body wall of A. japonicus decreased with the increase in Se-yeast. Among carbohydrates, the polysaccharide is accepted as the most valuable nutrient of A. japonicus. Since the polysaccharide content was not assayed, the negative effects of Se-yeast on the deposition of polysaccharide cannot be excluded and needs for further study. Se can affect glycaemic control through insulin signalling, glycolytic pathway and pyruvate metabolism in humans (Ewa et al., 2016). Studies on rats with diabetes found that Se supplementation improved the concentration of glucose and insulin in the plasma, and reversed the abnormal expression of gluconeogenesis-related genes (Mueller & Pallauf, 2006). It is acknowledged that seafood is dependable sources of mineral elements for humans. Supplementation of Se in the diets has been proven to be an effective strategy of accomplishing Se enrichment in the meat or eggs of broiler chickens (Lu et al., 2018; Wang & Xu, 2008). In this study, the Se content of the body wall significantly increased with the increase in dietary Se-yeast supplementation. This indicated that sea cucumbers were also ideal objectives of Se enrichment with high accumulating efficiency achieved by the supplementation of the Se-yeast.
In summary, a moderate level (0.5–1.0 mg/kg) of Se-yeast enhanced the growth performance, digestive enzyme activities, antioxidant capacity and nonspecific immunity of early juvenile A. japonicus. Besides, Se can be easily accumulated in the body wall of A. japonicus by the supplementation of Se-yeast in the diets.
ACKNOWLEDGEMENTS
This research was supported by the National Key R & D Program of China (Grant no.: 2018YFD0900400) and Young Elite Scientists Sponsorship (YESS20150157).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




