Effects of single-species microalgae diet on accumulation of lipid and carotenoid and expression of lipid-related genes in Nan'ao Golden Scallop Chlamys nobilis
Abstract
Nan'ao Golden Scallop, Chlamys nobilis, is an important scallop species in aquaculture and is rich in lipids and carotenoid. Previous studies have shown that microalgae diet has a significant effect on the accumulation of total lipid content (TLC) and total carotenoid content (TCC) in bivalves. However, the effects of single-species microalgae diet on accumulation of TLC and TCC at molecular level are unclear. In this study, we used C. nobilis as a bivalve animal model to assess the effects of single-species microalgae diet (Tetraselmis subcordiformis, Dicrateria zhanjiangensis and Chaetoceros mulleri) on the accumulation of lipids and carotenoids, and the expression of stearoyl-CoA desaturase (SCD) and scavenger receptor class B-like-3 (SRB-like-3) genes in C. nobilis. The results of this study indicated that high lipid microalgae diet promoted the accumulation of TCC in C. nobilis tissues. The expression of SCD and SRB-like-3 genes was highly affected by the lipid composition of diet, in which high expression of SCD and SRB-like-3 genes was correlated with low C16:1n-9 and/or C18:1n-9t and/or C16:1/C16:0 and/or C18:1/C18:0. In intestine and adductor muscle, the expression of SCD gene was positively correlated with accumulation of TCC, TLC and fatty acid content, suggesting that SCD gene may indirectly promote the accumulation of TCC (lipid soluble) by promoting lipid accumulation. For SRB-like-3 gene, the high expression of SRB-like-3 was only correlated to the high TCC in intestine, suggesting that SRB-like-3 may be a candidate gene for carotenoid absorption in C. nobilis and has nothing to do with the transport or accumulation of carotenoid. The findings of this study are of great significance for further understanding how microalgae diet affects the accumulation of TCC and TLC, and the expression of lipid-related genes in bivalves.
1 INTRODUCTION
Nan'ao Golden Scallop Chlamys nobilis is an important edible marine bivalves belonging to the Pectinidae family. It is naturally distributed in the coastal waters of Japan, Korea, Indonesia, Vietnam and the Southern Sea of China, (Wang et al., 1998; Tan et al., 2020a, Tan et al., 2020b). Since the early 1980s, C. nobilis aquaculture has developed into a large-scale industry in China, mainly due to its fast growth, short cultivation period, high amino acid and lipid nutritional quality, as well as carotenoid-rich advantages (Guo et al., 1999; Zheng et al., 2012a, Zheng et al., 2012b; Tan et al., 2019a, Tan et al., 2020c, Tan et al., 2021a, Tan et al., 2021b). Carotenoids are natural pigments that have been shown to be useful as natural antioxidants (Babin et al., 2010; Chien et al., 2003; Tan et al., 2019a; Tan et al., 2019b; Wang et al., 2006), which can increase the efficiency of immune responses and stimulate innate immune components (Blount et al., 2003; McGraw and Ardia, 2003; Tan et al., 2020d). In general, animals cannot biosynthesize carotenoids de novo, so they can obtain carotenoids either directly from food or partially modified them through metabolic reactions (Maoka, 2011). Carotenoids are lipid-soluble compounds, of which the absorption of carotenoids in humans (Goltz et al., 2012), rats (Mamatha & Baskaran, 2011), mice (Hessel et al., 2007) and salmon (Bjerkeng et al., 1999) has a positive correlation with the lipid content in food (Tan et al., 2021c; Tan et al., 2020e).
The growth performance and nutritional value of farmed bivalves are strongly influenced by the biochemical composition of the microalgae diet (Martínez-Fernández et al., 2004; Tan & Zheng, 2021). Various microalgae, including Dunaliella salina, Tetraselmis subcordiformis, Chlorella sp., Chaetoceros mulleri, Nitzschia closterium, Phae-odactylum tricornutum, Skeletonema costatum, Isochrysis zhangjiangensis and Isochrysis spp., are commonly used in marine bivalve aquaculture. Among them, I. zhanjiangensis, T. subcordiformis and Ch. mulleri are the most widely used in Chinese bivalve hatcheries (Chen et al., 2013). In addition, genes involved in fatty acid regulation, especially stearoyl-CoA desaturase (SCD) and scavenger receptor class B-like-3 (SRB-like-3), have been shown to be involved in carotenoid absorption (Hessel et al., 2007; Li et al., 2015; Liu et al., 2015).
SCD is a key enzyme that biosynthesizes palmitoleic acid (C16:1) and oleic acid (C18:1) from palmitic acid (C16:0) and stearic acid (C18:0), respectively, by introducing a double bond between the 9th and 10th carbon atoms (Denke & Grundy, 1992). SCD is primarily located on the endoplasmic reticulum membrane and has become a key regulator of lipid metabolism (Flowers & Ntambi, 2008). To date, a total of five SCD genes (SCD1 to SCD5) have been identified, most of which (SCD2, SCD3 and SCD4) have only been found in rodents (Miyazaki et al., 2003; Wang et al., 2005). Compared with vertebrates, information on SCD gene in invertebrates is very limited (Li et al., 2015). It has been shown that elevated expression levels of SCD genes are associated with lipid absorption (Hulver et al., 2005). Since carotenoids are lipid-soluble substances, high lipid absorption may indirectly promote accumulation of carotenoids.
On the other hand, SRB-like-3 also plays an important role in regulating lipid metabolism (Neculai et al., 2013). It is an integral membrane protein that can be found in many cell types and is known for its role in facilitating the uptake of cholesterol esters from high-density lipoproteins. Our previous research showed that SRB-like-3 is only expressed in golden scallops (rich in carotenoids), but not in brown scallops (low carotenoids), which suggests that the SRB-like-3 gene may be associated with carotenoid absorption (Liu et al., 2015). SRB has been shown to play a role in the absorption of lutein (Reboul et al., 2005), carotene (van Bennekum et al., 2005), zeaxanthin and xanthophylls (During et al., 2008), and lycopene (Moussa et al., 2008).
To date, it is unclear how different groups (Chlorophyta, Bacillariophyta and Chrysophyta) of single-species microalgae diets affect the accumulation of lipids and carotenoids and expression of SCD and SRB-like-3 genes in bivalves. Therefore, in the present study, Nan'ao Golden Scallop Chlamys nobilis was used as a bivalve animal model to assess the effects of single-species microalgae diet on the accumulation of lipids and carotenoids in bivalve in relation to SCD and SRB-like-3 gene expressions. The findings of this study can improve current understanding how microalgae diet affects the accumulation of total carotenoid content (TCC) and TLC, and the expression of lipid-related genes (SCD and SRB-like-3) in bivalves.
2 MATERIALS AND METHODS
2.1 Microalgae cultivation
Three species of microalgae were used in feeding trials, two of which were Tetraselmis subcordiformis (Chlorophyta) and Dicrateria zhanjiangensis (Chrysophyta) obtained from the Wenzhou laboratory and the Zhejiang Mariculture Research Institute, respectively. Another species of microalga, Chaetoceros mulleri (Bacillariophyta), was obtained from the Sanya Laboratory of South China Sea Fisheries Research Institute, Chinese Academy of Fisheries Sciences. All microalgae were separately inoculated in 250-mL conical flask containing sterile 1x f/2 medium (Guillard, 1975) and incubated at 20°C for 7 days under a light intensity of 1500 lux. The inoculum was gently shaken four times a day to keep the microalgae suspended. Subsequently, the microalgae were transferred to a 1 L container and then transferred to a 19-L container and incubated for 7 days each under the same environmental conditions. When the microalgae entered a stationary growth curve, the microalgae were cultured in a 90-L tank and stirred five times a day to keep the microalgae suspended. Microalgae with a stationary growth curve (about 7–10 days of incubation) were used for feeding experiments. Ten litres of each microalgae culture was harvested by filtered through 0.45-µm filter paper and then dried in a vacuum freeze-drying machine. The dried cell pellets were then ground into a fine powder using a mortar for further analysis. Dry cell weight, TCC and lipid analyses were performed in triplicate.
2.2 Experimental design
A total of 540 Nan'ao Golden Scallops (about six months of age with average size and weight were 5.10 ± 0.15 cm and 28.71 ± 1.30 g, respectively) were randomly collected from the Nan'ao Marine Biology Experimental Station of Shantou University, China (23.35°N, 116.68°E). Subsequently, the scallops were randomly divided into nine groups (60 scallops in each group) and maintained in nine 500-L tanks. Three of the nine scallop groups (three replicates) were randomly selected and fed a type of microalgae (T. subcordiformis, D. zhanjiangensis or Ch. mulleri) four times a day (4 times x 6 L phytoplankton culture x 0.16 mg/g DW). Water temperature, dissolved oxygen, salinity and pH were maintained at 26 ± 1°C, 6 mg/L, 31.0 ± 0.5 psu and 7.21 ± 0.27, respectively. Ten scallops were randomly collected from each group on days 1, 10, 25 and 45. The scallops were cut open; the adductor muscles, mantle and intestines of each scallop were extracted and dried separately in a vacuum freeze-drying machine. The same tissues from every three scallops were pooled (3 scallops x 3 replicate = 9; one extra sample was kept as spare) and then ground into fine powder using a mortar for further analysis.
2.3 Determination of TCC
Carotenoids of microalgae and C. nobilis tissues (adductor muscle and mantle) were extracted according to the method of Zheng et al. (2010). An amount of 0.20 g of freeze-dried tissue powder was added to 2 mL of acetone and incubated with a rotator (448 g/min) at room temperature of 25°C for 1 h in the complete dark (covered with aluminium foil). The sample was then centrifuged at 2800 g for 5 min, the supernatant was collected, and the absorbance was measured at wavelength 480 nm using a spectrophotometer (UV2501PC). The TCC was estimated using the extinction coefficient E(1%, 1 cm) of 1.900 as described by Zheng et al. (2010).
2.4 Determination of TLC and fatty acid composition (FAC)
Under the protection of N2 (purity: 99.99%), total lipid content of microalgae and the Nan'ao Golden Scallop tissues (adductor muscle and mantle) was extracted using chloroform/ methanol (2:1) and then was estimated according to Zheng et al. (2012b).
For fatty acid composition analysis, first fatty acid methyl esters were prepared under the protection of N2 according to the method of Morrison and Smith (1964). Briefly, 0.4 mg of C23:0 was used as an internal standard and added to the total lipid. A volume of 2 ml of NaOH-methanol solution (0.5 M) was then added to the mixture and incubate at 60°C for 20 min and then transesterified with 2 ml of boron trifluoride etherate (ca.14%, Acros Organics) at 60°C for 1 min. Then, 2 ml of hexane was added to the solution for fatty acid methyl esters extraction at 60°C for 1 min. After the solution was cooled to room temperature, 2 ml of saturated NaCl solution was added, and the supernatant containing methyl esters was filtered through a 0.45-μm filter paper. Subsequently, the extracted methyl ester was separated using a gas chromatograph (GC-17A, Shimadzu, Kyoto, Japan) equipped with a hydrogen flame ionization detector and a 30 m × 0.25 mm × 0.25 μm capillary column (VF-23ms, Varian). Nitrogen with air and hydrogen was used as carrier gas to support combustion, with a column flow rate was 1.1 ml/min and a split ratio of 30:1. The temperature of the injector and detector was set at 250°C. The column temperature was initially set at 140°C for 5 min, then gradually increased to 210°C at a rate of 3°C/min, and then finally increased to 230°C at a rate of 10°C/min. Individual peaks were identified by comparison with commercial available fatty acids standards (Sigma) and quantified using CLASS-GC10 GC workstation (Shimadzu). The absolute content of fatty acid content was calculated by gas chromatograms as (C23:0 weight· fatty acid area)/C23:0 area.
2.5 RNA isolation and reverse transcription
Total RNA was extracted from the adductor muscles, mantle and intestines of Nan'ao Golden Scallop, using the Trizol reagent (Invitrogen) following the manufacturer's protocol. The quantity, purity and integrity of RNA were determined by electrophoresis on a 1% agarose gel in 50 × TAE buffer (acetic acid 1 mol/L, Tris 2 mol/L, EDTA 0.1 mol/L, pH 8.0) and then measuring the UV absorbance ratio at 260 nm and 280 nm (Ultrospec 2100 pro, Amersham). Subsequently, cDNA was synthesized from 1 µg of total RNA (as template) using SuperScript III reverse transcriptase and Oligo-dT as primers following the manufacturer's instructions (Invitrogen).
2.6 Cloning of the full-length cDNA of SCD gene
Degeneration primers (SCDF and SCDR) of SCD were designed based on the alignment of SCD coding sequences (NCBI, USA) of different species, including Mizuhopecten yessoens, AGI48676.1; Danio rerio, NP_942110.2 and Gryllus bimaculatus, ALT55114.1. The PCR procedure was one cycle denaturation at 95°C for 3 min, 35 cycles at 94°C for 45 s, 50°C for 45 s and 72°C for 1 min, followed by extension at 72°C for 10 min. The obtained PCR products were separated on a 1% agarose gel, and then, a PCR purification kit (Sangon Biotech) was used to retrieve and purify the target cDNA. The purified cDNA with the SCD gene sequence was then ligated into pMD-18 T vector (TaKaRa) and transformed into competent Escherichia coli. The positive recombinants (blue-white colour colonies) grow on ampicillin-containing LB plates were selected and submitted to sequencing in both directions.
The SMART™ RACE cDNA amplification kit (Clontech) was used for 5′ and 3′ RACE PCRs to clone full-length DNA. Based on the obtained partial sequences, specific primers SCD31, SCD51 and nested primers SCD32, SCD52 were designed for 3′ RACE and 5′ RACE, respectively. The cDNA preparation and amplification conditions of RACE PCRs were performed following the manufacturer's instructions. The primers used for cloning are shown in Table 1. A touchdown PCR procedure was performed to obtain the 3′ end and 5′ end of SCD gene. The PCR procedure was 95°C for 2 min, followed by 35 cycles at 94°C for 30 s, 70–60°C for 30 s in the first 10 cycles decrementing 1°C/cycle, 60°C for 30 s for the remaining 25 cycles, 72°C for 60 s and a final extension step at 72°C for 10 min. The purified DNA fragments were then ligated into pMD-18 T vector (TaKaRa) and transformed into competent E. coli, and the fragments from positive clones were sequenced (Sangon Biotech). The 3′- and 5′-RACE PCR fragment sequences were then aligned to assemble the complete nucleotide sequence of the scallop putative SCD cDNA. The resulting sequences were verified by the amplification of the whole full length and further subjected to cluster analysis.
| Primer name | Sequence 5′−3′ |
|---|---|
| Degeneration primers for SCD | |
| SCDF | CACAGACTTTGGTCACATCGCTCGTA |
| SCDR | TGACAACCCAACCTGAAGCACGA |
| SCD RACE | |
| SCD51 | GAGTACCGTATTCACTGGCGGCGTAG |
| SCD52 | CAGCCATCCAATGTGGGAAAAGAAG |
| SCD31 | GGCGTGACGTTTGGTGCTGTAG |
| SCD32 | TGTTTGTCGTGCTTCAGGTTGG |
| Real-time PCR | |
| SCD-RT-R3 | CGCCTGTCCGTTCTTTCCTCATT |
| SCD-RT-F3 | TGTGGGGGTCCAAACCTTACG |
| SRB-RT-F | ATGAATCCCCGACGGATAACACA |
| SRB-RT-R | ATGGATTGACTGATGTGAGATGT |
| β-actin F | GCGGCAGTGGTCATCTCCT |
| β-actin R | GCCCTTCCTCACGCTATCCT |
2.7 Bioinformatics analysis
The BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to analyse the obtained SCD nucleotide sequence and deduced amino acid sequence. The Expasy-ProtParam online tool Compute PI/Mw program (http://web.expasy.org/compute_pi/) was used to determine the theoretical pI/Mw. The deduced SCD amino acid sequence was aligned with SCD amino acid sequence of other species using the Clustal X multiple alignment program (version 1.83) and Genedoc software. In addition, the protein motif features were analysed by the SMART program (Letunic et al., 2006). Phylogenetic tree was constructed using MEGA 5.0 software through the distance matrix by the Bootstrapped Neighbor-Joining (NJ) method (1000 pseudo-replicates; Tamura et al., 2011). All the analysed sequences in the tree were retrieved from the GenBank database.
2.8 Quantification real-time PCR analysis of SCD and SRB-like-3 genes in various tissues
The expression levels of SCD and SRB-like-3 genes in adductor, mantle and intestine were determined by SYBR Green quantitative real-time RT-PCR. RNA isolation was carried out as described above, and cDNA was synthesized by PrimeScript RT reagent kit with gDNA Eraser (TaKaRa). Quantitative real-time RT-PCR was conducted using the SYBR Premix Ex Taq II qRT-PCR Kit (TaKaRa) in an ABI 7300 real-time PCR system. For each assay, β-actin mRNA was used as an internal control, where primer β-actin F and β-actin R were used to amplify the β-actin gene. SCD-RT-R3 and SCD RT-F3 were used for SCD amplification, and SRB-RT-F and SRB-RT-R were used for SRB-like-3 amplification. The real-time PCR procedure was one cycle at 95°C for 1 min, followed by 40 cycles at 95°C for 15 s, and then 60°C for 60 s. At the end of each PCR, the amplified products were subjected to dissociation to confirm that only one PCR product was amplified. The expression levels of SCD and SRB were analysed using the comparative CT method (2−ΔΔCT method), and all data were reported as relative mRNA expression.
2.9 Statistical analysis
Results are reported as mean ± standard deviations (SD). Data were analysed using the SPSS Windows Statistical Package TM (version 21). Tests were judged to be significant at p < .05 level. Prior to analyses, the normality and variance homogeneity of all variables were tested by Kolmogorov–Smirnov and Levene's tests, respectively. One-way ANOVA was performed followed by Turkey multiple comparison tests (Turkey HSD) to test for significant differences in TCC, TLC, fatty acids (FA) and fatty acid content among phytoplankton. For bivalve tissues, one-way ANOVA was performed followed by Turkey multiple comparison tests (Turkey HSD) to test for significant differences for TCC, TLC, fatty acid content, SCD gene expression and SRB-like-3 gene expression among tissues. Pearson regression was used to estimate the correlation coefficients between fatty acid composition and the expression of genes (SCD and SRB-like-3) in different tissues (the fatty acid profile in algae represent fatty acid profile in intestine). In addition, correlation coefficients among SCD, SRB, TCC, TLC, FAC, SFA, MUFA and PUFA were also estimated using Pearson regression.
2.10 Ethics statement
The animals (oysters) were processed according to "the Regulations for the Administration of Affairs Concerning Experimental Animals" established by the Guangdong Provincial Department of Science and Technology on the Use and Care of Animals.
3 RESULTS
3.1 TCC, TLC and fatty acid profile (FAC) of three different single-species microalgae culture
The TCC, TLC and FAC contents of three single-species microalgae cultures are summarized in Table 2. The results of this study revealed that the TCC of Ch. mulleri (0.58 ± 0.06 µg/g) was significantly higher (p < .05) than that of D. zhanjiangensis (0.33 ± 0.03 µg/g) and T subcordiformis (0.32 ± 0.01 µg/g). The TLC of D. zhanjiangensis (20.05% ± 0.10%) and T subcordiformis (7.31% ± 0.03%) was significantly (p < .05) higher and lower, respectively, than that of Ch. mulleri (13.93% ± 0.10%). The FAC of D. zhanjiangensis (633.59 μg/g) was significantly higher than that of T. subcordiformis (498.26 μg/g) and Ch. mulleri (579.51 μg/g). The long-chain polyunsaturated fatty acids (LC-PUFA) of Ch. mulleri (24.37%) was significantly higher than those of T. subcordiformis (5.80%) and D. zhanjiangensis (8.61%).
| Algae species | TCC (µg/g DW) | TLC (%) | FAC (μg/g DW) |
|---|---|---|---|
| Dicrateria zhanjiangensis | 0.32 (0.01) | 20.05 (0.10) | 633.59 |
| Tetraselmis subcordiformis | 0.33 (0.03) | 7.31 (0.03) | 498.26 |
| Chaetoceros mulleri | 0.58 (0.06) | 13.93 (0.10) | 579.51 |
- Abbreviations: FAC, total fatty acid content; TCC, total carotenoid content; TLC, total lipid content.
For the fatty acid composition (Table 3), the relative abundance of C18:0 and C20:1n-9 in D. zhanjiangensis was significantly higher (p < .05), but the relative abundance of C18:2n-9 and C18:3n-6 was significantly lower than that of T. subcordiformis and Ch. mulleri. T. subcordiformis contain significantly higher (p < .05) relative abundance of C16:0 and C18:1n-9, but significantly lower relative abundance of C14:0 and C20:4n-6 than that of D. zhanjiangensis and Ch. mulleri. For Ch. mulleri, it has significantly higher (p < .05) relative abundance of C22:0 and C20:5n-3, but significantly lower relative abundance of C20:1n-9 than that of D. zhanjiangensis and T. subcordiformis. The (C16:1+C18:1)/ (C16:0+C18:0) ratio of D. zhanjiangensis was significantly lower (p < .05) than that of T. subcordiformis and Ch. mulleri.
| D. zhanjiangensis | T. subcordiformis | C. mulleri | |
|---|---|---|---|
| C14:0 | 6.07 (0.82) | 1.26 (0.09) | 5.39 (0.73) |
| C15:0 | 0.29 (0.02) | 0.18 (0.01) | 0.35 (0.03) |
| C16:0 | 17.24 (0.75) | 30.06 (1.35) | 18.36 (1.01) |
| C17:0 | 8.79 (0.66) | 7.75 (1.01) | 6.92 (0.90) |
| C18:0 | 26.23 (3.21) | 13.72 (1.11) | 9.38 (1.08) |
| C22:0 | 0.22 (0.01) | 0.27 (0.01) | 1.53 (0.55) |
| ΣSFA | 58.84 (7.56) | 53.24 (6.54) | 41.23 (5.55) |
| C16:1n-9 | 16.67 (1.12) | 1.01 (0.01) | 28.54 (2.34) |
| C18:1n-9 t | 6.61 (1.03) | 30.34 (6.78) | 1.31 (0.06) |
| C18:1n-9-c | 0.00 (0.00) | 5.53 (1.22) | 2.95 (0.85) |
| C20:1n-9 | 6.92 (2.21) | 2.87 (0.24) | 0.00 (0.00) |
| C22:1n-9 | 2.35 (0.91) | 1.21 (0.11) | 0.90 (0.21) |
| ΣMUFA | 32.55 (4.55) | 40.96 (5.01) | 33.70 (4.32) |
| C18:2n-9 | 0.32 (0.04) | 1.66 (0.15) | 1.96 (0.21) |
| C18:3n-6 | 0.08 (0.01) | 0.22 (0.03) | 0.19 (0.04) |
| C20:4n-6 | 0.30 (0.08) | 0.08 (0.01) | 0.31 (0.03) |
| C20:5n-3 | 5.02 (1.21) | 1.90 (1.10) | 19.94 (3.54) |
| C21:5n-6 | 1.83 (0.22) | 1.94 (0.31) | 0.89 (0.19) |
| C22:6n-3 | 4.06 (0.39) | 0.00 (0.00) | 1.08 (0.06) |
| ΣPUFA | 8.61 (1.93) | 5.80 (1.59) | 24.37 (5.89) |
| C16:1/C16:0 | 0.98 | 0.03 | 1.55 |
| C18:1/C18:0 | 0.25 | 2.61 | 0.45 |
| (C16:1+ C18:1)/ (C16:0+ C18:0) | 0.61 | 1.32 | 1.00 |
Note
- Unit of relative abundance of each fatty acid is %; unit for C16:1/C16:0, C18:1/C18:0 and (C16:1 + C18:1)/ (C16:0 + C18:0) is ratio.
3.2 Effect of single-species microalgae diet on the TCC, TLC and FAC of adductor and mantle of C. nobilis
The TCC, TLC and FAC of the adductor muscle and mantle of C. nobilis fed three different algae species are illustrated in Figures 1 and 2, respectively. The results of this study demonstrated that the TCC of the adductor muscle and mantle of C. nobilis fed D. zhanjiangensis was significantly higher (p < .05) than that of scallops fed T. subcordiformis and Ch. mulleri. However, no significant difference in TCC of adductor muscle and mantle of scallops was found between scallops fed Ch. mulleri and T. subcordiformis (p > .05).


In all groups, the TLC of the adductor muscle and mantle of C. nobilis remained unchanged in the first 25 days and then increased sharply on the 45th day, with the highest (p < .05) TLC of adductor muscle and mantle recorded in C. nobilis fed Ch. mulleri and T. subcordiformis, respectively.
As far as the FAC was concerned, a significant difference was found only on day 45, at this level, the FAC of adductor muscle of C. nobilis fed T. subcordiformis, and mantle of scallops fed D. zhanjiangensis were significantly lower and higher, respectively, than that of other tissue specimens (p < .05).
3.3 Effect of single-species microalgae diet on the fatty acid composition in adductor and mantle of C. nobilis
The fatty acid composition in the adductor muscle and mantle of C. nobilis is summarized in Table 4. The SFA content (mainly C16:0 and C18:0) of the adductor muscle and mantle of C. nobilis fed T. subcordiformis was significantly higher (p < .05) than that of respective tissues of C. nobilis fed D. zhanjiangensis and Ch. mulleri (p > .05). For MUFA, the MUFA content of adductor muscle (mainly C16:1n-9, C18:1n-9-c, C20:1n-9 t, C20:1n-9-c and C22:1n-9) and mantle (mainly C16:1n-9) of C. nobilis fed D. zhanjiangensis was significantly higher (p < .05) than that of C. nobilis fed T. subcordiformis and Ch. mulleri. For PUFA, the C20:2 content in adductor muscle of C. nobilis fed D. zhanjiangensis was significantly higher (p < .05) than that of C. nobilis fed T. subcordiformis and Ch. mulleri. The C18:2n-9 and C20:3n-3 in the mantle of C. nobilis fed T. subcordiformis were significantly higher (p < .05) than C. nobilis fed D. zhanjiangensis nd Ch. mulleri. The (C16:1+C18:1)/(C16:0+C18:0) ratio in adductor muscle (0.21) and mantle (0.19) of C. nobilis fed D. zhanjiangensis was significantly higher (p < .05) than that of respective tissues of C. nobilis fed T. subcordiformis (adductor muscle = 0.15; mantle = 0.14) and Ch. mulleri (adductor muscle = 0.16; mantle = 0.06).
| D. zhanjiangensis | T. subcordiformis | C. mulleri | ||||
|---|---|---|---|---|---|---|
| A | M | A | M | A | M | |
| C14:0 | 1.07 (0.05) | 1.15 (0.05) | 1.01 (0.03) | 1.04 (0.04) | 1.26 (0.06) | 1.13 (0.05) |
| C15:0 | 0.40 (0.03) | 0.58 (0.04) | 0.51 (0.03) | 0.67 (0.06) | 0.43 (0.07) | 0.57 (0.06) |
| C16:0 | 23.28 (2.21) | 21.74 (2.29) | 27.81 (1.18) | 25.22 (1.15) | 24.61 (2.22) | 20.10 (2.19) |
| C17:0 | 11.11 (1.12) | 12.11 (0.98) | 11.88 (1.11) | 12.24 (0.99) | 12.31 (1.56) | 10.32 (1.39) |
| C18:0 | 20.12 (2.51) | 20.73 (3.45) | 23.75 (1.33) | 23.19 (1.29) | 20.78 (2.78) | 18.77 (2.19) |
| C20:0 | 1.44 (0.03) | 1.38 (0.05) | 1.54 (0.06) | 1.28 (0.04) | 1.50 (0.01) | 3.99 (0.02) |
| ΣSFA | 57.42 (9.57) | 57.69 (9.62) | 66.50 (7.39) | 63.64 (7.73) | 60.89 (2.34) | 54.88 (2.31) |
| C16:1n-9 | 2.84 (0.11) | 2.68 (0.14) | 1.11 (0.09) | 1.15 (0.09) | 1.37 (0.09) | 0.45 (0.05) |
| C18:1n-9 t | 2.58 (0.78) | 2.78 (0.34) | 3.23 (0.35) | 3.34 (0.29) | 2.81 (0.31) | 1.26 (0.38) |
| C18:1n-9-c | 3.58 (0.18) | 2.70 (0.11) | 3.15 (0.08) | 2.16 (0.08) | 2.88 (0.15) | 0.78 (0.07) |
| C20:1n-9 t | 3.63 (0.12) | 5.31 (0.54) | 3.30 (0.19) | 4.28 (0.20) | 3.20 (0.21) | 1.71 (0.11) |
| C20:1n-9-c | 0.91 (0.09) | 0.73 (0.08) | 0.64 (0.09) | 0.58 (0.04) | 0.67 (0.05) | 0.26 (0.02) |
| C22:1n-9 | 2.25 (0.44) | 2.15 (0.39) | 1.87 (0.21) | 1.22 (0.29) | 1.48 (0.28) | 8.50 (1.51) |
| ΣMUFA | 15.79 (0.96) | 16.35 (1.44) | 13.30 (0.49) | 12.73 (0.45) | 12.41 (0.47) | 12.96 (0.69) |
| C18:2n-9 | 0.81 (0.09) | 0.77 (0.07) | 0.79 (0.09) | 0.93 (0.09) | 0.93 (0.11) | 0.72 (0.08) |
| C20:2 | 0.70 (0.08) | 0.47 (0.05) | 0.47 (0.08) | 0.45 (0.09) | 0.57 (0.07) | 0.55 (0.08) |
| C20:3n-3 | 0.53 (0.12) | 0.17 (0.03) | 0.30 (0.05) | 0.31 (0.03) | 0.47 (0.03) | 0.13 (0.01) |
| C20:4n-6 | 4.69 (0.33) | 6.88 (0.42) | 3.48 (0.09) | 5.82 (0.35) | 4.35 (0.38) | 7.53 (122) |
| C20:5n-3 | 5.79 (1.11) | 3.00 (0.90) | 4.19 (0.51) | 2.93 (0.44) | 5.01 (1.59) | 2.76 (0.38) |
| C22:6n-3 | 14.27 (1.97) | 14.67 (2.45) | 10.97 (1.11) | 13.20 (1.55) | 15.37 (2.10) | 12.85 (0.99) |
| ΣPUFA | 26.79 (0.48) | 25.96 (0.96) | 20.20 (1.48) | 23.64 (0.45) | 26.70 (3.75) | 24.54 (0.46) |
| C16:1/C16:0 | 0.12 | 0.12 | 0.04 | 0.05 | 0.06 | 0.02 |
| C18:1/C18:0 | 0.31 | 0.26 | 0.27 | 0.24 | 0.27 | 0.11 |
| (C16:1+ C18:1)/ (C16:0+ C18:0) | 0.21 | 0.19 | 0.15 | 0.14 | 0.16 | 0.06 |
- Abbreviations: A, adductor muscle; M, mantle; unit for C16:1/C16:0, C18:1/C18:0 and (C16:1+ C18:1)/ (C16:0+ C18:0) is ratiounit of relative abundance of each fatty acid is %.
3.4 Full-length cDNA of SCD and the deduced amino acid sequence
A 2421-bp full-length cDNA sequence of the C. nobilis SCD gene was obtained using RACE. The length of the 5′ terminal untranslated region (UTR) and the 3′ terminal UTR was 119-bp and 1312-bp, respectively (Figure 3). A canonical polyadenylation signal sequence AATAA prior to a poly-A tail was located within the 3’-UTR. This sequence contains an 990-bp open reading frame, which encodes 329 amino acid residues, with a theoretical molecular weight of about 37.54 Kda and an isoelectric point of 9.25.

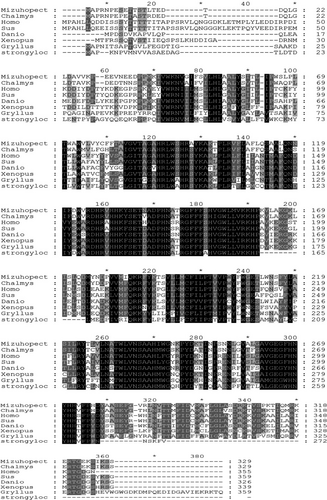
Alignment of cDNA and genomic DNA sequences indicated that the C. nobilis SCD gene contains four exons (45–67 aa, 71–93 aa, 191–208 aa and 284–303aa) and three introns. It contains three highly conserved histidine clusters: HXXHH, HXXXXH and HXXHH. The homology of SCD amino sequences from 7 species was analysed using ClustalX software (Figure 4). The results revealed that the SCD gene was highly conserved during evolution. The SCD gene was highly conserved in the middle sequence, and the homology of the sequences at both ends was low.
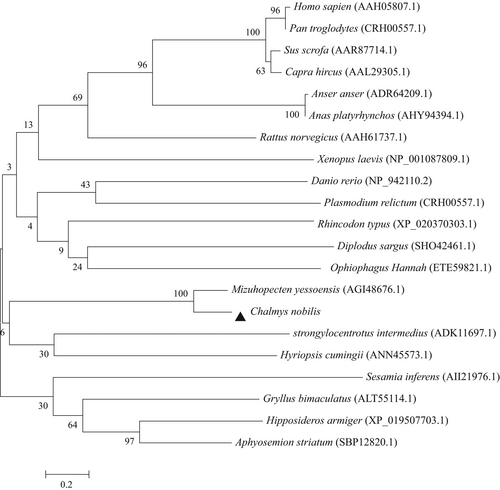
In order to investigate the relationship between the C. nobilis SCD and other SCD family members, a neighbour-joining tree was generated by aligning the conserved exons in SCD proteins from various phyla (Figure 5). Two distinct groups (vertebrate SCDs group and invertebrate SCDs group) were observed in the tree. The C. nobilis SCD share the highest identities of 85.71% with Mizuhopecten, followed by Xenopus laevis and Danio rerio with similarity >50% and shared 40%–50% identity with other species.
3.5 Relative expression of SCD and SRB-like-3 genes in tissues of C. nobilis fed with different single-species microalgae
The expression of SCD and SRB-like-3 genes in tissues of C. nobilis fed different microalgae species is illustrated in Figure 6. The relative expression of SCD gene in adductor muscle of C. nobilis fed T. subcordiformis was significantly higher (p < .05) than that of C. nobilis fed with D. zhanjiangensis and Ch. mulleri, while the relative expression of the SRB-like-3 gene was the highest in C. nobilis fed D. zhanjiangensis. The relative expressions of SCD and SRB-like-3 genes in mantle and intestine were significantly higher (p < .05) in C. nobilis fed Ch. mulleri and D. zhanjiangensis, respectively.
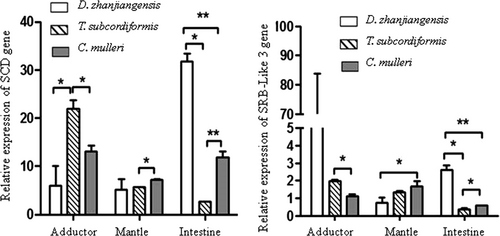
The relative expression order of SCD gene in the tissues of C. nobilis fed D. zhanjiangensis, T. subcordiformis and Ch. mulleri was intestine>adductor muscle & mantle, adductor muscle >mantle> intestine, and adductor muscle & intestine>mantle, respectively. For SRB-like-3 gene, the relative expression order in the tissues of C. nobilis fed D. zhanjiangensis, T. subcordiformis and Ch. mulleri was adductor muscle >intestine> mantle, adductor muscle >mantle> intestine, and mantle>adductor muscle>intestine, respectively. The correlation analysis between SCD and SRB-like-3 in adductor (r = −.83, p < .05) and intestine (r = .97, p < .05) was statistically significant.
3.6 Correlation coefficients among parameters
Correlation coefficients between fatty acid composition and genes (SCD and SRB-like 3) expression in different tissues of C. nobilis are summarized in Table 5. In general, the expression of SCD gene in all tissues and the expression of SRB-like-3 in adductor muscle and intestine of C. nobilis were negatively associated with C18:1n-9-c, C18:1/C18:0 and (C16:1+ C18:1)/(C16:0 + C18:0). In addition, SCD in the mantle was also negatively correlated to C18:1n-9 t, C16:1n-9, C20:4n-6 and C16:1/C16:0, while SRB-like-3 in the mantle was negatively correlated to C18:1n-9 t, C16:1n-9 and C18:0, but positively correlated to C16:0 and C16:1/C16:0.
| Adductor muscle | Mantle | Intestine | ||||
|---|---|---|---|---|---|---|
| SCD | SRB-like-3 | SCD | SRB-like-3 | SCD | SRB-like-3 | |
| C14:0 | −0.13 | −0.12 | −0.09 | 0.15 | −0.11 | 0.13 |
| C15:0 | −0.22 | −0.09 | 0.11 | 0.23 | −0.08 | −0.07 |
| C16:0 | 0.39 | 0.29 | 0.43 | 0.51 | −0.31 | −0.22 |
| C17:0 | −0.21 | 0.26 | −0.09 | −0.32 | 0.28 | −0.29 |
| C18:0 | −0.31 | 0.35 | −0.35 | −0.52 | 0.48 | 0.47 |
| C22:0 | 0.18 | 0.09 | −0.05 | 0.06 | −0.35 | −0.31 |
| C16:1n-9 | −0.45 | 0.49 | −0.65 | −0.78 | 0.29 | 0.19 |
| C18:1n-9 t | −0.44 | −0.39 | −0.59 | −0.62 | −0.45 | −0.39 |
| C18:1n-9-c | −0.66 | 0.49 | −0.78 | −0.87 | −0.78 | −0.73 |
| C20:1n-9 | −0.44 | 0.41 | −0.39 | −0.22 | 0.28 | 0.29 |
| C22:1n-9 | 0.23 | 0.43 | 0.19 | −0.42 | −0.21 | 0.23 |
| C18:2n-9 | 0.29 | −0.49 | 0.42 | 0.49 | −0.39 | −0.22 |
| C18:3n-6 | 0.49 | −0.49 | 0.22 | 0.39 | −0.31 | −0.49 |
| C20:4n-6 | −0.41 | 0.23 | −0.59 | 0.31 | −0.45 | 0.41 |
| C20:5n-3 | −0.23 | −0.23 | 0.22 | 0.29 | −0.31 | 0.18 |
| C21:5n-6 | 0.23 | −0.34 | 0.29 | 0.22 | −0.35 | −0.14 |
| C22:6n-3 | 0.11 | 0.29 | 0.09 | −0.02 | 0.47 | 0.35 |
| C16:1/C16:0 | −0.55 | 0.42 | −0.58 | −0.85 | 0.36 | 0.09 |
| C18:1/C18:0 | −0.51 | 0.49 | −0.71 | −0.53 | −0.91 | −0.88 |
| (C16:1 + C18:1)/(C16:0 + C18:0) | −0.52 | 0.44 | −0.61 | −0.72 | −0.89 | −0.85 |
Note
- Significant (p < .05) are in boldface.
The correlation analysis between parameters in intestine, adductor muscle and mantle were summarized in Tables 6–8, respectively. In intestine, the relative expression of SCD gene was positively correlated with SRB-like-3 (r = .88, p < .05), TCC (r = .75, p < .05), TLC (r = .78, p < .05) and FAC (r = .83, p < .05). The SRB-like-3 was positively correlated with TCC (r = .74, p < .05), TLC (r = .85, p < .05) and FAC (r = .75, p < .05), while TCC was positively correlated with TLC (r = .84, p < .05) and FAC (r = .74, p < .05).
| SCD | SRB | TCC | TLC | FAC | SFA | MUFA | PUFA | |
|---|---|---|---|---|---|---|---|---|
| SCD | 0.88 | 0.75 | 0.78 | 0.83 | 0.39 | 0.37 | 0.30 | |
| SRB | 0.84 | 0.85 | 0.75 | 0.07 | 0.06 | 0.35 | ||
| TCC | 0.84 | 0.74 | 0.36 | 0.43 | 0.18 |
Note
- Significant (p < .05) are in boldface.
| SCD | SRB | TCC | TLC | FAC | SFA | MUFA | PUFA | |
|---|---|---|---|---|---|---|---|---|
| SCD | −0.09 | 0.65 | 0.68 | 0.83 | 0.89 | 0.77 | 0.30 | |
| SRB | 0.34 | −0.25 | 0.15 | 0.07 | 0.06 | 0.55 | ||
| TCC | 0.74 | 0.54 | 0.66 | 0.43 | 0.18 |
Note
- Significant (p < .05) are in boldface.
| SCD | SRB | TCC | TLC | FAC | SFA | MUFA | PUFA | |
|---|---|---|---|---|---|---|---|---|
| SCD | −0.09 | 0.02 | −0.27 | −0.59 | −0.66 | −0.78 | 0.11 | |
| SRB | −0.093 | −0.73 | −0.03 | −0.53 | 0.31 | −0.69 | −0.48 | |
| TCC | 0.58 | 0.64 | 0.60 | 0.45 | 0.24 |
Note
- Significant (p < .05) are in boldface.
In the adductor muscle, the relative expression of SCD gene was positively correlated with TCC, TLC, FAC, SFA and MUFA. The relative expression of SRB-like-3 gene was positively associated with PUFA. For TCC, a strong positive correlation was observed between TCC and TLC (r = .74, p < .05), TCC and FAC (r = .54, p < .05) and TCC and SFA (r = .66; p < .05).
In the mantle, the relative expression of SCD gene was negatively associated with FAC (r = −.59, p < .05), SFA (r = −.66, p < .05) and MUFA (r = −.78, p < .05), while the relative expression of SRB-like-3 gene was negatively correlated with TCC (r = −.73, p < .05), FAC (r = −.53, p < .05) and MUFA (r = −.69, p < .05). The TCC was positively correlated with TLC (r = .58, p < .05), FAC (r = .64, p < .05) and SFA (r = .60, p < .05).
4 DISCUSSION
The microalgae (T. subcordiformis, D. zhanjiangensis and Ch. mulleri) used in this study are different types of microalgae commonly used in bivalve hatcheries (Chen et al., 2013). In this study, T. subcordiformis, D. zhanjiangensis and Ch. mulleri represent microalgae containing moderate TCC and high lipids, moderate TCC and low lipids, and high TCC and moderate lipids, respectively. The results of this study revealed that although the TCC of D. zhanjiangensis and T. subcordiform is similar, C. nobilis fed D. zhanjiangensis accumulated significantly higher TCC in the adductor muscle and mantle than that of respective tissues of C. nobilis fed T. subcordiform. The lipid content of D. zhanjiangensis is significantly higher than that of T. subcordiform, suggesting that the high microalgae lipid content could promote the absorption of carotenoids in the C. nobilis tissues. This observation was supported by a strong positive correlation between TCC and lipid content (TLC, FAC and SFA) in the adductor muscle and mantle of the C. nobilis. The findings of this study are consistent with those of Gouda et al. (2006), who reported that the accumulation of carotenoids in sea scallop Placopecten magellanicus is affected by the combination of carotenoid content and other nutrients in the feed. Similar observations have been recorded in humans (Goltz et al., 2012), rats (Mamatha & Baskaran, 2011), mice (Hessel et al., 2007) and salmon (Bjerkeng et al., 1999), in which high lipids, especially unsaturated fatty acids, promote the absorption of carotenoids.
There is no general trend of TLC and FAC accumulation in adductor muscle and mantle of the C. nobilis fed different algae. It is worth mentioning that the TLC and FAC in adductor muscle of scallops fed C. muller (moderate TLC and FAC) were significantly higher than those of C. nobilis fed D. zhanjiangensis with the highest TLC and FAC. Moreover, the TLC of the mantle of C. nobilis fed T. subcordiform (containing the lowest TLC and FAC) was significantly higher than that in C. nobilis fed D. zhanjiangensis and C. muller, where the mantle of scallops fed T. subcordiform appeared to selectively accumulate C16:0 and C18:0. We do not rule out that this complicated observation may be related to lipid transfer between bivalve tissues (Dridi et al., 2017). Since we only analysed the lipid content and fatty acid composition in adductor muscle and mantle, we cannot conclude whether this complicated observation is related to the transfer of lipids from tissue to tissue. In order to improve similar studies in the near future, it is strongly recommended to analyse the lipid content and fatty acid composition in as many tissues as possible.
The sequence of the SCD gene in C. nobilis was cloned and characterized for the first time. The protein encoded by this cDNA has more than 40% identity with vertebrate SCD and possesses all the main characteristic features of membrane-anchored desaturases derived from other species, indicating that these enzymes have conserved functional domains during evolution (Li et al., 2015). These include three highly conserved histidine boxes (HXXHH, HXXXXH and HXXHH) and four transmembrane domains (TMs) located at amino acids 45–67, 71–93, 191–208 and 284–303. Shanklin et al. (1994) revealed that in many living organisms, including mammals, fungi, cyanobacteria, insects and plants, histidine boxes are highly conserved regions of membrane-anchored desaturases. In the study of mutagenesis of rat δ9 desaturase, Fox et al. (1993) demonstrated that eight conserved histidine residues have a catalytic effect on its desaturase function and may be participated in the formation of catalytic sites.
Phylogenetic analysis showed that vertebrate SCD formed subclusters and then clustered with bivalve SCD, implying that bivalve SCD evolved separately from vertebrate SCD. This observation can be explained by the evolutionary history of living organisms. According to Castro et al. (2011), one duplicate SCD gene was lost in the first genome duplication, and the remaining gene formed the SCD1 and SCD5 after the second genome duplication. In teleost fish and some rodents, SCD5 has been replaced by other SCDs (Castro et al., 2011; Evans et al., 2008). Moreover, scallop SCDs formed a distinct group in this study. This finding is consistent with that of Li et al. (2015), suggesting that a single SCD gene found in scallops might represent an ancestral homologue that existed in vertebrates prior to genome duplication.
The relative expression of SCD and SRB-like-3 genes in different tissues of the C. nobilis fed different microalgae diets was analysed using qRT-PCR. The results showed that SCD and SRB-like-3 transcripts were detected in all the tissues analysed, but the expression of SCD and SRB-like-3 genes in C. nobilis is varied in different tissues. In general, the expression of SCD and SRB-like-3 genes in scallop tissues was high in low C16:1n-9 and/or C18:1n-9 t and/or C16:1/C16:0 and/or C18:1/C18:0 and/or (C16:1+ C18:1)/(C16:0+C18:0). Consistent with our results, Minville-Walz et al. (2012) found that C18:1 fatty acids control the expression of SCD1 at the transcriptional level, and the low relative abundance of C18:1n-9-c increases the delta-9 desaturation rate of SCD. Similarly, Henriquez-Rodriguez et al. (2017) demonstrated a clear inverse relationship between SCD expression level and MUFA content, especially C16:1 and C18:1. For the SRB gene, the results of Cardoso et al. (2020) are consistent with our results, in which SRB is inversely correlated with C18:1.
Our results also revealed that the relative expression of SCD gene in the intestine and adductor muscle of C. nobilis was positively associated with the accumulation of TCC, TLC, FAC, SFA (only in adductor muscle) and MUFA (only in adductor muscle). The results of this study are consistent with the findings of Li et al. (2015), who reported that the accumulation of lipids and TCC in adductor muscle of Yesso scallop was associated with increased expression of SCD. Ntambi and Miyazaki (2004) and Henriquez-Rodriguez et al. (2017) also reported a similar positive correlation between SCD expression and carotenoid. Therefore, we speculate that the SCD gene indirectly promoted the accumulation of TCC in adductor muscle of C. nobilis by promoting the accumulation of lipids. However, the opposite trend was observed in the mantle, where the relative expression of the SCD gene was inversely correlated with FAC, SFA and MUFA. It was found that high level of arachidonic acid (C20:4n-6) inhibited the expression levels of SCD mRNA (Nitambi, 1999; Sessler et al., 1996). Therefore, we speculate that the significantly higher C20:4n-6 level in mantle (6.74% ± 0.86%) than that in the adductor muscle (4.17% ± 0.62%) of the C. nobilis suppressed the expression levels of SCD gene, which was supported by a negative correlation recorded between expression levels of SCD and C20:4n-6 level in mantle of C. nobilis. Similar results have been reported in vertebrates, in which SCD expression negatively correlated with C20:4n-6 in rats (Bellinger, 2004) and beef cattle (Oliveira et al., 2014).
For the SRB-like-3 gene, the relative expression of the SRB-like-3 gene was positively correlated with PUFA content of the adductor muscle. This observation is consistent with Spady et al. (1999) and Moslehi et al. (2016) who reported that a diet rich in PUFA up-regulated the expression of SRB-like-3 gene. It is worth noting that, except for intestine, the up-regulation of SRB-like-3 expression was not associated with high TCC. Since the intestine is the main site for carotenoid absorption, we speculate that in C. nobilis, the SRB-like-3 gene only plays a role in carotenoid absorption, rather than the transportation or accumulation of carotenoids. Consistent with our previous study, inhibition of SRB-like-3 reduced TCC absorption in haemolymph, but did not affect the TCC accumulation in the adductor muscle (Liu et al., 2015). Our findings are also consistent with results reported in many other animals, in which SRB genes have been shown to play a role in the absorption of different carotenoids, such as lutein (Reboul et al., 2005), carotene (van Bennekum et al., 2005), zeaxanthin and xanthophylls (During et al., 2008), and lycopene (Moussa et al., 2008).
5 CONCLUSION
In a nutshell, the results of this study revealed that microalgae diets affect the lipid composition and expression of lipid-related genes (SCD and SRB-like-3) in bivalve tissues. In general, a high lipid microalgae diet can promote the accumulation of TCC in C. nobilis. It was also found that the expression of SCD and SRB-like-3 genes in C. nobilis was relatively high under low C16:1n-9 and/or C18:1n-9 t and/or C16:1/C16:0 and/or C18:1/C18:0 conditions. In the intestine and adductor muscle of C. nobilis, the expression of SCD gene was positively correlated with the accumulation of TCC and TLC, suggesting that SCD gene may indirectly promote the accumulation of TCC (lipid soluble) by promoting lipid accumulation. For SRB-like-3 gene, the high expression of SRB-like-3 gene was only associated with the high intestinal TCC, suggesting that SRB-like-3 may be a candidate gene for carotenoid absorption in C. nobilis, but not involved in transport or accumulation of carotenoids. The findings of this study are important to improve understanding of how microalgae diets affect accumulation of TLC and TCC, and expression of SCD and SRB-like-3 genes in bivalves.
ACKNOWLEDGEMENTS
The authors declare no conflicts of interest. Present study was financially supported by the National Key R&D Program of China (2018YFD0901400), National Natural Science Foundation of China (31872563), China Modern Agro-industry Technology Research System (CARS-49).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




