High dietary lipid level decreases the immunity and disease resistance of abalone Haliotis discus hannai and affects the perilipin-2/TLR4, JNK and Keap1/Nrf2 pathways
Yanlin Guo and Dong Huang are contributed equally to this work.
Abstract
A 100-day feeding trial was conducted to investigate the influences of dietary lipid levels on the immunity and mechanism in abalone Haliotis discus hannai Ino. Abalones (initial weight: 10.98 ± 0.05 g) were fed with graded levels of dietary lipid, which were 15.73, 23.41, 31.72, 38.25, 46.35, 55.63, 61.70 and 67.19 g/kg. Results showed that compared with the treatment with 38.25 g/kg of dietary lipid, 67.19 g/kg of dietary lipid increased the cumulative mortality of abalone after a challenge with Vibrio parahaemolyticus. Meanwhile, 67.19 g/kg of dietary lipid decreased the activities of lysozyme (LZ) and acid phosphatase (ACP) in serum, and the mRNA levels of β-defensin and mytimacin 6 in hepatopancreas of abalone. The mRNA levels of tumour necrosis factor α (tnfα), activator protein 1 (ap-1), nuclear factor κB (nf-κb), interleukin 1 receptor-associated kinase-4 (irak4), myeloid differentiation primary response protein MyD88 (myd88), toll-like receptor 2 (tlr2), tlr4 and perilipin-2, and the protein level of interleukin-1β (IL-1β), TNFα, NF-κBp65, AP-1, MyD88 and Perilipin-2 in hepatopancreas were up-regulated by 67.19 g/kg of dietary lipid. However, the mRNA levels of arginase and inhibitor of κBα (iκbα) were down-regulated. Besides, 67.19 g/kg of dietary lipid up-regulated the mRNA levels of executor apoptosis-related cysteine peptidase 3 (caspase-3), caspase-7 and B-cell lymphoma protein-2-associated X protein (bax), and the protein levels of caspase-3 and c-Jun N-terminal kinases (JNK) in hepatopancreas. Moreover, high dietary lipid level down-regulated the mRNA levels of copper/zinc superoxide dismutase (cuznsod), manganese superoxide dismutase (mnsod), catalase (cat), glutathione peroxidase (gpx), glutathione-S-transferase (gst) and NF-E2-related factor 2 (nrf2), while up-regulated the mRNA and protein levels of Kelch-like ECH-associated protein 1 (Keap1) in hepatopancreas. It was concluded that a high level (67.19 g/kg) of dietary lipid decreased the immunity of abalone, which might be related to the aggravated inflammation and apoptosis, and decreased anti-oxidative capacity under Perilipin-2/TLRs/MyD88/IRAK4/(NF-κB or AP-1), JNK/Bcl-2/Bax and Keap1/Nrf2 signalling.
1 INTRODUCTION
In recent years, with the rapid development of aquaculture industry, highly intensive culture is an inevitable choice to increase the output of aquatic product. However, higher culture density usually leads to decreased growth performance and immunity, elevated susceptibility to bacterial infection, higher mortality and so on (Castillo-Vargasmachuca et al., 2012). Lipid, as an essential nutrient, contributes to improving growth performance and immunity of aquatic animals (Lin & Shiau, 2003; Mai et al., 1995; Xie et al., 2019). High-lipid content diets have been increasingly used in aquaculture because of its protein-sparing effects (Jannathulla et al., 2019; Yigit et al., 2002), which not only reduced nitrogen waste but also decreased feed cost. Nevertheless, a high levels of dietary lipid resulted in anomalous lipid deposition and metabolic disturbance in body, which is frequently accompanied by decreased immunity in fish and shrimp (Jia et al., 2020; Li et al., 2020; Yuan et al., 2016). Hence, understanding how high-lipid level influences the immunity and possible signalling is vital for healthy culture of aquatic animals.
Immunity of aquatic animals is tightly related to inflammation (Guardiola et al., 2014; Uribe et al., 2011). Toll-like receptors (TLRs) were proved to play important roles in the regulation of inflammation (Rebl et al., 2010). According to a previous studies in fish (Rauta et al., 2014; Shan et al., 2018), TLRs triggered cell signalling cascades through myeloid differentiation primary response protein MyD88 (MyD88)-interleukin 1 receptor-associated kinase-4 (IRAK4) complex, induced the subsequent nuclear localization of nuclear factor κB (NF-κB) and activator protein 1 (AP-1) and finally induced the expression of pro-inflammatory cytokines such as tumour necrosis factor (TNF-α) and interleukin 1 (IL-1). Perilipin-2 is a lipid droplet associated protein, which featured in the regulation of metabolism (Imai et al., 2012). In recent years, it was found that Perilipin-2 also played important roles in the regulation of inflammation in mammals (McManaman et al., 2013; Norman et al., 2018). Hence, Perilipin-2 has drawn more attention because of its potential role in inflammation of aquatic animals.
Apoptosis and anti-oxidation also influence the immunity of aquatic animals (García-Valtanen et al., 2017; Guo et al., 2017; Hoole et al., 2003). It has been reported that c-Jun N-terminal kinases (JNK) could induce the phosphorylation of B-cell lymphoma-2 (Bcl-2) and B-cell lymphoma protein-2-associated X protein (Bax) and then stimulate the release cytochrome C from the mitochondrial inner membrane (Guo, Chen, et al., 2020). This series of celling signalling cascade finally leads to the activation of the executor apoptosis-related cysteine peptidase (caspases), such as caspase-3 and caspase-7 (Dhanasekaran & Reddy, 2008, 2017), which induce apoptosis. Besides, a previous study has demonstrated the important roles of NF-E2-related factor 2 (Nrf2) in anti-oxidative process (Sayin et al., 2017). After degradation of the binding protein Kelch-like ECH-associated protein 1 (Keap1), Nrf2 will accumulate, translocate to the nucleus and induce transcription of anti-oxidative enzymes (Stewart et al., 2011; Sykiotis & Bohmann, 2008).
A previous study indicated that high dietary lipid level decreased the growth performance and increased the lipid deposition in abalone Haliotis discus hannai (Guo, Huang, et al., 2020). However, the influences of high-lipid level on disease resistance, inflammation, apoptosis and anti-oxidation, and the underlying regulation mechanisms in abalone are still unclear. The present study aims at investigating the effects of high dietary lipid level on the inflammation, apoptosis and oxidative damages in abalone. It provides basal data to improve the theory of lipid nutrition and immunity, and guidance for healthy culture of abalone.
2 MATERIALS AND METHODS
All animal care and handling procedures in present study were approved by the Animal Care Committee of Ocean University of China.
2.1 Experimental diets
Ingredients and proximate compositions as well as the fatty acid compositions of the experimental diets are shown in Tables S1 and S2 (Guo, Huang, et al., 2020). Eight experimental diets with graded lipid levels (15.73, 23.41, 31.72, 38.25, 46.35, 55.63, 61.70 and 67.19 g/kg) were used. All ingredients were mixed using an electronic mixer and then made into 9 cm2 flakes through a tablet compressing machine. After dried, the flakes were stored in the sealed bag at −20℃ until use.
2.2 Feeding trial
Abalones were purchased from a fishery company in Fuzhou, China. Prior to the feeding trial, abalones were acclimatized to the experimental environment in the natural sea area in Pingtan County, Fuzhou, China, for two weeks. Then, a total of 1440 abalones (initial body weight: 10.98 ± 0.05 g) were randomly assigned to 24 sea cages (three replicates per treatment and sixty abalones per replicate). Each diet was randomly fed to triplicated cages once every two days. Uneaten diets and faeces were removed before the next feeding. During the 100-day feeding trial (from October 2018 to January 2019), the water temperature was 23.39 ± 4.85℃, the pH value was 8.04 ± 0.14, and the dissolved oxygen was 6.76 ± 0.93 mg/L.
2.3 Sample collection
At the end of the feeding trial, all abalones were not fed for 72 h. After that, abalones were removed from cags and anaesthetized by 5% ethyl alcohol thereafter. Cut open the foot muscle of abalone along the middle from the anterior, and then cut the cephalic arterial sinus. After that, serum was collected and immediately frozen in liquid nitrogen. Then, the hepatopancreas of abalone was collected, washed with cold saline (0.86% NaCl) and finally frozen in liquid nitrogen.
2.4 Challenge test
After the feeding trial, due to the site condition limitation, 4 groups of abalone representing lower (15.73 g/kg), middle (31.72 and 38.25 g/kg) and higher (67.19 g/kg) dietary lipid levels, respectively, were used for a challenge test with Vibrio parahaemolyticus infection in a static aquaculture system. The V. parahaemolyticus was provided by the Laboratory of Aquatic Animal Diseases and Immunology of Ocean University of China. Twelve abalones were randomly collected from each cage and used as one replicate (each treatment had three replicates). And then, abalones were intramuscularly injected with 100 μl V. parahaemolyticus [(concentration of 1.0 × 108 colony-forming units (CFU)/ml]. The injected bacterial concentration was a lethal dose 50 (LD50) according to the pretest. The challenge test lasted for 6 days. During the challenge test, the abalone were not fed and mortalities were recorded twice daily at 8:00 and 18:00.
2.5 Serum parameters
The activities of lysozyme (LZ), acid phosphatase (ACP), superoxide dismutase (SOD) and catalase (CAT), and concentrations of malondialdehyde (MDA) and glutathione (GSH) in 6 serum samples per treatment were determined using the commercial assay kit (Nanjing Jiancheng Bioengineering Institute, China).
2.6 Real-time polymerase chain reaction (PCR) analysis
Total 6 RNA samples per treatment were isolated from the hepatopancreas using RNAiso Plus Kit (9109, Takara Biotech), and the RNA was dissolved in DEPC treated water. Then, the quality of RNA was assessed using agarose gel electrophoresis. Single-stranded cDNA was prepared from total RNA by reverse transcription using a PrimeScript RT reagent Kit (RR047A; Takara Biotech). PCR specific primers are listed in Table 1. Real-time PCR assays were carried out in a quantitative thermal cycler (Quant Studio 5, Applied Biosystems). The final reaction mixture contained 15 μl containing 7.5 μl 2× SYBR Green Realtime PCR Master Mix (Q711-02, Vazyme Biotech), 0.3 μl each of primers (10 μmol/L) and 1 μl of cDNA mix. β-actin was used as an endogenous reference to normalize the template amount. Real-time PCR temperature profile was 95℃ for 30 s followed by 40 cycles of 10 s at 95℃ and 30 s at 58℃. To confirm the specificity and purity of all PCR products, melt curve analysis was carried out after amplification: 95℃ for 15 s, 60℃ for 1 min and 95℃ for 1 s. Results of gene expression were analysed using the 2−ΔΔCT method according to Livak and Schmittgen (2001). Normalized gene expression for the 1.57% lipid diet group was set at 1.
| Target gene | Primer sequence forward (5′→3′) | Primer sequence reverse (5′→3′) | Accession number |
|---|---|---|---|
| β-defensin | CGTCACCTGCGACCTGCTG | CAGAATCACCGAAGGAGTTGCC | FJ864724.1 |
| mytimacin 6 | ACAATGTTCCTCGCTATCGTCGT | TCATCCAGCAGTCAGAAGCATCC | MF066909.1 |
| tnfα | TGGACTCAATGGAACGACAGG | GCCCTTCTCATACCGCATCC | EU863217.1 |
| arginase 1 | CGTACTACATAGCTGAGGAGGTG | CCATAGAAACGGGACATGACTT | MT919298 |
| ap-1 | TGTGTCGTCTGCTTCACGTT | TGTGGCTCCTCTTTCAACCG | GU186850.1 |
| nf-κb | TGCTGGTTGTATGTGGGAGGAC | GAGGTCTCGTTGTCGGATGGTC | GQ903763.1 |
| iκbα | GGAGGGACAGAGGGAGGATT | GACCCGTCCACCATGTCATT | JX235971.1 |
| ikkα | ACATGTCCAGACGCCCAA | CCAAGACCAACAAGCACAC | MT919293 |
| irak4 | TGCAACTGCCAGATTAGCACCT | TGACGTGCCGATCACAACCA | KU351646.1 |
| myd88 | TTGCCCTACACAATGCGAGT | CCTCCGGTCAATGATACGTTC | KU351389.1 |
| tlr2 | CCTGGAGCAAATCATTCCCA | ATTGCCAAGATCTAAGCTTCGT | MT919299 |
| tlr4 | GGACGCCGACATCAGACACAT | CAGCCACTTCAGAGACGACAGAG | MT919300 |
| prilipin-2 | ACCGACAAAGTCTTATGGCAC | TCCATGCCAGGTTCACCA | MT919297 |
| caspase-3 | AAATTCTTGAAACCGCAGACAC | ACCAACAAGTGCGATACAGT | FJ864720.1 |
| caspase-7 | TGAAGAAATACAGCACGACCA | TACTACGGCCATCAATCTGGG | MT919294 |
| bcl-2 | TGGTCGCTTTGTTTACTTTCGG | AGGGTTCTCGTTTCGCTTCTC | MT482025 |
| bax | CGGAGCACATTTCTCAACGTA | AAGTAAAAGAGGGCAACCAC | MT501464 |
| cuznsod | CTTCAACCCCTTTCGGCAAG | ACCAGCATGTCTGTTTTCGT | DQ530214.1 |
| mnsod | ATTTCACTCCAGCCATCCCT | ACCATTTGGGCTAAGCACCT | DQ821491 |
| cat | GGAACCCTTTCGACCTCACC | ATCTGTTTCCACGTCAGCAA | DQ821496.1 |
| gpx | TTTCGACCGTCCAACTTGCT | CAAAGTTCCAGTACACGTCCC | GU254066.1 |
| gst | CAAAGTTCCAGTACACGTCCC | TCCGTTGTTGTCGCCTCC | DQ530212.1 |
| nrf2 | CGACAGCAGCAGCAGCAATG | GGTGAGGACATCTGGAGGCATC | MT919295 |
| keap1 | CGACCATGTCCACTAACCGTATTG | TAGCAGTCCATAGCGATAACACCAG | MT919296 |
| β-actin | GGCCTCTCTGTCAACCTTCC | CGGACTCATCGTACTCCTGC | AY380809.1 |
- Abbreviations: ap-1, transcription factor AP-1; bax, B-cell lymphoma protein-2-associated X protein; bcl-2, B-cell lymphoma-2; caspase, apoptosis-related cysteine peptidase; cat, catalase; cuznsod, Cu/Zn superoxide dismutase; gpx, glutathione peroxidase; gst, glutathione-S-transferase; irak4, interleukin 1 receptor-associated kinase-4; iκbα, inhibitor of κBα; keap1, Kelch-like ECH-associated protein 1; mnsod, Mn superoxide dismutase; myd88, myeloid differentiation primary response protein MyD88; nf-κb, nuclear factor κB; nrf2, NF-E2-related factor 2; tlr, toll-like receptor; tnfα, tumour necrosis factor.
2.7 Western blot analysis
Protein homogenates sample (3 hepatopancreas per treatment) preparation, antibodies and western blotting were processed as refer to previous studies (Guo, Huang, et al., 2020). A Thermo Fisher assay kit (78833; Thermo Fisher Scientific) was used for the extraction of nuclear protein. Protein concentrations were determined by a Bradford assay kit (P0006, Beyotime Biotechnology). Protein samples (30 µg per lane) were separated by SDS-PAGE and transferred to a PVDF membrane for western analysis. Then, the PVDF membrane was blocked for 50 min using 5% non-fat dry milk–TBST at room temperature and then incubated with primary antibody in 1% non-fat dry milk–TBST overnight at 4℃. GAPDH (AB-P-R001, 1:500, Goodhere Biotechnology) was used as a control protein for IL-1β (WL00891, 1:500, Wanleibio), TNFα (WL01581, 1:500, Wanleibio), MyD88 (WL02494, 1:500, Wanleibio), perilipin-2 (15295-1-AP, 1:500, Proteintech), caspase-3 (WL02117, 1:500, Wanleibio), JNK (WL01295, 1:500, Wanleibio) and Keap1 (WL03285, 1:1500, Wanleibio), while nuclear Lamin B1 (WL01775, 1:500, Wanleibio) was used as a control protein for Nuclear NF-κBp65 (#8242, 1:1000, Cell Signaling Technology) and Nuclear AP-1 (#9165, 1:1000, Cell Signaling Technology). After being washed, the PVDF membrane was incubated for 1 h with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (sc-2004, 1:5000, Santa Cruz Biotechnology) in 1% non-fat dry milk–TBST. The immune complexes were visualized using ECL reagents (P0018FM, Beyotime Biotechnology). The western bands were quantified using NIH Image 1.63 software. Different treatments were expressed relative to the level of control group. This experiment was repeated at least three times, and similar results were obtained each time.
2.8 Statistical analysis
Statistical analysis was performed using SPSS 25.0 (SPSS) and Microsoft Excel 2010. The results are represented with the means ± SD. Normality and homoscedasticity assumptions were confirmed before statistical analysis. All evaluated variables were subjected to an ANOVA to determine whether dietary lipid levels significantly (p < .05) affected the observed responses. When significant differences (p < .05) were found, means were compared using Tukey's test.
3 RESULTS
3.1 Cumulative mortality after Vibrio parahaemolyticus infection
As shown in Figure 1, on the sixth day after infection, the cumulative mortality of abalone in the treatment with 67.19 g/kg of dietary lipid was significantly higher than that in the group with 38.25 g/kg of dietary lipid (p < .05). There were no significant differences in the cumulative mortalities among the treatments with 15.73, 31.72 and 38.25 g/kg of dietary lipid.
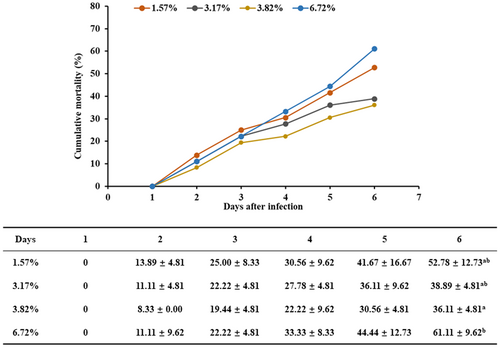
3.2 Serum biochemical index
As shown in Table 2, LZ and ACP activities increased with dietary lipid level up to 46.35 g/kg and then decreased. MDA concentrations in the treatments with 61.70 and 67.19 g/kg of dietary lipid were significantly higher than other groups (p < .05), and the highest MDA concentration was observed in the treatment with 61.70 g/kg of dietary lipid (p < .05). Activities of SOD in the treatments with 55.63, 61.70 and 67.19 g/kg of dietary lipid were significantly lower than other groups (p < .05), and the lowest activity was observed in the group with 67.19 g/kg of dietary lipid (p < .05). Activity of CAT and concentration of GSH increased with dietary lipid level up to 31.72 g/kg and then decreased.
| Dietary lipid levels (g/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 15.73 | 23.41 | 31.72 | 38.25 | 46.35 | 55.63 | 61.70 | 67.19 | |
| LZ (U/ml) | 250.56 ± 11.12ab | 262.44 ± 11.92ab | 266.73 ± 13.75b | 259.32 ± 22.73ab | 268.51 ± 22.32b | 256.66 ± 4.74ab | 238.95 ± 6.43a | 236.44 ± 8.49a |
| ACP (U/ml) | 3.48 ± 0.23b | 3.67 ± 0.21b | 3.95 ± 0.34bc | 3.86 ± 0.13bc | 4.74 ± 0.30d | 4.30 ± 0.37cd | 3.15 ± 0.16b | 2.59 ± 0.19a |
| MDA (nmol/ml) | 0.48 ± 0.04a | 0.48 ± 0.03a | 0.45 ± 0.01a | 0.46 ± 0.03a | 0.46 ± 0.03a | 0.44 ± 0.02a | 1.87 ± 0.13c | 1.74 ± 0.07b |
| SOD (U/ml) | 141.09 ± 10.75b | 140.53 ± 10.30bc | 140.50 ± 11.13bc | 141.44 ± 3.63b | 141.12 ± 6.94b | 121.58 ± 10.82a | 122.49 ± 6.40a | 123.95 ± 10.86ab |
| CAT (U/ml) | 5.63 ± 0.28c | 5.61 ± 0.43c | 6.68 ± 0.18d | 6.58 ± 0.18d | 3.95 ± 0.16b | 3.81 ± 0.25b | 3.23 ± 0.18a | 3.34 ± 0.22a |
| GSH (μmol/L) | 50.69 ± 4.29bc | 47.88 ± 3.37b | 58.39 ± 4.79d | 54.99 ± 2.45cd | 45.12 ± 1.87b | 35.58 ± 2.33a | 30.84 ± 1.95a | 32.46 ± 1.80a |
Note
- Means in the same row sharing with different superscript letters were significantly different (p < .05) as determined by Tukey's test.
- Abbreviations: ACP, acid phosphatase; GSH, glutathione; LZ, lysozyme; MDA, malondialdehyde.
3.3 Antibacterial peptides and inflammation related parameters
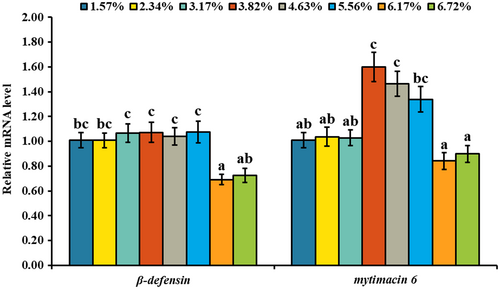
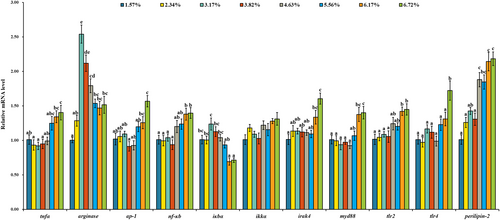
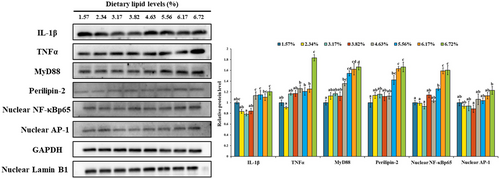
As shown in Figure 2, there was no significant change of β-defensin mRNA level with dietary lipid level increasing from 15.73 to 55.63 g/kg, but it was down-regulated with the further increase of dietary lipid level. The mRNA level of mytimacin 6 was up-regulated with dietary lipid level up to 38.25 g/kg and then down-regulated. Compared with the treatment of 38.25 g/kg dietary lipid, 67.19 g/kg dietary lipid level significantly down-regulated the mRNA levels of β-defensin and mytimacin 6 (p < .05). As shown in Figure 3, there were no significant change of tnfα, nf-κb, irak4, myd88, tlr2, tlr4 and perilipin-2 mRNA levels with dietary lipid up to a certain level and then up-regulated. Compared with the treatment of 38.25 g/kg dietary lipid, 67.19 g/kg dietary lipid level significantly up-regulated the mRNA levels of tnfα, nf-κb, irak4, myd88, tlr2, tlr4 and perilipin-2 (p < .05), while their mRNA levels in the treatment of 15.73 g/kg dietary lipid were not significantly different with 38.25 g/kg dietary lipid. The mRNA levels of arginase and inhibitor of κBα (iκbα) were up-regulated with dietary lipid level up to 38.25 g/kg, and then down-regulated gradually. With dietary lipid increasing from 15.73 to 38.25 g/kg, the mRNA level of ap-1 was down-regulated and then up-regulated with the continuous increase of dietary lipid level. Compared with the treatments of 15.73 and 38.25 g/kg dietary lipid, the mRNA level of ap-1 in the group of 67.19 g/kg dietary lipid was significantly up-regulated (p < .05). However, the mRNA level of ikkα was independent of dietary lipid level. As shown in Figure 4, with the increase of dietary lipid level, protein of IL-1β, TNFα, MyD88, Perilipin-2, NF-κB and AP-1 was gradually up-regulated. Compared with the treatments of 15.73 and 38.25 g/kg dietary lipid, the protein level of IL-1β, TNFα, MyD88, Perilipin-2, NF-κB and AP-1 was significantly up-regulated by the treatment of 67.19 g/kg dietary lipid (p < .05).
3.4 Apoptosis-related parameters
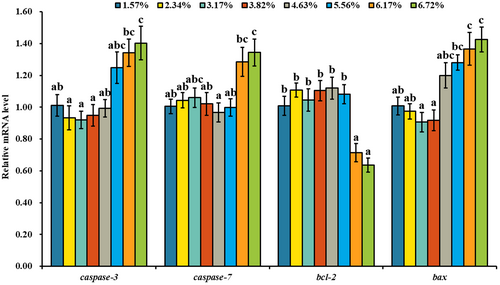
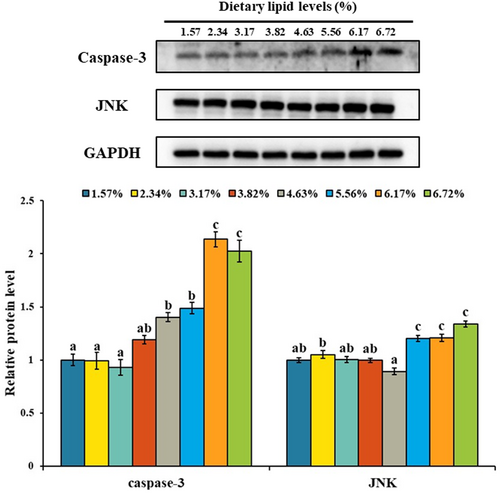
As shown in Figure 5, there was no significant change of caspase-3, caspase-7 and bax mRNA levels with dietary lipid up to a certain level and then up-regulated. The highest mRNA levels of caspase-3, caspase-7 and bax were observed in the treatment of 67.19 g/kg dietary lipid (p < .05), and the lowest mRNA level of caspase-3, caspase-7 and bax was observed in the groups of 31.72, 46.35 and 31.72 g/kg dietary lipid (p < .05). The mRNA level of bcl-2 in the treatments of 61.70 and 67.19 g/kg dietary lipid was significantly lower than other treatments (p < .05). As shown in Figure 6, the protein level of caspase-3 was gradually up-regulated with the elevation of dietary lipid level. And compared with the treatments of 15.73 and 38.25 g/kg dietary lipid, 67.19 g/kg dietary lipid level significantly up-regulated the protein level of caspase-3 (p < .05). The protein level of JNK in the treatments of 55.63, 61.70 and 67.19 g/kg dietary lipid was significantly higher than other treatments (p < .05).
3.5 Anti-oxidation related parameters
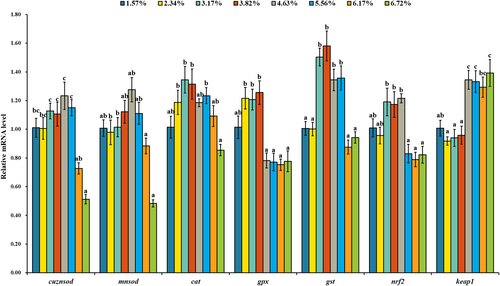
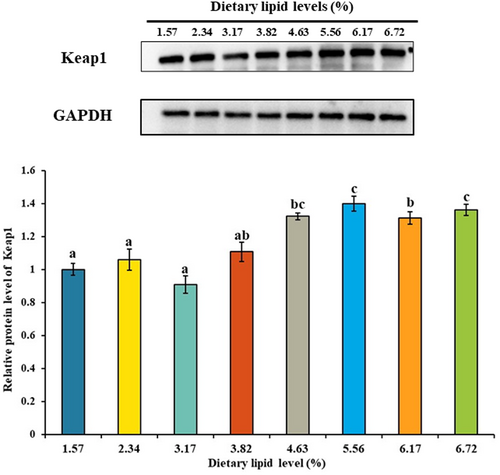
As shown in Figure 7, with dietary lipid level up to 46.35, 46.35, 31.72, 38.25, 38.25 and 46.35 g/kg, the mRNA levels of copper/zinc superoxide dismutase (cuznsod), manganese superoxide dismutase (mnsod), cat, glutathione peroxidase (gpx), glutathione-S-transferase (gst) and nrf2 were gradually up-regulated and down-regulated thereafter. With dietary lipid level increasing from 15.73 to 38.25 g/kg, the mRNA level of keap1 showed no significant change, and with the further increase of dietary lipid level, the mRNA level of keap1 was up-regulated. The highest mRNA level of keap1 was observed in the treatment of 67.19 g/kg dietary lipid (p < .05), and the lowest mRNA level was observed in the treatment of 23.41 g/kg dietary lipid (p < .05). As shown in Figure 8, the protein level of Keap1 was gradually up-regulated with the increase of dietary lipid level. The highest protein level of Keap1 was observed in the treatment of 55.63 g/kg dietary lipid (p < .05), and the lowest protein level was observed in the group of 31.72 g/kg dietary lipid (p < .05).
4 DISCUSSION
Generally, normal immunity is required for increasing the growth performance of aquatic animals (Van Nguyen et al., 2019). Our previous study demonstrated that compared with the optimal dietary lipid level (38.25 g/kg), the higher dietary lipid level (67.19 g/kg) decreased the weight gain and daily increment in shell length of abalone (Guo, Huang, et al., 2020). A similar result was found in the previous study (Lee et al., 2016), in which diet with high-lipid level decreased the weight gain of abalone Haliotis discus (Reeve, 1846). In addition, immunity could be reflected by disease resistance of fish and shrimp (Magnadottir, 2010; Miao et al., 2017). It was demonstrated that V. parahaemolyticus causes outbreaks of vibriosis associated with warm water, leading to decreased immunity of abalone (Cai et al., 2006; Lu et al., 2017; Travers et al., 2008). In the present study, compared with optimal dietary lipid level (38.25 g/kg), high dietary lipid level (67.19 g/kg) increased cumulative mortality of abalone after V. parahaemolyticus infection. All the above-mentioned data indicated that a high level of dietary lipid decreased the immunity of abalone.
Abalone lacks an adaptive immune response (Xue et al., 2008), and this makes the innate immune responses more important in defending against bacterial infection. LZ and ACP are also important components of innate immune responses (Ivanina et al., 2014; Uribe et al., 2011). Besides, antibacterial peptides, such as β-defensin and mytimacin 6, are all important components of innate immune responses which is essential in defending against bacteria, fungi and enveloped viruses (Hoar et al., 1997; Suzuki & Iida, 1992; Wang, Zhang, et al., 2015). In the present study, compared with the 38.25 g/kg of dietary lipid level, the higher dietary lipid level (67.19 g/kg) decreased the activities of LZ and ACP in serum and down-regulated the mRNA levels of β-defensin and mytimacin 6 in the hepatopancreas of abalone. Similar results were found in the serum of Nile tilapia Oreochromis niloticus (Lim et al., 2009) and serum of Chinese mitten crab Eriocheir sinensis (Wang, Chen, et al., 2015). These data indicate that high-lipid level impaired immunity by suppressing the innate immune responses of abalone.
A previous study has pointed out the important roles of inflammation in immunity of fish (Wu et al., 2016). IL-1β and TNFα are pro-inflammatory cytokines, and up-regulation of their gene expression is frequently related to triggered inflammation in fish (Zuo et al., 2013). In addition, NF-κB is a transcription factor, which could transfer into the nuclear localization accompanied by the degradation of IκBα through IKK complex, and the nuclear localization of NF-κB could up-regulate pro-inflammatory cytokine (such as IL-1β) expression in mammals (Liu et al., 2017). Besides, arginase was proven to play important roles in the anti-inflammatory process, which could be down-regulated by transcription factor AP-1 in mammals (Hannemann et al., 2017; Monticelli et al., 2016). In the present study, compared with 38.25 g/kg of dietary lipid level, the higher dietary lipid level (67.19 g/kg) up-regulated the mRNA levels of tnfα, nf-κb and ap-1, and the protein level of IL-1β, TNFα, nuclear NF-κBp65 and nuclear AP-1. Meanwhile, it down-regulated the mRNA levels of arginase and iκbα in hepatopancreas of abalone. It has been reported that a high-fat diet (103 g/kg) up-regulated the mRNA levels of TNFα and NF-κB and down-regulated the mRNA level of IκBα in brain of blunt snout bream Megalobrama amblycephala (Dai et al., 2018). Wang et al. (2016) reported that high dietary lipid level (180 g/kg) up-regulated the protein level of nuclear NF-κB and AP-1 in the liver of large yellow croaker Larmichthys crocea. These data indicate that compared with the 38.25 g/kg of dietary lipid level, the higher dietary lipid level (67.19 g/kg) induced inflammation in hepatopancreas of abalone, which might be related to NF-κB and AP-1 signalling. However, the higher dietary lipid level (67.19 g/kg) had no significant influence on the mRNA level of IKKα. The possible reason might be related to the different functions of the three catalytic subunits of the IKK complex (i.e. IKKα, IKKβ and IKKγ). IKKβ could activate the canonical pathway through NF-κBp65, whereas IKKα could activate the non-canonical pathway NF-κBp52 in humans (Bollrath & Greten, 2009). In the present study, it is hypothesized that high dietary lipid level (67.19 g/kg) activated the canonical pathway rather than the non-canonical pathway. Further investigation is needed.
In addition, TLRs which expressed in antigen presenting cells serve as key pattern recognition receptors with central roles in induction of inflammatory cytokines (Kawai & Akira, 2007). The activation of TLRs could recruit MyD88 and IRAK4 and then activate TAK1 pathway, and finally induce the nuclear translocation of NF-κB and AP-1 (Kawai & Akira, 2007; Korneev et al., 2017). Moreover, Perilipin-2, a ubiquitously expressed CLD-associated protein that regulates neutral lipid storage and metabolism in eukaryotic cells, has drawn attention because of its important roles in the regulation of inflammation. In a study of mice, the deletion of Perilipin-2 down-regulated the hepatic mRNA levels of TLR2 and TLR4 (Orlicky et al., 2019). Compared with the 38.25 g/kg of dietary lipid level, in the present study, the higher dietary lipid level (67.19 g/kg) up-regulated the mRNA levels of irak4, myd88, tlr2, tlr4 and perilipin-2, and the protein level of MyD88 and Perilipin-2 in the hepatopancreas of abalone. Similar results were found in a previous study. Jia et al. (2020) demonstrated that high-fat (210 g/kg) diet up-regulated the mRNA levels of TLR2 and MyD88 in liver of tilapia after 90 days of feeding. Arias-Jayo et al. (2018) pointed out that a high-fat diet up-regulated the mRNA levels of MyD88, TLR2 and TLR4 in intestine of zebrafish Danio rerio. The study of Libby et al. (2016) indicated that high-lipid level (210 g/kg) up-regulated hepatic gene expression of Perilipin-2 in mice. In addition, a high level of n-6 polyunsaturated fatty acids (PUFAs) in the present diet might be also related to the aggravated inflammation. It has been reported that in liver of large yellow croaker, a high level of n-6 PUFAs (375.2 g/kg) up-regulated the mRNA levels of TNFα, IL-1β and MyD88. All the data above indicated that compared with 38.25 g/kg of dietary lipid level, high dietary lipid level induced inflammation in hepatopancreas of abalone, which might be related to Perilipin-2/(TLR2 or TLR4)/MyD88/IRAK4/(NF-κB or AP-1) signalling.
Apoptosis and anti-oxidation are also crucial for the immune of aquatic animals (García-Valtanen et al., 2017; Guo et al., 2017; Hoole et al., 2003). In regard to apoptosis, caspase-3 and caspase-7 are executioners, and their up-regulation aggravates apoptosis (Cohen, 1997; Vince et al., 2018). It has been reported that expression of caspase-3 and caspase-7 could be down-regulated by anti-apoptotic protein Bcl-2 and up-regulated by pro-apoptotic protein Bax in human (Fan et al., 2005). Besides, in PC-12 cells, JNK activation could down-regulate the expression of Bcl-2 and up-regulate the expression of Bax (Lu, Miao, et al., 2017). Compared with the 38.25 g/kg of dietary lipid level, in the present study, the higher dietary lipid level (67.19 g/kg) up-regulated the mRNA levels of caspase-3, caspase-7, bax and the protein level of caspase-3 and JNK and down-regulated the mRNA levels of bcl-2 in the hepatopancreas of abalone. Similar results were found that high-fat (103 g/kg) diets up-regulated the mRNA levels of caspase-3 and Bax in blunt snout bream (Dai et al., 2019). Jia et al. (2020) reported that high-fat (210 g/kg) diet up-regulated the mRNA level of JNK in the liver of tilapia. These data indicate that the impaired immunity of abalone could be caused by aggravated apoptosis, which related to JNK/(Bcl-2 and Bax)/caspase signalling.
In fish, increased lipid deposition could increase the degree of lipid peroxidation and increased MDA concentrations are commonly considered an indicator of lipid peroxidation caused by oxidative stress (Garcia et al., 2020; Xie et al., 2021). The anti-oxidative enzymes (AOEs) play important roles in protecting fish from stress (Mu et al., 2020). CuZnSOD, MnSOD, CAT, GPx and GST are all important anti-oxidative enzymes (Klein et al., 2017; Zhang et al., 2019). In addition, Keap1/Nrf2 signalling is vital in the regulation of anti-oxidative enzymes. After the dissociation of Nrf2 from Keap1, Nrf2 translocated into the nucleus and induce the transcription of AOEs (Hashimoto, 2018). Results of the present study showed that compared with the 38.25 g/kg of dietary lipid level, the higher dietary lipid level (67.19 g/kg) increased the concentration of MDA and decreased the activities of SOD and CAT, and concentration of GSH in serum of abalone. Besides, the higher dietary lipid level (67.19 g/kg) down-regulated the mRNA levels of cuznsod, mnsod, cat, gpx, gst and nrf2 and up-regulated the mRNA and protein levels of Keap1 in hepatopancreas of abalone. Zhang et al. (2013) reported that high dietary lipid level (140 g/kg) decreased activity of GPx in hepatopancreas of Pacific white shrimp Litopenaeus vannamei. Ni et al. (2016) confirmed that excessive level of dietary lipid (80.1 g/kg) down-regulated the mRNA levels of CuZnSOD, MnSOD, GPx and Nrf2 and up-regulated the mRNA levels of Keap1 in head kidney and spleen of grass carp. Besides, a high level of n-3 PUFAs in the present diet might be also related to the decreased anti-oxidative capacity of abalone. In the study of white shrimp L. vannamei, a high level of n-3 PUFAs (34.4 g/kg) decreased activities of phenoloxidase and SOD in gill (Yang et al., 2019). These data indicate that impaired immunity of abalone could be caused by decreased anti-oxidative capacity, which related to Keap1/Nrf2/AOEs signalling.
5 CONCLUSIONS
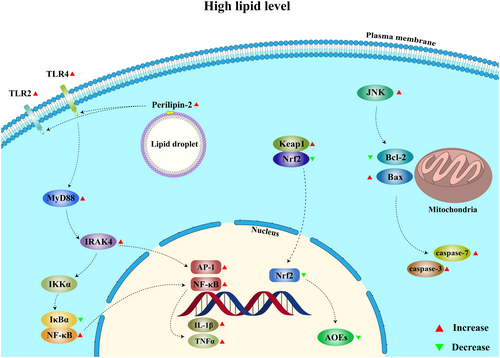
Results from the present study showed that high dietary lipid level (67.19 g/kg) impaired the immunity and increased the cumulative mortality of abalone H. discus hannai (initial weight: 10.98 ± 0.05 g) after a challenge test with V. parahaemolyticus. The aggravated inflammation and apoptosis, and decreased anti-oxidative capacity induced by the high dietary lipid level were regulated by the signalling pathways including the Perilipin-2/TLRs/MyD88/IRAK4/(NF-κB or AP-1), JNK/Bcl-2/Bax and Keap1/Nrf2 (Figure 9).
ACKNOWLEDGEMENT
This study was financially supported by the National Key R & D Program of China (2018YFD0900400) and the China Agriculture Research System of MOF and MARA.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




