Assessment of microalgae concentrate as diet for hard clam, Mercenaria mercenaria, larvae
Abstract
The on-site culture of live microalgae represents a major challenge for bivalve hatchery operations due to high cost and labour demands. This study evaluated the efficacy of commercially available microalgae concentrates as a partial or complete replacement diet for live microalgae for culture of the hard clam, Mercenaria mercenaria, larvae. Larvae were fed from D-larval stage to post-set, 2–14 days post fertilization (DPF). Larvae fed the complete replacement diets were significantly smaller than those fed partially replaced or live microalgae diets. Foot development was significantly delayed in larvae fed complete replacement diets compared to partially replaced or live microalgae diets. Although early survival (at 5 DPF) was similar among the treatments, final survival (at 14 DPF) varied significantly among treatments. The average final survival was 69%–77% for larvae fed live microalgae, 60%–61% for larvae fed partially replaced diets and 14%–16% for larvae fed complete replacement diets. These results suggest that a live microalgae diet could be partially replaced by a concentrate to feed hard clam larvae. However, complete replacement diet may be used only for a short duration (up to 3 days) without affecting survival.
1 INTRODUCTION
Bivalve aquaculture (such as oysters, mussels, clams and scallops) offers the most sustainable option for seafood production since they do not require external feed input for grow out. Bivalve aquaculture leaves less of an environmental footprint than other forms of aquaculture (i.e. finfish and crustacean) and helps to restore coastal ecosystems (Willer & Aldridge, 2020). Bivalves are nutritionally rich in omega-3 fatty acids and micronutrients, including iron, zinc, vitamin A and vitamin B12 (Tan et al., 2020; Wright et al., 2018). Even though bivalve aquaculture is an attractive option from ecological and human nutrition perspectives, the growth of this industry is slower than that of finfish and crustacean culture operations. In the last decade, aquaculture production of bivalve species had an annual increase of 2.7% per year, whereas carnivorous fish species had an annual increase of 8.4% (FAO, 2018). Grow-out of bivalves does not require any feed input, thereby making it the most sustainable source of animal protein, yet seed production is heavily reliant on a supply of live microalgae. The need for live microalgae during larval production is considered a major bottleneck for the expansion of bivalve hatcheries necessary for the growth of the industry. Live microalgae production is costly and labour intensive, representing 30%–50% of the operational cost for a bivalve hatchery (Oostlander et al., 2020). Additionally, a single microalgae diet is not optimal to support different stages of bivalve development, thereby necessitating the need for maintaining multiple microalgae species (Utting & Spencer, 1991). The demand for an alternative diet was identified in 1990 (Coutteau & Sorgeloos, 1992), and several options have been explored, such as microalgae concentrate, microalgae paste, dried microalgae, microencapsulated diet, yeast, bacteria, single-cell detritus, cornmeal, cheese whey, gelatine acacia spray bead, emulsion and liposome (Ehteshami et al., 2017; Knauer & Southgate, 1999; Robert & Trintignac, 1997; Tanyaros et al., 2016).
Microalgae concentrates appear to be the most promising of alternative diets for bivalve larvae production based on reported literature. Production involves concentrating mass cultures of microalgae by high-speed centrifugation and flocculation which can condense microalgae 200- to 800-fold (Heasman et al., 2000; Knuckey et al., 2006). The recent development of commercially available microalgae concentrates could facilitate the production of bivalve larvae by minimizing the need for an on-site live microalgae culture facility (Reed & Henry, 2014). Microalgae concentrate can be stored at 2–4℃ for 1–4 months, thereby providing ‘off the shelf’ convenience (Camacho-Rodríguez et al., 2016; Welladsen et al., 2014). The use of microalgae concentrates as a dietary source has shown promise for some invertebrate larvae, including the eastern oyster, Crassostrea virginica (Rikard & Walton, 2012), winged pearl oyster, Pteria penguin (Wassnig & Southgate, 2016), giant clam, Tridacna noae (Southgate et al., 2017) and sea cucumber, Holothuria scabra (Militz et al., 2018). Southgate et al. (2016) produced winged pearl oyster larvae using microalgae concentrate as the only dietary source. Even though survival rates of larvae were relatively low at the end of the larval cycle (1%–13%), these studies have laid the foundation to test the efficacy of microalgae concentrate for larvae production in other bivalve species.
The hard clam, Mercenaria mercenaria, is the most important bivalve aquaculture species on the East Coast of the United States, generating $56 million in annual sales (USDA, 2018). Hard clam seed production is almost entirely reliant on hatchery-produced seed (FAO, 2018). Hard clam hatcheries depend on live microalgae production for larvae culture, which is a long-standing challenge from technical and economic perspectives. Small-scale hatcheries often lack the infrastructure and technical capacities for on-site microalgae production; therefore, the efficacy of an alternative “off the shelf” dietary source would greatly facilitate small-scale hatchery development. Hatcheries with on-site algae production capabilities may experience “crashes” of their algae cultures for various reasons from time to time, resulting in the need to temporarily reduce the feed rate. Furthermore, natural disasters, such as hurricanes, are regular events on the East Coast of the United States where many hard clam hatcheries are located. Associated power outages can negatively impact microalgal production. Recovery to full-scale production may not occur for weeks. The use of an alternative dietary source as a supplement or a replacement for live microalgae during such periods would allow for continuous larval production despite the lack of live algae. This study aims to determine whether microalgae concentrates can be used as a complete or partial replacement to a live microalgae diet for hard clam larvae culture.
2 MATERIAL AND METHODS
An ethical approval was not required for experiments with marine bivalve (invertebrate) larvae according to guidelines for the use of animals in research at Florida Atlantic University.
2.1 Larvae acquisition and rearing
This experiment was conducted in the aquaculture facility of Florida Atlantic University's Harbor Branch Oceanographic Institute (FAU-HBOI). Seaventure Clam Co., a commercial hatchery located on the FAU-HBOI campus, supplied the hard clam larvae for this study (October 2020). Larvae were fed Tahitian strain, Isochrysis galbana, (T. Iso) in the hatchery for 2 days post fertilization (DPF), prior to experimental initiation. Larvae were then transferred to 6-L plastic containers at a stocking density of 3.5 larvae/ml having three replicate containers per treatment. The average length of larvae at the start of the experiment was 96.5 ± 5.4 µm. Salt well water treated with UV sterilization and filtered through a 1-μm bag was used for larvae rearing. Temperature, salinity and pH were maintained at 25–26℃, 27–29 ppt and 8.0–8.3 respectively. Culture water was completely exchanged every 24 hr. Larvae from each plastic container were transferred to a downweller at 7 DPF once foot development was noted (pediveliger stage). For each treatment, three replicate downwellers were placed inside a 60-L tank containing UV-treated, 1 μm filtered salt well water.
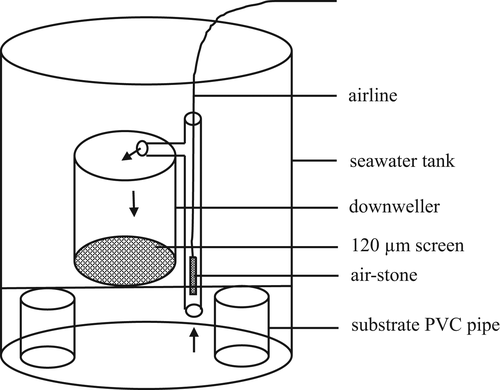
The design of the downweller is described in Hassan et al. (2021). Briefly, a downweller was made of PVC pipe (6-inch diameter × 9-inch length) with a 120-µm screen fitted at the bottom with PVC coupling. Each downweller was placed 3 inches off the bottom of the tank using PVC pipe and a plastic grid (Figure 1). An air-stone was strung inside a 1-inch pipe which was placed outside the downweller. The aeration created airlifted water flow in the downweller (Figure 2).
2.2 Live microalgae
Two microalgae species were used in this study: T. Iso and Chaetoceros gracilis. Microalgae inoculums were purchased from Algagen (https://www.algagen.com/). Batch cultures were produced in the indoor algae culture facility of the Seaventure Clam Co hatchery. Flagellates were cultured with F/2 nutrient media, whereas diatoms were cultured with a combination of F/2 and metasilicates. The temperature was maintained at 20℃ and pH at 8.0–8.2 by CO2 injection.
2.3 Microalgae concentrate
Two microalgae concentrate products were purchased from Reed Mariculture: “Isochrysis 1800®” and “Shellfish diet 1800®”, which were stored at 4℃ for the duration of the study. The products were used individually in feeding treatments. Microalgae suspensions were prepared using the recommended addition of 1-ml concentrate to 400-ml sterilized salt well water in a clean glass jar, followed by gentle mixing. To remove any clumps, a 20-μm sieve was used to filter the microalgae suspensions prior to larval container/tank addition.
2.4 Weight standardization of microalgae
Size variations of algae were standardized based on the dry weight and cell density (Abbas et al., 2018; Mamat & Alfaro, 2014). Triplicate 100-ml microalgae samples were filtered through preweighed 1.2-μm GF filters, washed with 50 ml 0.5 M ammonium formate to remove salt residue and dried in the oven at 70℃ for 18 hr. Cell density was determined using a haemacytometer and a light microscope. Based on dry weight, 1 cell of T. Iso ≈ 0.53 cell of C. gracilis, ≈ 0.74 cell of Isochrysis 1800®, ≈ 0.42 cell of Shellfish diet 1800®.
2.5 Experimental Diets
At the beginning of the experiment, larvae were fed 10,000 cells/larvae with T. Iso (or equivalent cell number based on dry weight standardization for other algae). The daily ration was equally divided and fed twice daily, morning and afternoon. The feed ration was increased 20% every day, and larvae were cultured until 14 DPF. The experiment consists of six treatments (Table 1).
| Treatment | Diet type | Diet (inclusion level) |
|---|---|---|
| 1 | Live microalgae | Isochrysis galbana T. Iso (100%) |
| 2 | Live microalgae | T. Iso (50%) + Chaetoceros gracilis (50%) |
| 3 | Partial replacement | T. Iso (50%) + Isochrysis 1800® (50%) |
| 4 | Partial replacement | T. Iso (25%) and C. gracilis (25%) + Shellfish diet 1800® (50%) |
| 5 | Complete replacement | Isochrysis 1800® (100%) |
| 6 | Complete replacement | Shellfish diet 1800® (100%) |
2.6 Assessment of growth, development and survival
Percentage of foot development and survival in each replicate was calculated based on a subsample of at least 50 larvae. A total of 10- to 20-ml larvae subsample was concentred and observed under microscope using 1-ml Sedgewick Rafter Counting Chambers. Survival of larvae was calculated based on the number of live larvae present in the subsamples at each sampling date. Larvae with an empty shell and no movement were considered dead.
2.7 Statistical analysis
Assumptions of normality and homogeneity of variances were tested for data by the Shapiro–Wilk and Levene's statistical test. Percentage data (foot development and survival) were arcsine transformed before analysis. Treatment effects on growth, foot development and survival were analysed using one-way analysis of variance (ANOVA). Tukey's post-hoc test was applied when a significant difference (p < .05) was found among the response variables. All the data were analysed using SPSS v. 27 (IBM).
3 RESULTS
3.1 Growth
Significant differences in larval size were observed among the treatments beginning 5 DPF (p < .05; Table 2). Larvae fed live T. Iso and C. gracilis (T2) were significantly larger than larvae in other treatments. Larvae fed 100% microalgae concentrate (T5, T6) were significantly smaller than larvae in other treatments. Larvae fed the partial replacement diets (T3, T4) were smaller than those fed a live microalgae diet but larger than those fed a complete replacement diet (Table 2). No significant difference was noted in larval size of those fed two microalgae concentrate products.
| Treatment | Age of larvae | |||
|---|---|---|---|---|
| 5 DPF | 8 DPF | 11 DPF | 14 DPF | |
| 1 | 128.7 ± 8.8b | 167.4 ± 11.1b | 218.8 ± 19.0b | 308.8 ± 25.5b |
| 2 | 138.4 ± 9.0a | 185.7 ± 11.3a | 247.4 ± 17.6a | 349.0 ± 26.4a |
| 3 | 123.6 ± 7.9c | 158.2 ± 10.5c | 204.7 ± 16.7c | 283.6 ± 20.6c |
| 4 | 128.6 ± 8.1b | 166.2 ± 10.1b | 214.2 ± 15.3b | 291.2 ± 22.0c |
| 5 | 119.9 ± 7.7cd | 143.5 ± 9.5d | 169.0 ± 13.8d | 214.2 ± 15.6d |
| 6 | 118.8 ± 7.6d | 138.9 ± 9.9d | 164.1 ± 16.2d | 209.4 ± 19.5d |
Note
- Different letters in the same column indicate significant differences among treatments (one-way ANOVA, α = .05, a > b > c > d).
- Abbreviation: DPF, days post fertilization.
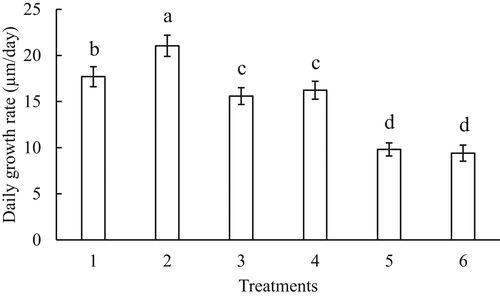
The daily growth rate of larvae was significantly different among treatments (F = 337.2, df = 5, p < .05). The highest daily growth rate was found in larvae fed a live T. Iso and C. gracilis (21.0 µm/day, T2). The slowest growth rate was found in larvae fed solely Isochrysis 1800® (9.8 µm/day, T5) or Shellfish diet 1800® (9.4 µm/day, T6). Larvae fed partial replacement diets achieved higher growth rates than complete replacement diets, but the growth rate was suboptimal compared to live microalgae diets (Figure 3).
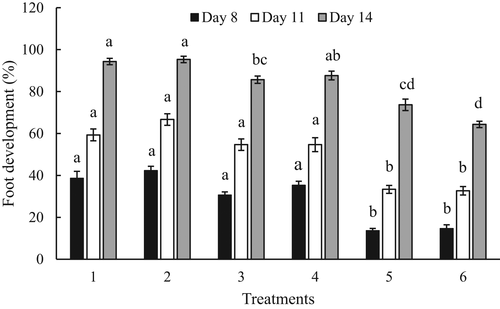
3.2 Development
Foot development began at 7 DPF (pediveliger stage). Significant differences in the percentage of foot development were found among treatments on 8 DPF (F = 30.2, df = 5, p < .05), 11 DPF (F = 20.8, df = 5, p < .05) and 14 DPF (F = 24.4, df = 5, p < .05; Figure 4). Larvae fed the complete replacement diets (T5, T6) represented a significantly lower percentage of foot development than those fed partial replacement (T3, T4) or live microalgae diets (T1, T2). Rate of foot development among larvae fed live microalgae and the partial replacement diets were similar until 11 DPF but significantly different on 14 DPF. The average rate of foot development on 14 DPF was 94%–95% for live microalgae (T1, T2), 85%–87% for partial replacement diets (T3, T4) and 64%–73% for complete replacement diets (T5, T6; Figure 4).
3.3 Survival
There was no significant difference in survival up to 5 DPF among the dietary treatments (F = 337.2, df = 5, p = .15). However, significant differences in survival were found on 8 DPF (F = 16.7, df = 5, p < .05), 11 DPF (F = 32.1, df = 5, p < .05) and 14 DPF (F = 97.2, df = 5, p < .05; Table 3). Larvae fed either a live or partial replacement diet had a similar survival rate up to 11 DPF. Larvae fed the live microalgae diet, consisting of T. Iso and C. gracilis (T2), had the highest final survival rate (76.6%), whereas those fed complete replacement diets (T5, T6) had the lowest survival rate (13.6%–15.7%). There was no statistical difference in larval survival among treatments fed Isochrysis 1800® versus Shellfish diet 1800®, either fed as a partial replacement or complete replacement.
| Treatment | Age of larvae | |||
|---|---|---|---|---|
| 5 DPF | 8 DPF | 11 DPF | 14 DPF | |
| 1 | 89.3 ± 4.7 | 81.8 ± 3.0a | 77.2 ± 4.3a | 69.8 ± 5.2ab |
| 2 | 91.5 ± 2.2 | 86.9 ± 2.8a | 81.7 ± 3.2a | 76.6 ± 4.2a |
| 3 | 88.7± 3.5 | 81.5 ± 1.8a | 69.8 ± 2.1a | 60.1 ± 4.5b |
| 4 | 87.1 ± 2.1 | 79.0 ± 4.0a | 70.7 ± 7.2a | 61.2 ± 6.0b |
| 5 | 87.1 ± 5.1 | 58.4 ± 2.8b | 35.0 ± 6.6b | 15.7 ± 4.8c |
| 6 | 81.2 ± 5.8 | 63.0 ± 5.3b | 40.7 ± 8.9b | 13.6 ± 2.3c |
Note
- Different letters in same the column indicate significant differences among the treatments (one-way ANOVA, α = .05, a > b > c).
- Abbreviation: DPF, days post fertilization.
4 DISCUSSION
Studies on the successful culture of invertebrate larvae using alternative diets are limited. This is the first known study of hard clam larvae production using commercially available microalgae concentrate diets. Results of this study indicate that hard clam larvae can be cultured using microalgae concentrates as a partial or complete replacement diet to live microalgae; however, growth and survival would be lower compared to a live microalgae diet. Larvae fed the partial replacement and complete replacement diets had 12% and 35% smaller shell length, respectively, than those fed live microalgae. Similarly, larvae fed the partial replacement and complete replacement diets had 12% and 60% lower survival, respectively, than those fed live microalgae.
Although feeding microalgae concentrate decreased the growth of hard clam larvae, the growth of hard clam larvae was comparable to literature reported growth rates of other species fed commercially available microalgae concentrates. In this study, larvae fed Isochrysis 1800® reached 214 µm size on 14 DPF with a growth rate of 9.8 µm/day. Southgate et al. (2016) reported winged pearl oyster larvae fed Isochrysis 1800® reached 240 µm size on 17 DPF at a growth rate of 10.3 μm/day. As such, hard clam larvae fed Isochrysis 1800® in this study reached 143.5 µm size at 8 DPF, whereas Pacific oyster larvae fed concentrate diets reached 107.6 µm size at 7 days post hatch (Ponis et al., 2003). The justification of such growth comparison is arguable due to variations of natural growth among the species used in different studies, but this comparison provides a proxy measurement of growth rate being the microalgae concentrate as only diet source.
In this study, clam larvae fed a partial replacement diet had a lower growth rate than if fed a live microalgae diet. Similarly, larval growth was impacted when dried Tetraselmis was used to replace 25%–50% live microalgae in black-lip pearl oyster, Pinctada margaritifera, cultured up to 13 days (Doroudi et al., 2002). Total replacement of a live microalgae diet produced lower shell growth in black-lip pearl oyster compared to a 50% or 75% replacement diet. However, the authors noted less impact of replacement diets when larvae aged over 13 days (Doroudi et al., 2002). Heasman et al. (2000) found 10%–15% lower growth using microalgae concentrate of Pavlova lutheri in combination with Chaetoceros calcitrans compared to a live microalgae diet consisting of Tahitian Isochrysis, P. lutheri and C. calcitrans. Supplementing live microalgae with microalgae concentrates produced similar growth in Pacific oyster larvae compared to a live microalgae diet (Brown & Robert, 2002). In that study, the diets consisted of live T. Iso combined either with fresh or concentrated Chaetoceros calcitrans forma pumilum (1:4 ratio of T. Iso and C. calcitrans). Interestingly, Ponis et al. (2003) reported better growth and survival of Pacific oyster larvae fed microalgae concentrates than live microalgae over a 7-day feeding period (live versus concentrated P. lutheri + T. Iso and T. Iso + C. calcitrans). One potential difference is that these studies used freshly prepared microalgae concentrates within 1–2 weeks of preparation as opposed to commercially available microalgae concentrates used in the current study which are generally used within several months after preparation.
In this study, poor survival (13.6%–15.7%) was found when hard clam larvae were fed solely microalgae concentrates (Isochrysis 1800® or Shellfish diet 1800®). Previous studies have reported a low survival rate of larvae when commercially available microalgae concentrate was fed exclusively (Table 4). For example, Southgate et al. (2016) reported 6.4% survival of winged pearl oyster, Pteria penguin, larvae fed 1:1 mix of Isochrysis 1800® and Pavlova 1800®. Duy et al. (2016) reported 6.7%–13.7% survival of sandfish, Holothuria scabra, fed Isochrysis 1800®, Pavlova 1800® or Thalassiosira weissflogii1200® fed as a single species or a ternary diet. Militz et al. (2018) reported 1.1% survival of sandfish larvae fed Isochrysis 1800® and Shellfish diet 1800®. Wassnig and Southgate (2016) reported higher survival (54.1%) of winged pearl oyster larvae, but this study evaluated survival of D-stage larvae at 7 DPF, whereas other studies reported survival at the end of the larval cycle.
| Species | Product used | Feeding protocol | Outcome | References |
|---|---|---|---|---|
| Pteria penguin (bivalve) | Isochrysis 1800® and Pavlova 1800® | (a) 1:1 ratio of Isochrysis 1800® and Pavlova 1800® at 1 to 20 × 103 cells/ml from 2 to 17 DPF, twice per day | (a) Survival rate 6.4% | Southgate et al. (2016) |
| (b) 1:1 ratio of Isochrysis 1800® and Pavlova 1800® at 20 to 60 × 103 cells/ml from 17 to 105 DPF, twice per day | (b) Survival rate 4.7% | |||
| Isochrysis 1800®, Pavlova 1800® and Shellfish Diet 1800® | (a) 1:1 ratio of Isochrysis 1800® and “Pavlova 1800 at 5, 10 or 15 × 103 cells/ml from 2 to 7 DPF, once daily | (a) Up to 54.1% survival | Wassnig and Southgate (2016) | |
| (b) 1:1:1 ratio of “Isochrysis 1800®”, “Pavlova 1800®” and “Shellfish Diet 1800®” at 10, 15 or 20 × 103 cells/ml from 8 to 10 DPF, once daily | (b) Up to 35.3% survival | |||
| Holothuria scabra (echinoderm) | Isochrysis 1800® | (a) 10,000 cells/mL per day on 2 DPF with 10% daily increase in cell number up to 25 DPF, fed three times a day | (a) Survival rate 6.7% | Duy et al. (2016) |
| Pavlova 1800® | (b) 10,391 cells/ml per day on 2 DPF with 10% daily increase in cell number up to 25 DPF, fed three times a day | (b) Survival rate 6.7% | ||
| TW 1200® | (c) 3926 cells/ml per day on 2 DPF with 10% daily increase in cell number up to 25 DPF, fed three times a day | (c) Survival rate 13.7% | ||
| Ternary diet | (d) 1:1:1 ratio of three products based on dry weight, fed three times a day | (d) Survival rate 8.5% | ||
| Isochrysis 1800® and Shellfish diet 1800® | Isochrysis 1800® fed on 2–5 days at 10,000–25,000 cells/ml, mix of Isochrysis 1800® and Shellfish diet 1800® on 6–25 days at 25,000 cells/ml, Shellfish diet 1800® fed on 25–40 days at 30,000 cells/ml, fed twice daily | Survival rate 1.1% | Militz et al. (2018) |
- Abbreviation: DPF, days post fertilization.
Differences in the performance of larvae in response to a live or microalgae concentrate diet are likely to be a function of multiple factors, such as nutritional quality, physical properties of the cell or capacity of larvae to ingest and digest. Microalgae cells can be damaged, and nutrients can leach from the algae cells due to processing and preservation. Loss of motility, cellular damage and incomplete resuspension were observed following preparation of microalgae concentrate by flocculation (Ponis et al., 2003). Brown (1995) reported an 11%–94% loss of the ascorbic acid content of microalgae concentrates due to processing and storage.
Along with nutritional value, variation in larval performance can also arise due to the preference of ingestion for living particles. Pacific oyster larvae grazed twice the number of fresh P. lutheri cells than concentrated cells (Ponis et al., 2003). Rejection of non-living particles was observed in Pacific oysters and the bay mussel when dried saltmarsh cordgrass particles (3–20 µm) were provided along with live algae (Ward et al., 1998). Hard clam larvae transition from velum-based suspension feeding to gill-based feeding at the pediveliger stage (the velum present at the early pediveliger stage disappears, and a siphon is formed as larvae metamorphose to post-set). To avoid larval mortality during metamorphosis, the energy requirement must meet via stored energy in body tissue or an external dietary source. The biochemical composition and the tissue energy content in the bear paw clam, Hippopus hippopus, indicated a caloric loss in larval tissue during metamorphosis (Southgate, 1988). In this study, the high mortality rate of hard clam after 8 DPF indicates that the energy requirement was not met when fed a complete replacement diet. However, larvae fed a live microalgae and partial replacement diet might have had better energy storage prior to metamorphosis which resulted in a relatively low mortality rate.
Microalgae concentrate cells are non-motile and negatively buoyant, indicating a greater chance of settling at the tank bottom compared to live microalgae (Braley et al., 2018). In this study, higher settlement of uneaten food was visually observed in the containers fed concentrated microalgae than those fed live microalgae; however, analysis of bacterial populations in the water was not conducted. A higher bacterial load might be expected in larval culture system fed microalgae concentrate compared to those fed live microalgae due to an increased chance for the proliferation of bacterial populations as a higher number of cells settle to the bottom of culture tanks. Small clumps and strands of microalgae were also observed even though care was taken to prevent clumping by passing the concentrated microalgae through a 20-µm screen prior to tank addition. Southgate et al. (2016) reported similar observations with clumping when cultured winged pearl oysters were fed microalgae concentrate. Microalgae concentrate negatively influenced survival from pediveliger to juvenile stage of giant clam larvae, which was assumed to be due to potential fouling in the tanks by uneaten microalgae concentrate that created an unsuitable environment (Braley et al., 2018).
5 ECONOMIC FEASIBILITY AND CONCLUSION
For microalgae concentrate diets to be considered in routine hatchery production, their use must be cost-effective compared to the use of live microalgae. Live microalgae production includes recurring labour and chemicals/nutrients cost plus capital investment for the culture facility. Concentrated microalgae, on the other hand, provides “off the shelf” convenience. The average cost for culturing one million eastern oyster, Crassostrea virginica, larvae using a concentrated microalgae diet was 6.8 USD for the 14-day larvae culture period (Rikard & Walton, 2012). However, use of concentrated microalgae may require additional labour costs due to the need for more frequent and thorough cleaning of tanks and equipment due to fouling. The use of concentrated microalgae may have future potential due to cost-effectiveness at a smaller scale, but its utilization in large-scale hatchery production is currently unlikely due to the poor survival seen in hard clam when concentrated microalgae was the sole food source. Braley et al. (2018) determined that additional management procedures put in place to deal with the associated fouling that occurred with the use of microalgae concentrates, such as antibiotics and more frequent water exchange did not result in improved production compared to a live microalgae diet. As such, incorporating microalgae concentrates into semi-intensive or intensive hatchery culture facilities did not provide any production benefit for giant clam larvae (Braley et al., 2018). On the other hand, the use of microalgae concentrates for a temporary replacement or a supplement to live microalgae when algae production is suboptimal shows promise (Ponis et al., 2003).
Even though inferior growth and survival of larvae were achieved by feeding microalgae concentrate, production of larvae using microalgae concentrate as the sole dietary source is considered a step forward for hard clam aquaculture. This study demonstrated the applicability of using microalgae concentrate diet for hard clam larvae production for emergency use during food shortage due to natural disasters or shortfalls in live microalgae production. Further studies should attempt to improve growth and survival by investigating the biological (such as ingestion, digestion and assimilation ability at different life stages) and environmental factors (such as minimize fouling in the tanks, clumping and bacterial load) during larval culture.
ACKNOWLEDGEMENTS
This research was supported by sponsored research agreement of Florida Atlantic University (FAU-SRA # 19-284). Seaventure Clam Co. sponsored funds for this research. We thank Eddie Perri and Richard Baptiste for help on system design. This is FAU contribution no # 2301.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this article. Raw data are also available from the corresponding author upon reasonable request.




