Effects of dietary manganese supplementation on growth performance, antioxidant capacity, immune function and intestinal microbiota in Pacific white shrimp Litopenaeus vannamei
Lefei Jiao and Tianmeng Dai are co-first authors and contributed equally to this study.
Abstract
Water manganese (Mn) is apparently insufficient to meet aquatic animals’ metabolic requirement, and their food is considered as the major Mn source. Currently, researches reporting the effects of dietary Mn inclusion on physiological and metabolic function in Litopenaeus vannamei (L. vannamei) are very scarce. Therefore, 8-week feeding trail was conducted to determine dietary Mn requirement for L. vannamei and further evaluate the effects of dietary Mn supplementation on tissue Mn concentration, antioxidant enzyme activity, nonspecific immune enzymes activity and intestinal microbiota composition. Our results demonstrated that the optimum dietary Mn requirement for L. vannamei was determined to be 44.83 mg/kg based on the per cent weight gain; supplementing 39.57 mg/kg, 54.57 mg/kg and 72.67 mg/kg Mn significantly increased (p < .05) the activity of serum lysozyme (LZM), nitric oxide synthase and alkaline phosphatase, the activity of antioxidative capacity and glutathione in serum and hepatopancreas of L. vannamei. Supplementing 111.97 mg/kg Mn significantly increased (p < .05) hepatopancreas Mn concentration and serum LZM activity. Intestinal microbiota analysis showed that supplementing 39.57 mg/kg and 111.97 mg/kg Mn significantly influenced (p < .05) intestinal microbiota composition and function. These findings might help provide a scientific basis for the commercial application of Mn in L. vannamei.
1 INTRODUCTION
Manganese (Mn) is an essential micronutrient for the living organisms to maintain normal physiological and metabolic function, consisting of growth, reproduction, immunity, etc. (Kimura, 2016). Normally, water Mn is apparently insufficient to meet aquatic animals’ metabolic requirement and their food is considered as the major Mn source (NRC, 2011). Dietary Mn requirement and its importance have been intensively reported for freshwater fish and marine fish species in the nutrition researches (Prabhu et al., 2016). However, limited information is available on the Mn nutrition of crustacean, which only been reported in Chinese mitten crabs (Eriocheir sinensis) (Zhao et al., 2016), new rice shrimp Neocaridina heteropoda, river prawn Macrobrachium nipponense and Indian white shrimp Penaeus indicus (Ali., 2000; Wang et al., 2010; Ding et al., 2020).
Intensive culture of Pacific white shrimp Litopenaeus vannamei (L. vannamei) has developed rapidly and L. vannamei has become the most important crustacean species in aquaculture of many countries (Sharawy et al., 2020). Several experiments have been conducted to determine several mineral requirements and nutritional function for L. vannamei, including zinc (Wu & Chen, 2005), copper (Yuan et al., 2019) and iron (D. Allen Davis et aI., 1992). However, studies reporting the effects of dietary Mn inclusion on physiological and metabolic function in L. vannamei are very scarce. As a component of Mn-SOD superoxide dismutase (Mn-SOD), it has been suggested that Mn help crustaceans maintain antioxidant and immunity function (Chen et al., 2010). To our best knowledge, only one recent paper has demonstrated the optimal dietary Mn requirement for juvenile L. vannamei, ranging from 23.90 mg/kg to 32.26 mg/kg Mn (Cai et al., 2016).
The complex communities of microorganisms that colonize the gastrointestinal tract play an important role to the host's health (Gerritsen et al., 2011). Previous researches mentioned above are conducted largely for examining dietary Mn requirement based primarily on growth performance and antioxidant function; there is a lack of researches investigating the effects of Mn supplementation on the intestinal microbiota in aquatic animals. Inconsistent results have been reported in terms of the Mn's effect on the intestinal microbiota homeostasis using different animal models (Choi et al., 2020; Ding et al., 2020). Therefore, examining the effects of dietary Mn levels on the intestinal microbiota homeostasis may provide a new guide for Mn supplementation in L. vannamei.
Hence, the aim of this study was to evaluate the dietary Mn requirement in L. vannamei based on growth performance and further compare the specific effects of different Mn levels on tissue Mn concentration, antioxidant capacity, nonspecific immune function and intestinal microbiota homeostasis in this species.
2 MATERIALS AND METHODS
2.1 Ethics statement
All experimental procedures were complied with the Standard Operation Procedures (SOPs) of the Guide for Use of Experimental Animals of Ningbo University. The study was approved by the Ethics-Scientific Committee for Experiments on Animals of Ningbo University.
2.2 Experimental diet preparation
The six diets were formulated to contain different Mn levels (MnSO4.H2O as the Mn source): the control group was fed the basal diet without extra Mn supplementation, and the other five treatment groups were supplemented with 15, 30, 45, 60 and 100 mg/kg Mn, respectively. Correspondingly, the measured Mn supplementations in each diet were 10.04, 27.18, 39.57, 54.57, 72.67 and 111.97 mg/kg, respectively. The ingredient and content of the basal diet are listed in Table 1. All ingredients were purchased from Ningbo Tianbang Feed Co. Ltd. (Ningbo, China), and pellet feeds were sealed in vacuum-packed bags and stored at −20 ℃ until use.
| Ingredients (g/kg) | |
|---|---|
| Fish meal (Peru) | 300 |
| Casein | 100 |
| Soy protein concentrate | 80 |
| Krill meal | 150 |
| Wheat flour | 277 |
| Fish oil | 15 |
| Soy oil | 15 |
| Soy lecithin | 20 |
| Mineral premixa | 15 |
| Vitamin premixb | 10 |
| Ca (H2PO4)2 | 15 |
| Choline chloride | 3 |
| Nutrient levelsc | |
| Crude protein (%) | 45.38 |
| Crude lipid (%) | 8.59 |
| Manganese (mg kg−1) | 10.04 |
- a Mineral premix (per kg diet): 12 mg/kg Fe as C6H5O7Fe·5H2O; 25 mg/kg Cu as CuSO4·5H2O; 0.1 mg/kg I as KIO3; 350 mg/kg Mg as MgSO4·7H2O;2000 mg/kg K as K2SO4; 300 mg/kg Na as NaCl; 65 mg/kg Ca as C6H10O6Ca.5H2O; 5 mg/kg Co as CoSO4·7H2O; 60 mg/kg Zn as ZnSO4.7H2O.
- b Vitamin premix (per kg diet): 120 mg/kg D-Ca pantothenate; 200 mg/kg inositol, 60 mg/kg menadione, 100 mg/kg nicotinic acid, 60 mg/kg pyridoxine 50 mg/kg hydrochloride, 60 mg/kg thiamin nitrate, 100 mg/kg tocopherol, 0.1 mg/kg cyanocobalamin, 6 mg/kg biotin, 10 mg/kg folic acid, 5000 mg/kg retinyl acetate, 2000 IU/kg cholecalciferol.
- c Nutrient levels were measured values (dry matter basis).
2.3 Shrimp culture and condition monitoring
This experiment was carried out in Ningbo Marine Fishery Science and Technology Innovation base. Healthy L. vannamei were reared in a semi-intensive culture pond with running aerated water at ambient temperature prior to the experiment. A total of 600 L. vannamei (average initial weight 0.95 ± 0.01 g) were distributed into six dietary treatments for eight weeks at random, each of which had four 300-L cylindrical fibre-glass tanks (filled with 200 L of water) of 25 shrimp per tank. Shrimp were fed three times a day (6–8% of biomass) at 6:00 h (30% of the diets), 12:00 h (40% of the diets) and 18:00 h (30% of the diets). Daily amount of feed was adjusted once every 2 weeks according to the weight of shrimp in each tank. The dead shrimp were removed, weighed and recorded immediately, and above 60% of seawater in each tank was exchanged an hour before the first feeding every morning.
2.4 Sample collection
Following the eight-week feeding period, the number of shrimps in each tank was counted and weighed to obtain the final body weight (FBW), per cent weight gain (PWG), specific growth rate (SGR), survival rate and feed efficiency (FE). PWG, % = [final body weight (g) − initial body weight (g)]/initial body weight (g) × 100; SGR, % day−1 = [Ln (final body weight) − Ln (initial body weight)] × 100/days; FE = weight gain (g, wet weight)/feed consumed (g, dry weight).
Ten shrimp tissues in each tank were randomly collected and were mixed as one sample. Haemolymph samples were collected from the pericardial cavity using a 1-ml syringe and placed into 1.5-ml centrifuge tubes overnight at 4℃ before centrifugation (1811 g, 10 min). The supernatant was transferred into 1.5-ml centrifuge tubes and stored at −80℃ until analysis. Muscle and hepatopancreas samples were collected in a sterile procedure and rapidly frozen in a −80℃ freezer for further analysis. The intestine was collected from the shrimp with tweezers with faeces removed. Then, the intestines were homogenized, stored in a sterile cryotube and frozen in a −80℃ freezer.
2.5 Experimental parameters measured
2.5.1 Analysis of tissue Mn concentration
Tissue Mn concentration was measured as described by a previous study (Jiao et al., 2020). Briefly, 0.1 g tissue samples were mixed with 5.0 ml sulfuric acid and heated on the electric plate at 320℃. After carbonization, approximately 2–3 drops of nitric acid were added every half hour. Cool it to the room temperature and constant volume to a 50-mL volumetric flask when the liquid became clear and bright. The Mn concentration in muscle and hepatopancreas was measured using iCAP 6500 Thermo Fisher ICP-OES (Thermo Fisher Scientific, Waltham, MA, USA).
2.5.2 Analysis of antioxidant enzyme activity and nonspecific immune enzymes activity
Hepatopancreas samples were homogenized with 0.89% physiological saline and then centrifugated at 4000 rpm for 10 min at 4℃ (Eppendorf centrifuge 5418R, Germany). The supernatant of haemolymph and hepatopancreas was collected in 200-μl Eppendorf tubes and stored at −80℃ until the analysis of enzyme activity.
The activity of glutathione (GSH), catalase (CAT), superoxide dismutase (SOD) and antioxidative capacity (AOC), alkaline phosphatase (ALP), lysozyme (LZM), nitric oxide synthase (NOS) were measured using diagnostic reagent kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) according to the manufacturer's instructions.
2.5.3 Analysis of intestinal microbiome
We selected Mn10.04, Mn39.57 (optimum dosage level), Mn111.97 (high dosage level) groups to investigate the effect of Mn supplementation on the intestinal microbiota composition in L. vannamei. Total genome DNA was extracted using CTAB/SDS method as described previously (William et al., 2012) and later tested its concentration and purity using a NanoDrop ND-1000 spectrophotometer (ND-2000; Gene Company Ltd). According to the concentration, DNA was diluted to 1 ng/μl using sterile water. The V4-V5 region of 16S rRNA gene was amplified using 515F-907R primer with the barcode. All PCRs were carried out in 30 μL reactions with 15 μl of Phusion®High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μm of forward and reverse primers and 10 ng template DNA. The PCR program was conducted at an initial denaturation step at 98℃ for 1 min, followed by 30 cycles of 98℃ for 10 s, 50℃ for 30 s and 72℃ for 60 s. PCR products were visualized on a 2% agarose gel and samples with bright main strip between 400 and 450 bp were mixed in equidensity ratios and purified with GeneJET Gel Extraction Kit (Thermo Scientific). Sequencing libraries were generated using NEB Next®Ultra™DNA Library Prep Kit and its quality was measured on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Later, sequencing library was sequenced on an Illumina MiSeq platform and 250 bp/300 bp paired-end reads were generated.
Paired-end reads were merged using FLASH (V1.2.7, http://ccb.jhu.edu/software/FLASH/) when the original DNA fragments are shorter than twice the length of reads. Sequence analyses were performed by UPARSE software (Uparse v7.0.1001, http://drive5.com/uparse/) and sequences with ≥97% similarity were assigned to the same OTUs.
Alpha diversity indices (Simpson, Chao, Shannon and Goods coverage) were calculated with QIIME (version 1.7.0). The microbial community distribution of samples was visualized based on the community composition at phylum and genus levels. The phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) (Galaxy Version 1.0.0) was used to predict bacterial gene function from microbial composition based on 16S rRNA genes and infer from Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation database (Langille et al., 2013). Then, the results were imported into STAMP (version 2.1.3) software for the statistical analysis (Parks et al., 2014).
2.6 Statistical analysis
One-way analysis of variance (ANOVA) was conducted using IBM SPSS statistics 26.0 (SPSS Inc., Chicago, IL, USA). Tukey's multiple range test was conducted to compare the differences of growth performance, tissue Mn concentration, antioxidant enzyme activity and nonspecific immune enzymes activity among different Mn levels. Taking the weight gain rate as the evaluation index, the optimal amount of Mn requirement was determined using the two slope broken-line model (K. R. Robbins and Southern 2006). The independent t test was chosen to compare the differences of intestinal microbiota composition and KEGG functional analysis between two groups. Effects were considered significant at p < .05.
3 RESULT
3.1 Growth performance and feed utilization
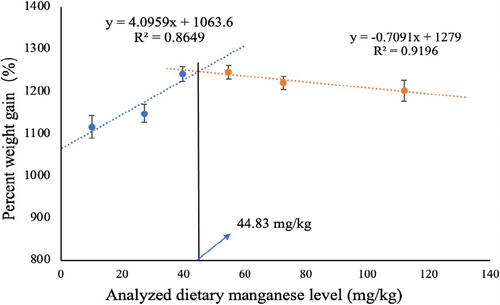
Table 2 shows the effects of dietary Mn levels on growth performance and feed utilization in L. vannamei. FBW, PWG and SGR were significantly affected by dietary Mn levels (p < .05). Compared with the control group, supplementing 39.57 mg/kg, 54.57 mg/kg and 72.67 mg/kg Mn significantly increased (p < .05) FBW, PWG and SGR, while supplementing 27.18 mg/kg and 111.97 mg/kg Mn had no effect on FBW, PWG and SGR in L. vannamei. Survival rate or FE did not reach statistical significance among all treatments (p > .05). Further two slope broken-line regression analysis showed that the optimum dietary Mn requirement based on PWG was determined to be 44.83 mg/kg (Figure 1).
| Parameters | Mn10.04 | Mn27.18 | Mn39.57 | Mn54.57 | Mn72.67 | Mn111.97 |
|---|---|---|---|---|---|---|
| IBWa (g) | 0.95 ± 0.01 | 0.94 ± 0.01 | 0.95 ± 0.00 | 0.95 ± 0.01 | 0.95 ± 0.01 | 0.95 ± 0.00 |
| FBWb (g) | 11.59 ± 0.44c | 11.77 ± 0.47bc | 12.81 ± 0.33a | 12.81 ± 0.39a | 12.59 ± 0.32ab | 12.42 ± 0.50abc |
| PWGc (%) | 1116.3 ± 52.72c | 1147.33 ± 42.43bc | 1241.69 ± 35.66a | 1245.07 ± 33.16a | 1220.53 ± 32.18ab | 1201.80 ± 48.52abc |
| SGRd (% d−1) | 4.46 ± 0.08 c | 4.51 ± 0.06 bc | 4.64 ± 0.05 a | 4.64 ± 0.04 a | 4.61 ± 0.04 ab | 4.58 ± 0.07 abc |
| Survival rate | 0.92 ± 0.07 | 0.86 ± 0.04 | 0.83 ± 0.11 | 0.87 ± 0.06 | 0.93 ± 0.05 | 0.90 ± 0.05 |
| FEe | 0.36 ± 0.02 | 0.34 ± 0.01 | 0.36 ± 0.03 | 0.38 ± 0.01 | 0.40±0.01 | 0.38 ± 0.01 |
- Values are presented as the means ± SD (n = 4). Values in the same line with different superscripts are significantly different (p ˂ .05).
- a IBW, initial body weight.
- b FBW, final body weight.
- c PWG, per cent weight gain.
- d SGR, specific growth rate.
- e FE, feed efficiency.
3.2 Tissue Mn concentration
Table 3 shows the effects of dietary Mn levels on Mn concentration in muscle and hepatopancreas of L. vannamei. Compared with the control group, supplementing 72.67 mg/kg and 111.97 mg/kg Mn significantly increased (p < .05) Mn concentration in hepatopancreas of L. vannamei, while supplementing 27.18 mg/kg, 39.57 mg/kg and 54.57 mg/kg Mn had no effects (p > .05) on Mn concentration in hepatopancreas of L. vannamei. Muscle Mn concentration was not affected (p > .05) by different Mn supplementation in L. vannamei.
| Parameters | Mn10.04 | Mn27.18 | Mn39.57 | Mn54.57 | Mn72.67 | Mn111.97 |
|---|---|---|---|---|---|---|
| Muscle (mg/kg) | 0.37 ± 0.07 | 0.38 ± 0.03 | 0.41 ± 0.06 | 0.40 ± 0.05 | 0.40 ± 0.10 | 0.49 ± 0.14 |
| Hepatopancreas(mg/kg) | 3.14 ± 0.21c | 3.30 ± 0.26c | 3.45 ± 0.36c | 3.57 ± 0.34bc | 4.21 ± 0.45ab | 4.80 ± 0.25a |
- Values are presented as the means ± SD (n = 4). Values in the same line with different superscripts are significantly different (p ˂ .05).
3.3 Antioxidant enzyme activity
Figure 2 shows the effects of dietary Mn levels on antioxidant enzyme activity in serum and hepatopancreas of L. vannamei. Compared with the control group, supplementing 39.57 mg/kg, 54.57 mg/kg and 72.67 mg/kg Mn significantly increased (p < .05) the activity of AOC and GSH in serum and hepatopancreas and CAT in hepatopancreas of L. vannamei. Supplementing 39.57 mg/kg significantly increased (p < .05) the activity of CAT in hepatopancreas of L. vannamei. Supplementing 27.18 mg/kg and 111.97 mg/kg Mn had no effects (p > .05) on antioxidant enzyme activity in serum and hepatopancreas of L. vannamei.

3.4 Nonspecific immune enzymes activity
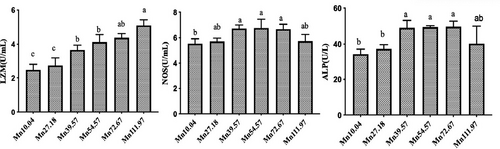
Figure 3 shows the effects of dietary Mn levels on nonspecific immune enzymes activity in serum of L. vannamei. Compared with the control group, supplementing 39.57 mg/kg, 54.57 mg/kg, 72.67 mg/kg Mn significantly increased (p < .05) the activity of LZM, NOS and ALP in serum of L. vannamei, while supplementing 111.97 mg/kg Mn significantly increased (p < .05) the activity of LZM in serum of L. vannamei. Supplementing 27.18 mg/kg Mn had no effects (p > .05) on the activity of nonspecific immune enzymes in serum of L. vannamei.
3.5 Intestinal microbiota
3.5.1 Characteristics of sequencing results
As shown in Table 4, a total of 360146 high-quality reads were produced, with an average of 45955 ± 14241 in Mn10.04 group, 37012 ± 6416 in Mn39.57 group and 37081 ± 3147 in Mn111.97 group. Good's coverage showed that the sequencing data was sufficient to cover the diversity of the bacterial communities in each group. No significant differences (p > .05) were observed in OTUs, diversity index (Shannon and Simpson) and richness index (Chao) among three groups.
| Item | Reads | OTUs | Simpson | Shannon | Chao | Goods coverage |
|---|---|---|---|---|---|---|
| Mn10.04 | 45955 ± 14241 | 1613 ± 113 | 0.97 ± 0.00 | 4.87 ± 0.17 | 2084 ± 186 | 98.05% ± 0.00 |
| Mn39.57 | 37012 ± 6416 | 1459 ± 178 | 0.97 ± 0.01 | 5.02 ± 0.36 | 1729 ± 218 | 98.62% ± 0.00 |
| Mn111.97 | 37081±3147 | 1410 ± 79 | 0.95 ± 0.02 | 4.60 ± 0.40 | 1772 ± 141 | 98.45% ± 0.00 |
- Values represent the mean ± SD (n = 4).
3.5.2 Intestinal microbiota composition
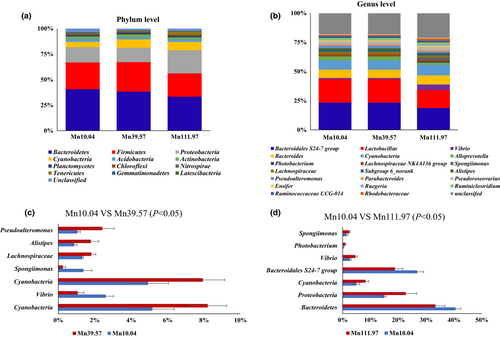
Figure 4 shows the effects of dietary Mn levels on the relative abundance of intestinal microbiota composition at phylum (raw data are shown in Table S1) and genus levels (raw data are shown in Table S2) in L. vannamei. At the phylum level, compared with the control group, supplementing 39.57 mg/kg Mn significantly increased (p < .05) the relative abundance of Cyanobacteria, while supplementing 111.97 mg/kg Mn significantly increased (p < .05) the relative abundance of Cyanobacteria, Proteobacteria and Tenericutes and decreased (p < .05) the relative abundance of Bacteroidetes in L. vannamei. At the genus level, supplementing 39.57 mg/kg Mn significantly increased (p < .05) the relative abundance of Lachnospiraceae, Alistipes and Pseudoalteromonas and decreased (p < .05) the relative abundance of Vibrio and Spongiimonas. Supplementing 111.97 mg/kg Mn significantly increased (p < .05) the relative abundance of Vibrio, Photobacterium and Spongiimonas and decreased (p < .05) the relative abundance of Bacteroidales S24–7 in L. vannamei.
3.5.3 Functional analysis
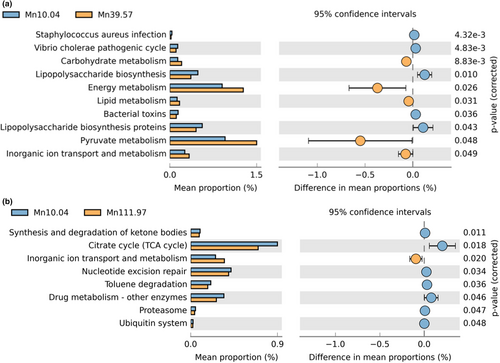
As shown in Figure 5 (raw data are shown in Table S3), compared with the control group, supplementing 39.57 mg/kg Mn significantly increased (p < .05) KEGG pathways mainly involved in inorganic ion transport and metabolism, energy metabolism, carbohydrate metabolism, lipid metabolism and pyruvate metabolism and decreased (p < .05) KEGG pathways mainly involved in bacterial toxins, lipopolysaccharide biosynthesis proteins, Staphylococcus aureus infection, Vibrio cholerae pathogenic cycle. Supplementing 111.97 mg/kg Mn significantly increased (p < .05) KEGG pathways mainly involved in inorganic ion transport and metabolism and decreased (p < .05) KEGG pathways mainly involved in synthesis and degradation of ketone bodies, citrate cycle, nucleotide excision repair, toluene degradation, drug metabolism-other enzymes, proteasome and ubiquitin system.
4 DISCUSSION
The present study demonstrated that the growth performance of L. vannamei was significantly influenced by the dietary Mn levels, and shrimp feeding 39.57 mg Mn/kg diet achieved the best growth performance. Further two slope broken-line regression analysis showed that the optimum dietary Mn requirement based on PWG was determined to be 44.83 mg/kg (Mn content in basal diet was included), similar result was observed by Cai et al (2016) who reported that the optimal dietary Mn supplementation for juvenile L. vannamei ranged from 23.90 mg/kg to 32.26 mg/kg Mn, and growth performance was not affected with increased dietary Mn levels in L. vannamei (67.90 mg/kg) (Cai et al., 2016). The PWG of L. vannamei in our study is over 1116%, which is higher than that in Cai's study (approximately 500 ~ 600%). We speculated that shrimp need more nutrients to support its rapid growth, so the optimal requirement for Mn in our study is higher than that in Cai's study. In addition, feed nutrition levels (protein level, lipid level, etc.), initial weight and breeding density might be partially responsible for the difference in optimal manganese requirement in L. vannamei. Moreover, we observed that shrimp fed 54.57, 72.67 and 111.97 mg Mn/kg, which was higher than the dietary Mn requirement, did not display a significant reduced growth performance in L. vannamei. Macrobrachium nipponense fed the 150 mg/kg Mn diets also did not display a significant reduced growth performance (Ding et al., 2020). Fuller and Benevenga (2004) demonstrated that farmed animals are more tolerant to Mn than other elements, and the outer signs of weight loss and reproductive failure occur only when the concentration of Mn in the feed exceeds 1000 mg/kg (Fuller and Benevenga, 2004). In general, this observation seems to suggest that L. vannamei could tolerate up to 111.97 mg/kg of Mn, without affecting growth performance.
Reactive oxygen species (ROS) is potentially toxic oxygen metabolite formed as a by-product of oxidative metabolism, and its accumulation can initiate extensive intracellular oxidative damage (Apel & Hirt, 2004). Antioxidant enzymes such as GSH and CAT participated in protecting cells against oxidative damage (Mates, 2000). Previous studies have described the correlation between dietary Mn levels and antioxidant status in aquatic animals (Cai et al., 2016; Ding et al., 2020; Tan et al., 2012). In consistent with the growth performance results, we found that supplementing 39.57 mg/kg, 54.57 mg/kg and 72.67 mg/kg Mn significantly increased the antioxidant enzyme activity (AOC and GSH in serum and hepatopancreas and CAT in hepatopancreas) in L. vannamei, indicating that Mn supplementation could improve the antioxidant function. Moreover, 111.97 mg/kg Mn supplementation had no effects on antioxidant enzyme activity in serum and hepatopancreas of L. vannamei. Similarly, Cai et al. (2016) reported that AOC and Mn-SOD activities were higher in the hepatopancreas of prawns fed with 44.48 mg/kg Mn when compared with 15.71 mg/kg Mn (Cai et al., 2016).
Animals have evolved mechanisms to regulate tissue Mn levels by controlling Mn absorption, transportation and excretion (Aschner & Aschner, 2005). We analysed tissue Mn concentration and found that muscle Mn content was not affected by the dietary Mn supplementation in L. vannamei. Similar results were reported that Mn content in muscle increased with Mn supplementation levels ranging from 5.4 mg to 22 mg/kg and then stabilized with further increase in Mn levels in Macrobrachium nipponense (Ding et al., 2020). Whole-body Mn concentrations in fish increased at first and then plateaued once the dietary Mn requirement was met (Maage et al., 2000). Hence, Mn concentration in muscle was not affected with increasing dietary Mn levels in L. vannamei may be due to the homeostatic status after achieving the requirement. However, we found that hepatopancreas Mn concentration was not influenced when L. vannamei fed 27.18–54.57 mg/kg Mn diet and increased when L. vannamei fed 72.67 or 111.97 mg/kg Mn diet, which was different from muscle Mn deposition results. Similarly, Mn deposition of whole body increased with the increase in Mn levels (3.1 mg/kg-19.5 mg/kg) in juvenile yellow catfish Pelteobagrus fulvidraco (Tan et al., 2012). Heavy metals accumulate primarily in the hepatopancreas, and its deposition could be easily influenced by exogenous supplemental metal levels. (Xiong et al., 2020).
Like other invertebrates, nonspecific immunity is shrimp's main defence against pathogens (Jia et al., 2014; Li & Xiang, 2013). LZM, ALP and NOS are identified as important immune indices of shrimp. NO, which is produced from L-arginine catalysed by the enzyme NOS, has been shown to be beneficial for defencing against pathogens (Chakravortty & Hensel, 2003). LZM not only help body tissue to hydrolyse bacterial cell walls, but also induce and regulate the synthesis and secretion of other immune factors, and ALP is an important part of lysosomal enzymes of crustaceans (Liu et al., 2019). Mn is described as a trace mineral associated with better immunity or to functions that support immunity (Bortoluzzi et al., 2020). A lack or an excess of Mn intake can lead to a weakened immune system, and induce inflammation in fish (Aliko et al., 2018; Jiang et al., 2015). So far, no studies had been conducted to determine the nonspecific immune effects of dietary Mn supplementation in shrimp, which made our comparison quite difficult. In our study, we found that the activity of NOS and ALP was increased in serum of L. vannamei with the increase of Mn supplementation (39.57–72.67 mg/kg Mn). Moreover, higher LZM activity was observed with the increase of Mn supplementation (39.57–111.97 mg/kg Mn) in serum of L. vannamei. A high-cited review has summarized that the change of LZM activity in defence mechanisms is far more complex than considered originally (Saurabh & Sahoo, 2008). In healthy body status, higher lysozyme activity was observed in fish fed with probiotics Lactobacillus rhamnosus, Carnobacterium maltaromaticum B26 and Carnobacterium divergens B3, which has the close association with increasing protection against a variety of fish diseases (Kim & Austin, 2006). Enhanced serum lysozyme activity, on the other hand, was also observed in fish after bacterial infection (Hikima et al., 2001). Therefore, variation in the serum lysozyme activity seems to be influenced by the body condition, which might be further discussed combining other indexes.
Currently, understanding how dietary metals modulate host–microbe interactions has become a hot topic in numerous types of animals (Lopez & Skaar, 2018). The intestinal microbiota encounters a broad range of unabsorbed luminal Mn concentration via a diet. It remains unclear whether Mn could influence the intestinal microbiota composition and its metabolic functions, and relevant literature reports are still rare (Chi et al., 2017; Ding et al., 2020). Our result demonstrated for the first time that different Mn supplementation levels could significantly influence intestinal microbiota composition and function in L. vannamei. In this study, we observed that supplementing 39.57 mg/kg Mn significantly influenced intestinal microbiota composition, which was identified as the increased relative abundance of potential beneficial bacteria (Lachnospiraceae, Alistipes, Pseudoalteromonas) and decreased relative abundance of opportunistic pathogens (Vibrio and Spongiimonas). Lachnospiraceae family has been identified to have anti-inflammatory properties, and also helps kill pathogens in the digestive tract (Vacca et al., 2020). Alistipes participates in glucose metabolism through producing succinic acid as the main metabolic end product of glucose fermentation and iso-C15: 0 as their major long-chain fatty acid (Song, 2006). Pseudoalteromonas is identified as a potential probiotics to control infectious diseases against Vibrio harveyi for abalone and crab culture (Morya et al., 2014; Offret et al., 2019). Vibrio and Spongiimonas are well-known pathogenic bacterium, which are associated with causing economic loss in the aquaculture system (Vezzulli et al., 2013; Pedrosa-Gerasmio et al., 2020) Moreover, we observed that supplementing 111.97 mg/kg Mn significantly increased the relative abundance of opportunistic pathogens Vibrio, Photobacterium, Spongiimonas and decreased the relative abundance of Bacteroidales S24–7 in L. vannamei. Members of the Bacteroidales S24–7 family are associated with host–microbiome interactions that impacts the gut function and health, and contribute to the alleviation of inflammation and insulin resistance (Ormerod et al., 2016; Zhang et al., 2018). As the aetiological agent of photobacteriosis, Photobacterium is responsible for important economic losses in cultured fish worldwide (Andreoni & Magnani, 2014). Interestingly, we found that the relative abundance of Cyanobacteria was significantly increased in the intestine of L. vannamei with the increase in Mn supplementation level. It has been reported that Mn is an essential element required by Cyanobacteria, and Cyanobacteria is capable of binding high levels of dissolved Mn which might be used ameliorate metal toxicity of polluted waters (Keren et al., 2002).
Further KEGG functional analysis demonstrated that supplementing 39.57 mg/kg or 111.97 mg/kg Mn significantly increased KEGG pathways mainly involved in inorganic ion transport and metabolism, which might be associated with increased Mn supplementation. Supplementing 39.57 mg/kg Mn significantly influenced KEGG pathways mainly involved in energy metabolism (carbohydrate, lipid, pyruvate) and pathogen infection (bacterial toxins, lipopolysaccharide biosynthesis proteins, Staphylococcus aureus infection, Vibrio cholerae pathogenic cycle) in L. vannamei. Moreover, supplementing 111.97 mg/kg Mn significantly decreased energy metabolisms (citrate cycle, nucleotide excision repair, toluene degradation, proteasome and ubiquitin system), and metabolic capacity to environmental substances (synthesis and degradation of ketone bodies, drug metabolism-other enzymes) in L. vannamei. Mn participates in normal lipid and carbohydrate metabolism through the activity of pyruvate carboxylase. A second function of Mn has been identified as member of SOD enzyme which is required for additional protection from oxidative stress associated with inflammatory responses to some infections (Bortoluzzi et al., 2020). However, Mn could dose dependently induce the decline of energy metabolism and disturb ion homeostasis when animals are exposed to high Mn concentration (Shao et al., 2012; Zhang et al., 2008). This result indicated that the influence of Mn on the physiological and metabolic function might be regulated through intestinal microbiota in L. vannamei.
In the present study, the following conclusions can be drawn that (1) the optimum dietary Mn requirement for cultured L. vannamei was determined to be 44.83 mg/kg based on PWG in formulated feed; (2) dietary Mn supplementation significantly affected the growth performance, Mn deposition in hepatopancreas, antioxidant enzymes activities, nonspecific immune response and intestinal microbiota homeostasis in L. vannamei; (3) although there was no clear growth retardation, high dietary Mn (111.97 mg/kg) supplementation disturbed intestinal microbiota homeostasis and increased susceptibility to pathogens in L. vannamei.
ACKNOWLEDGEMENTS
This research was supported by National Key R & D Program of China (2018YFD0900400), Ningbo Public Welfare Science and Technology Project (202002N3041), Industrial Chain Collaborative Innovation Project of the Demonstration Work on Innovative Development of the Marine Economy of the State Oceanic Administration (NBHY-2017-S2), K. C. Wong Magna Fund in Ningbo University.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




